Short communication
Effect of fruit maturity on efficiency of
1-methylcyclopropene to delay the ripening of bananas
D.R. Harris
a,b,*, J.A. Seberry
b, R.B.H. Wills
a, L.J. Spohr
baCentre for Food Industry Research and De6elopment,Faculty of the Central Coast,Uni6ersity of Newcastle,PO Box127,
Ourimbah,NSW2258,Australia
bHorticultural Research and Ad6isory Station,NSW Agriculture,Locked Bag26,Gosford,NSW2250,Australia Received 7 March 2000; accepted 25 July 2000
Abstract
Three bunches of unripe ‘Williams’ banana fruit of different maturity, 173, 156 and 71 days from bunch emergence, were harvested. Fruit from the top, bottom and middle hands from each bunch were fumigated for 24 h with 1-methylcyclopropene (1-MCP) at 0, 5, 50 or 500 nl l−1at 20oC. All fruit were then stored at 20oC in air containing
0.1ml l−1ethylene and the time taken for each fruit to ripen (green life) was noted. The green life of fruit treated with
500 nl l−11-MCP varied with fruit maturity. In the two most mature bunches it was 27.992.3 days, 4-fold longer
than fruit fumigated with 0 nl l−11-MCP (6.790.6 days). In the least mature bunch, green life was 39.793.0 days,
1.5-fold longer than fruit fumigated with 0 nl l−11-MCP (25.792.5 days). Most fruit treated with 500 nl l−11-MCP
showed an unacceptable uneven skin colouration when ripe. There was no significant effect on green life of 1-MCP at 50 nl l−1and 5 nl l−1. Other fruit from these bunches were not exposed to 1-MCP and were held in ethylene-free
air until ripe. In the two most mature bunches, these fruit had a significantly shorter green life (11.295.6 days in hand 1; 18.994.1 days in hands 4 and 6) than fruit that were fumigated with 500 nl l−11-MCP. In the least mature
bunch, however, these fruit had a significantly longer green life (56.095.9 days) than 1-MCP treated fruit. Since the effectiveness of 1-MCP varied with fruit maturity and in any commercial consignment there is a mixture of fruit maturity, it is concluded that 1-MCP has limited commercial potential for the storage of unripe ‘Williams’ bananas. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Banana; Ripening; 1-methylcyclopropene; Maturity; Ethylene
www.elsevier.com/locate/postharvbio
1. Introduction
1-Methylcyclopropene (1-MCP) has been re-ported to delay or reduce ethylene induced effects on senescence in a variety of potted flowering
* Corresponding author. Tel.:+61-2-43481946; fax:+ 61-2-43481910.
E-mail address:[email protected] (D.R. Har-ris).
plants and cut flowers (Serek et al., 1994, 1995a, 1996; Porat et al., 1995; Sisler et al., 1996a; Heyes and Johnston, 1998; Newman et al., 1998). Effects of 1-MCP on fruit and vegetables include inhibit-ing the ripeninhibit-ing of tomatoes (Serek et al., 1995b; Sisler et al., 1996b), delaying senescence of straw-berries (Ku et al., 1999) and broccoli (Ku and Wills, 1999) and inhibiting the degreening of or-anges while not suppressing other ethylene-in-duced effects such as chilling injury (Porat et al., 1999).
While 1-MCP has been shown to delay the ripening of bananas, the effect of 1-MCP is quite variable among the reported studies. Sisler et al. (1996b) showed that 24 h fumigation with 10 nl l−1
1-MCP was sufficient to protect fruit for 11 – 12 days at 25oC against 18 h exposure to 1000 ml l−1ethylene, while bananas treated with 0.7 nl l−1 1-MCP did not respond for 7 days and 0.4 nl
l−1 1-MCP did not give any protection. Golding
et al. (1998) reported that 6 h fumigation with 450 ml l−1 1-MCP extended the green life (time to ripen) of bananas held in air from 20 – 30 days, and from 2 – 20 days when held continuously in 500 ml l−1propylene at 20oC. They also reported that 1 h exposure to 45 ml l−1 1-MCP extended the green life of bananas when applied 6 or 12 h after the start of exposure to 500 ml l−1 propyl-ene, but not after 24 h exposure. Macnish et al. (1998) demonstrated a 4.4-fold extension in shelf life of ‘Cavendish’ fruit at 20oC, treated with 15
ml l−1
1-MCP for 12 h and then exposed to 100 ml l−1 ethylene for 12 h, compared with fruit not
treated with 1-MCP but similarly exposed to eth-ylene. Jiang et al. (1999a) demonstrated that a 24 h exposure to either 500 or 1000 nl l−1
1-MCP at 20oC extended the green life of ‘Cavendish’
ba-nanas from 16 – 31 days in the absence of ethylene, compared with untreated controls. Jiang et al. (1999b) showed that a 1 h exposure to 1000 nl l−1
1-MCP at 20o
C eliminated the effects of a 24 h exposure to 100ml l−1ethylene for at least 5 days (the duration of the experiment) in ‘Cavendish’ fruit and that a 12 h exposure to 50 nl l−11-MCP
was just as effective. In these studies no reference was made to fruit maturity which could have an interactive effect on ripening, since sensitivity to ethylene increases with increasing physiological age of bananas (Liu, 1976).
This study examined the effectiveness of 1-MCP to protect preclimacteric bananas of different ma-turity from continuous exposure to levels of ethyl-ene, commonly encountered in the market place prior to gas ripening. Ethylene at 0.1 ml l−1 was used as the background concentration as Wills et al. (1999) found it to be present at that level around preclimacteric bananas in commercial sit-uations. Our hypothesis was that there was a significant interaction between the effectiveness of 1-MCP and the maturity of the banana fruit.
2. Materials and methods
Three bunches of banana fruit (Musa sp., AAA group, Cavendish subgroup, cultivar ‘Williams’) were collected from a commercial grower in the Macksville district of New South Wales, Australia on 28 January 1999. The bunches (designated as A, B and C) at harvest were estimated from the grower’s records to be 173, 156 and 71 days, respectively, from bunch emergence. Hands were numbered from 1 (largest and most proximal hand) to 6 or 7 (most distal) and represent fruit age. Finger diameter was measured for each finger on each hand. Callipers were placed two-thirds down the finger towards the blossom end, and the minimum diameter obtainable was recorded. Hands 1 (oldest), 4 and 6 (youngest) were selected from bunches B and C; and hands 1, 4 and a mixture of 6 and 7 were selected from bunch A. The mixture of hands 6 and 7 were then treated as one hand. Wing fruit were removed from the hands. The remaining fruit were then cut into singles and dipped in a solution containing 400 mg l−1a.i. of the fungicide thiabendazole (‘Tecto
90’, Merck, Sharp and Dohme, Granville, NSW, Australia) for 2 min and then dried with a paper towel. Fourteen fingers from each hand were se-lected for uniformity. Three fruit from each hand were fumigated at 20oC in sealed 10 l plastic
buckets for 24 h with air containing 0, 5, 50 or 500 nl l−1 1-MCP. Fruit were then placed singly
into a 1.8 l glass jar and ventilated at 60 ml min−1
with humidified air containing 0.1ml l−1 ethylene at 20oC. The remaining two fruit from each hand
into 1.8 l glass jars and ventilated at 60 ml min−1
with humidified ethylene-free air.
The source of 1-MCP was Ethyblock®(Floralife,
Burr Ridge, Illinois). One gram of Ethyblock powder releases 1.83 ml 1-MCP gas at 20°C when dissolved in 20 ml of aqueous 2% KOH (Floralife, Ethyblock product specification sheet). Calculated amounts of powder were weighed into 20 ml vials and the vials placed in 10 l buckets. KOH solution (15 ml) was added to the vials and the buckets were immediately sealed. Ethylene-free air was obtained by passing dried, compressed air through a series of 4×5 l chambers filled with Purafil®
(Purafil, At-lanta, Georgia).
Air streams containing 0.1ml l−1ethylene were generated by mixing metered flows of a standard containing 100 ml l−1 ethylene in nitrogen (BOC Gases, Sydney) with air. Ethylene was monitored at the inlet ports of representative glass jars using gas chromatography as previously described in Wills et al. (1999). Both air and the ethylene mixture were humidified at 20oC by passing through 25 l plastic
drums3
4filled with water.
The carbon dioxide level in the effluent gas stream from each jar was measured daily by passing through an Infra Red Gas Analyser (Horiba, Kyoto). Calibrations were performed at regular intervals throughout the experiment using a stan-dard carbon dioxide gas mixture (BOC Gases, Sydney) and carbon dioxide-free air. Respiration rates were calculated from the carbon dioxide emissions, and the end of green life was determined for each banana by attainment of the respiratory climacteric. Fruit were then rated on a subjective scale for colour development. The peel colour of fruit was scored 1 (normal yellow colour), 2 (persis-tent green tip) and 3 (blotchy green and yellow).
The influence of fruit maturity and 1-MCP treatment on green life was modelled using a mixed linear regression approach (Searle, 1971) which allowed the separation of variance components into fixed and random effects. Natural logarithms of green life were taken to normalise the data. Analysis of loge(green life) was conducted using the REML
directive in Genstat 5, Release 4.1, 3rd edition. In a preliminary analysis, no difference was found between the green life of fruit from the
bunches with maturity levels of 156 and 173 days. Data from these two bunches were combined, so that there were now two replications of a ‘most mature’ maturity level, as well as the original ‘least mature’ 71 days maturity.
The blocking strata are given by: bunch/hand/ ba-nana, where bunch, hand and banana are factors with 3, 3 and 14 levels respectively. The ANOVA decomposition of the strata are as follows: bunch — maturity, residual; bunch.hand — age, residual;
bunch.hand.banana — treatment, treatment.
maturity, treatment.age, treatment.maturity.age, residual.
The model was given by
loge(green life)
=maturity+A+maturity.A+maturity.A.hand
+bunch+bunch.hand,
where the italicised terms were included in the model as random effects and A=1-MCP treat-ment. Treatment effects were examined for signifi-cance using Wald tests (Rao, 1973), while treatment means were compared using the least significant difference (LSD) technique at the 5% level and then back-transformed into original units.
The effect of bunch on the relationship between finger diameter and hand number was modelled using linear regression of grouped data also using Genstat.
3. Results and discussion
All the terms fitted as fixed effects in the model (Maturity, 1-MCP treatment, Maturity×1-MCP treatment and Maturity×1-MCP treatment×
Table 1
Wald tests for fixed effects of fruit maturity and 1-MCP concentration on green life of ‘Williams’ bananas at 20oC
d.f.
Fixed term Wald statistic x2probability
Maturity 647.4 1 ***
1-MCP gave a 4-fold increase in green life, com-pared with the respective control fruit held in 0.1 ml l−1 ethylene. In the ‘least mature’ bunch, the increase in green life was in the order of 1.5-fold only. This is a much higher level than 0.7 nl l−1
1-MCP for 24 h claimed to be effective by Sisler et al. (1996b). Comparison of fruit exposed to 500 nl l−1 1-MCP and low ethylene with fruit held in
ethylene-free air showed that in the ‘most mature’ bunches, the 1-MCP treatment was more effective in extending green life than removing ethylene from the atmosphere (Table 2). The 1-MCP treat-ment was, however, not as effective as ethylene removal in the ‘least mature’ bunch where the green life was 1.5-fold longer in the fruit held in ethylene-free air. The results demonstrate that in our study, the effectiveness of 1-MCP to extend the postharvest life of bananas varied significantly according to the maturity of the fruit. As fruit matures and green life decreases, 1-MCP becomes relatively more effective in delaying ripening al-though the absolute time to ripen still decreases.
1-MCP at 500 nl l−1had a deleterious effect on
fruit appearance, with uneven colouring of the skin of ripe fruit at a level considered unaccept-able for marketing (score\2) (Table 3). Only three of the nine hands of fruit were acceptable after treatment with 500 nl l−11-MCP and
subse-quent exposure to 0.1 ml l−1 ethylene. While the use of 500 nl l−11-MCP was effective in
prolong-was no effect of hand number within any matu-rity×1-MCP treatment combination.
The relationship between finger diameter and hand number was dependent on fruit maturity. The following linear regression model accounted
for 60% of the variation in diameter: y=
42.53090.337−0.99590.084x for bunches A and B; y=35.48790.498−0.42990.134x for bunch C, where y=finger diameter (mm) and x=hand number (1 – 7).
Green life was affected by 1-MCP only at the highest concentration tested (Table 2). Exposure to 5 and 50 nl l−1
1-MCP had no significant effect on the green life of any fruit from the same hand not treated with 1-MCP. There was a signifi-cant increase in green life due to 500 nl l−1
1-MCP. In the ‘most mature’ bunches, 500 nl l−1
Table 2
Effect of fruit maturity and 1-MCP concentration on green life (Days) of ‘Williams’ bananas at 20oC1
Hand no. Ethylene free2 After fumigation with 1-MCP (nl l−1)3 Bunch maturity
1Analysis performed on log
e(Green life) and back transformed into original units (days). 2Not fumigated with 1-MCP and held continuously in ethylene-free air.
31-MCP fumigation for 24 h followed by continuous exposure to 0.1
ml l−1ethylene.
4Means with the same letter are not significantly different according to LSD at the 5% level. LSD=0.2832 of log
Table 3
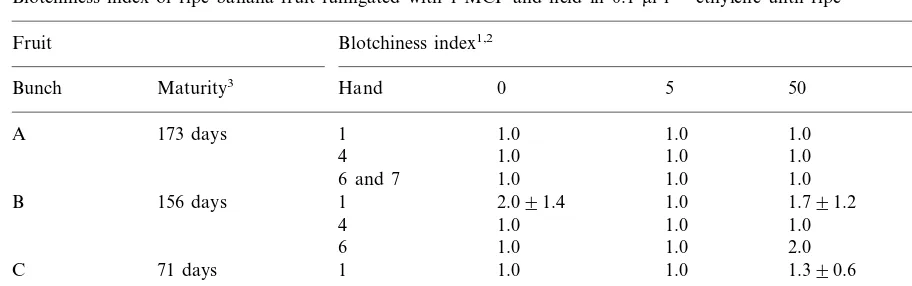
Blotchiness index of ripe banana fruit fumigated with 1-MCP and held in 0.1ml l−1 ethylene until ripe
Blotchiness index1,2 Fruit
Bunch Maturity3 Hand 0 5 50 5004
1 1.0
A 173 days 1.0 1.0 2.390.6
4 1.0 1.0 1.0 1.790.6
6 and 7 1.0 1.0 1.0 2.0
156 days
B 1 2.091.4 1.0 1.791.2 2.790.6
4 1.0 1.0 1.0 2.590.7
6 1.0 1.0 2.0 2.0
71 days
C 1 1.0 1.0 1.390.6 2.590.7
4 1.390.6 1.0 2.0 2.790.6
6 1.0 1.0 1.790.6 3.0
11, normal, yellow colour; 2, persistent green tip, 3, persistent green throughout the fruit.
2Values are mean9standard deviation. Where no standard deviations are shown, all data in the set were normal. 3Days after bunch emergence.
41-MCP concentration (nl l−1).
ing green life of fruit of both levels of maturity, when the fruit eventually ripened its colour was generally uneven and blotchy in appearance. Un-even ripening of ‘Williams’ bananas treated with 1-MCP was also observed by Golding et al. (1998) when they used 45ml l−11-MCP for 1 h. 1-MCP would, thus, seem to have limited potential as a commercial treatment to delay the ripening of ‘Williams’ bananas. This is not only due to un-even colour development, but also because in any commercial consignment of fruit, there is a mix-ture of fruit maturity. Since the effectiveness of 1-MCP to protect bananas from the effects of exogenous ethylene depends on the maturity of the fruit, it is highly likely that a consignment of 1-MCP-treated fruit in a ripening room would ripen at different times, resulting in ‘mixed ripe’ fruit.
It is known that in tomatoes, 1-MCP prevents the accumulation of a number of mRNAs coding for the expression of ACC synthase, ACC oxidase and the ethylene receptor involved in positive feedback regulation of autocatalytic (System 2) ethylene production (Nakatsuka et al., 1998). The longer ripening time of the ‘most mature’ fruit when treated with 500 nl l−1 1-MCP, compared
to fruit stored in ethylene-free air, suggests that the accumulation of mRNAs required for the transition from system 1 to system 2 ethylene
production was blocked or retarded by 1-MCP and the fruit did not respond to the low level of exogenous ethylene in this treatment. Fruit in the ethylene-free control, however, had sufficient quantities of these mRNAs that even the very low levels of ethylene in this treatment were enough to quickly initiate ripening. Even so, there were sig-nificant differences in green life among hands in the most mature bunches, consistent with the findings of Liu (1976). The opposite effect in the ‘least mature’ bananas, that is, a shorter ripening time when treated with 500 nl l−1 1-MCP
com-pared to fruit stored in ethylene-free air, suggests that although both treatments were initially less sensitive to ethylene than the ‘most mature’ fruit, the mRNAs required for the transition from sys-tem 1 to syssys-tem 2 ethylene production were pro-duced by this fruit after 1-MCP treatment. Presumably a continuous exposure to exogenous ethylene would stimulate the production of these mRNAs, or prevent the accumulation of mRNAs involved in negative feedback regulation, thus reducing the effectiveness of this treatment com-pared with holding fruit in ethylene-free air. Golding et al. (1998) have suggested that when new ethylene receptors are synthesised or are ca-pable of ethylene reception after 1-MCP
treat-ment, a continuous presence of propylene
These findings demonstrate the need for regard to fruit maturity when quantifying the benefits of 1-MCP treatment on bananas.
References
Golding, J.B., Shearer, D., Wyllie, S.G., McGlasson, W.B., 1998. Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biol. Technol. 14, 87 – 98.
Heyes, J.A., Johnston, J.W., 1998. 1-Methylcyclopropene ex-tends cymbidium orchid vaselife and prevents damaged pollinia from accelerating senescence. New Zealand J. Crop Hort. Sci. 26, 319 – 324.
Jiang, Y., Joyce, D.C., Macnish, A.J., 1999a. Extension of the shelf life of banana fruit by 1-methylcyclopropene in com-bination with polyethylene bags. Postharvest Biol. Tech-nol. 16, 187 – 193.
Jiang, Y., Joyce, D.C., Macnish, A.J., 1999b. Responses of banana fruit to treatment with 1-methylcyclopropene. Plant Growth Reg. 28, 77 – 82.
Ku, V.V.V., Wills, R.B.H., 1999. Effect of 1-methylcyclo-propene on the storage life of broccoli. Postharvest Biol. Technol. 17, 127 – 132.
Ku, V.V.V., Wills, R.B.H., Ben-Yehoshua, S., 1999. 1-Methyl-cyclopropene can differentially affect the postharvest life of strawberries exposed to ethylene. HortScience 34, 119 – 120. Liu, F.W., 1976. Banana response to low concentrations of
ethylene. J. Amer. Soc. Hort. Sci. 101, 222 – 224. Macnish, A.J., Joyce, D.C., Hofman, P.J., 1998.
1-Methylcy-clopropene delays ripening of ‘Cavendish’ banana fruit. In: Australasian Postharvest Horticulture Conference, Pro-ceedings, 28 September – 3 October 1997. University of Western Sydney, Hawkesbury, NSW, Australia, pp 282 – 284.
Nakatsuka, A., Murachi, S., Okunishi, H., Shiomi, S., Nakano, R., Kubo, Y., Inaba, A., 1998. Differential ex-pression and internal feedback regulation of 1-aminocyclo-propane-1-carboxylate synthase,
1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Phys-iol. 118, 1295 – 1305.
Newman, J.P., Dodge, L.L., Reid, M.S., 1998. Evaluation of ethylene inhibitors for postharvest treatment ofGypsophila paniculataL. HortTechnology 8, 58 – 63.
Porat, R., Shlomo, E., Serek, M., Sisler, E.C., Borochov, A., 1995. 1-Methylcyclopropene inhibits ethylene action in cut phlox flowers. Postharvest Biol. Technol. 6, 313 – 319. Porat, R., Weiss, B., Cohen, L., Daus, A., Goren, R., Droby,
S., 1999. Effects of ethylene and 1-methylcyclopropene on the postharvest qualities of ‘Shamouti’ oranges. Posthar-vest Biol. Technol. 15, 155 – 163.
Rao, C.R., 1973. Linear Statistical Inference and its Applica-tion. 2nd ed. Wiley, New York.
Searle, S.R., 1971. Linear Models. John Wiley & Sons, New York.
Serek, M., Sisler, E.C., Reid, M.S., 1994. Novel gaseous ethylene binding inhibitor prevents ethylene effects in pot-ted flowering plants. J. Amer. Soc. Hort. Sci. 119, 1230 – 1233.
Serek, M., Sisler, E.C., Reid, M.S., 1995a. Effects of 1-MCP on the vase life and ethylene response of cut flowers. Plant Growth Regul. 16, 93 – 97.
Serek, M., Sisler, E.C., Reid, M.S., 1995b. 1-Methylcyclo-propene, a novel gaseous inhibitor of ethylene action, improves the life of fruits, cut flowers and potted plants. Acta Hort. 394, 337 – 345.
Serek, M., Sisler, E.C., Reid, M.S., 1996. Ethylene and the postharvest performance of miniature roses. Acta Hort. 424, 145 – 149.
Sisler, E.C., Dupille, E., Serek, M., 1996a. Effect of 1-methyl-cyclopropene and methylenecyclopropane on ethylene binding and ethylene action on cut carnations. Plant Growth Regul. 18, 79 – 86.
Sisler, E.C., Serek, M., Dupille, E., 1996b. Comparison of cyclopropene, 1-methylcyclopropene, and 3,3-dimethylcy-clopropene as ethylene antagonists in plants. Plant Growth Regul. 18, 169 – 174.
Wills, R.B.H., Harris, D.R., Seberry, J.A., 1999. Delayed ripening of bananas through minimisation of ethylene. Tropical Agric. 76, 279 – 282.