Procedia Chemistry 19 ( 2016 ) 441 – 446
1876-6196 © 2016 The Authors. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Peer-review under responsibility of School of Materials and Mineral Resources Engineering, Universiti Sains Malaysia doi: 10.1016/j.proche.2016.03.036
ScienceDirect
5th International Conference on Recent Advances in Materials, Minerals and Environment (RAMM) & 2nd
International Postgraduate Conference on Materials, Mineral and Polymer (MAMIP), 4-6 August 2015
Preparation and Characterization of Microwave-absorption of Sarulla North
Sumatra Zeolite and Ferric Oxide-filled Polyurethane Nanocomposites
Golfrid Gultom
a,b, Basuki Wirjosentono
c, Kerista Sebayang
d, Masno Ginting
ea University of Sumatera Utara, Physics Post Graduate Program, Medan 20155, Indonesia b Polytechnic of Chemical Industry Technology, Medan 20228, Indonesia c University of Sumatera Utara, Chemistry Department, Medan 20155, Indonesia
dUniversity of Sumatera Utara, Physics Department, Medan 20155, Indonesia e Indonesian Institute of Science, Research Center for Physics, Serpong 15314 , Indonesia
Abstract
Microwave-absorptive polymeric composite materials are becoming important to protect interference of any communication sy stems due to the increase in the use of microwave-inducing devices. In this work, the microwave-absorptive polyurethane composites are prepared using natural zeolites of Sarulla North Sumatra and commercial ferric -oxide as fillers. Weight ratio of the natural zeolite to ferric oxide were varied (18:2; 16:4; 14:6; 12:8 and 10:10) by weight. The fillers are prepared using ball milling technique and characterized using Particle Size Analyzer for particle size distribution. The nanocomposites, prepared using in-situ reaction of polyethylene glycol and toluene diisocyanate, is characterized for physical and mechanical properties using tensile strength, thermal properties with TGA techniques, as well as morphological and chemical properties using scanning electron microscopy. Composition and loading of the nanofillers against polyurethane matrices is 20% by weight. Microwave-absorption properties of the nanocomposites is characterized using 8-12 GHz frequency. Tensile strengths of the natural zeolite-ferric oxides polyurethane nanocomposites shows higher values when matrices filled with lower ferric-oxide, which could be due to the nanozeolites have functioned as reinforcement for the polyurethane matrix through polar-polar interaction between the filler surfaces with the matrices. The microwave absorption properties, which investigated by Vector Network Analyzer, of the nanocomposites filled in polyurethane with the ratio of nanozeolite to ferric oxide filler of 12:8 shows reflection loss of – 13.2 dB. This condition was observed at 11.1 GHz.
© 2016 The Authors. Published by Elsevier B.V.
Peer-review under responsibility of School of Materials and Mineral Resources Engineering, Universiti Sains Malaysia.
Keywords: Polyurethane, Nanozeolites, Microwave-absorption
*Corresponding author Tel: +6281361245428 Email: [email protected]
© 2016 The Authors. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Electromagnetic interference is worsening with the rapid development of wireless communications and circuit devices. The high frequency electromagnetic wave is drawing more attention, due to the explosive growth in the utilization of telecommunication devices in industrial, medical and military application 1,2.
In the past decades, the spinel ferrite have been utilized as the most frequent absorbing materials in various forms. The absorbing characteristics of materials depend on the frequency, layer thickness, complex permittivity (Hr) and complex permeability
(Pr). All the parameters H’, H’’, P’ and P’’ are found to increase with the increased of ferrite contents and it is found that the
absorption properties in the composites are greatly improved with the increasing of ferrite contents in the polymer matrix4. But in
other side, it will increase the mass of the absorbing materials. Conducting polymer composites with micro/nanostructured have attracted a significant academic and technological attention because of their unique physical properties and potential applications 5-8.
Zeolite mineral is a compound of aluminium silicate hydrate with alkali metal which is group of several types of minerals. Zeolite is aluminosilicate with a framework structure enclosing cavities occupied by a large ions and water molecules, both of which have considerable freedom of movement, permitting ion-exchange and reversible dehydration.
Natural zeolite is natural mineral that composed of crystalline silica (SiO2) and alumina (Al2O3), with cavities of metal ions,
which is usually alkali and alkaline or earth metals, and water molecules. Unique characteristic, include very stable with very high adsorption capacity and selectivity and have large active pore structure (microporous) and has a high specific surface area. Natural resources have the potential to be further processed into products that can be used for broad applications, among others, as supporting the catalyst or catalysts, and slow release substances. Zeolite crystal structure of alumina silicate shaped frame (framework) three-dimensional, having cavities and channels as well as containing metal ions such as Na, K, Mg, Ca and Fe as well as water molecules.
Sarulla natural zeolite chemical composition analysis using XRF showed the dominant chemical compounds are SiO2 and
Al2O3 as showed in Table 1.
In this paper in-situ reaction of polyethylene glycol and toluene diisocyanate with nanofillers of zeolite and ferric oxide is used to fabricate PU nanocomposites. The effect of alumino silica as the higher compound in natural zeolite combined with ferric oxide as the most frequent absorbing materials were observed as microwave absorption.
2. Experimental
The fillers used for nanocomposite fabrication are natural zeolite and commercial ferric-oxide (J-Fe2O3). The polyurethane
matrix used was a commercial type which contains two-part urethane monomers i.e. 60% wt polyethylene glycol and 40% wt toluene diisocyanate.
3. Result and Discussion
Fig.1 shows the X-Ray Diffraction spectrum of Sarulla natural zeolite. Observed from the XRD spectrum shows intensity at 2θ
= 29.76; 27.67 and 27.92. Maximum peak occur at 29.76 with FWHM 0.124 which is at the aluminium silicate phase. Mordenite standard have a high intensity at 2θ = 27; 25.63 and 23. Compare with Sarulla zeolite, it shows that natural zeolite is mordenite, but another peak at natural zeolite at 29.76 shows that crystaline at Sarulla natural zeolit is not only modernit but also mixed with other crystalline and other impurities
Fig. 1. X-Ray diffraction of Sarulla natural zeolite.
Fig.2. (a) SEM micrograph of Sarulla natural nanozeolite; (b) SEM micrograph of PU filled zeolit and ferric oxide 50 x magnification; (c) SEM micrograph of PU filled zeolit and ferric oxide 500 x magnification.
b
c
a
Pore
Fig.2(a) above shows the surface of the zeolite have a crystal lattice in the form of pores of the zeolite surface , this is in-line with the report stating that zeolite microporous crystalline solid is hollow and grooved and has a pore size of 3 to 10Ǻ called as molecular sieves. SEM photograph shows that dark colors are pores or cavities and a bright color is a natural zeolite nanoparticles, particle size of > 50 nm stated that zeolite is the mordenite type. Fig.2(b) show the PU nanocomposites foams distribution and Fig.2(c) shows where zeolit and ferric oxide distributed in PU matrices.
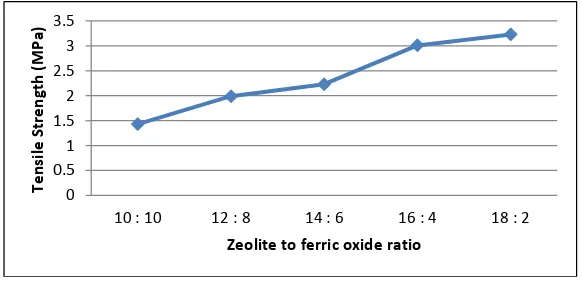
Based on the measurement data, it is shows that the best mechanical properties of tensile strength at 3.23 MPa with natural zeolite to ferric oxide ratio is 18:2, larger ferric oxide ratio will affect to the tensile strength degradation. This is probably due to the polar to polar interaction between nanozeolite and PU matrices. The layer silicates in nanometer-sized natural zeolites can be dispersed randomly and evenly that give structure to the nano composites. Silicate layers that exist in scattered individual zeolite has a large surface contact area which caused to a strong bind with the matrix PPG and TDI and further gives effect to the increase in tensile strength.
Fig.3. Polyurethane nanocomposite filled natural zeolite and ferric-oxide tensile strength.
Termogram (TGA) curve analysis shows samples of PU with no filler, PU with ratio of nanozeolite and ferric-oxide of 16:4 (PU A-2) and PU with ratio of nanozeolite and ferric-oxide of 10:10 (PU A-5) have lost its weight when the temperature increased, all samples will release the water in the compounds at the temperature of 200oC. Decomposition of PU at 336oC and 410oC will
lose its weight of 27.07% and 40.68% respectively. PU A-2 is decomposed at 335oC and 406oC and the weight is reduced of
23.17% and 28.74 % respectively. After heating to the temperature of 680oC, PU will have 16.44% residue, PU A-2 with 29.03 %
residue and PU A-5 with 30.18 % residue. Thermal stability will increase according to the increase of ferrite oxide content in the composite.
Fig. 4. TGA curve of PU- natural nanozeolite and ferric oxide. 0
0.5 1 1.5 2 2.5 3 3.5
10 : 10 12 : 8 14 : 6 16 : 4 18 : 2
Ten
si
le Str
e
ngth
(M
Pa)
Reflection loss measurement using Vector Network Analyzer for polyurethane nanocomposites reinforcement with 20% wt nanofillers of natural zeolite and ferric-oxide ratio of 18:2; 16:4; 14:6; 12:8 and 10:10 as a function of frequency in X-band (8-12 GHz) shows in Fig.5 PU nanocomposites with ratio of natural zeolite and ferric-oxide ratio of 12:8 fillers have the optimum reflection loss of -13.2 dB at 11.1 GHz. The reflection loss of less than -10 dB means the microwave absorption more than 90%. The microwave absorption being dependent on the ratio of SiO2 and Al2O3 which is to dominant compounds of natural zeolite and
ferric-oxide, the content of ferric-oxide in natural zeolite will modify the reflection loss and microwave absorption of the nanocomposite and will affect the complex permittivity and complex permeability of the nanocomposite. To satisfy the zero-reflection condition where maximum absorption would occur, the impedance of the composite should be 1 to prevent zero-reflection. This can be ideally achieved when the materials present complex permittivity equal to complex permeability. Ferrite itself usually presents electromagnetic characteristics of complex permeability less than complex permittivity. Minimum loss occurs when the thickness is about an odd multiple of one quarter of the wavelength of the incident frequency 4.
Fig.5. Reflection loss of polyurethane natural zeolite and ferric oxide nanocomposites
4. Conclusion
Polyurethane filled nanozeolite and ferric-oxide has prepared using in-situ reaction. Natural zeolite and ferric oxide ratio as a filler in PU matrices will cause a different effect in mechanical properties and microwave absorption properties. Tensile strength properties show a better value when matrices filled with larger ratio of natural zeolite. Binding of zeolite with PU will increase the mechanical properties. The nanocomposite absorb microwave at X band frequency for > 90 % absorption. This is due to the effect of aluminum silicate and ferric oxide binding with polar – polar reaction between filler and matrices. These reaction cause difference in input impedance and this will make a difference in the resonance at high frequency. Ferrite-oxide particle have a great microwave absorption characteristic, but in this case, the ratio between zeolite and ferric-oxide will affect the best microwave absorption.
References
1. Guo Z, Lee SE, Kim H, Park S, Hah HT, Karki AB, D.P. Young, Fabrication, characterization and microwave properties of polyurethane nanocomposites reinforced with iron oxide and barium titanate nanoparticles. Acta Materialia 2009; 57: 267-277.
2. Kong I, Ahmada S, Abdullah M, DavidHui, Yusoff AN, DwiPuryanti. Magnetic and Microwave Absorbing Properties Of Magnetite-Thermoplastic Natural Rubber Nanocomposites . J Magnetism Magnetic Mat 2010; 322: 3401–3409.
3. Kato Y, Sugimoto S, Shinohara K, Tezuka N,Kagotani T, Inomata K.. Magnetic Properties and Microwave Absorption Properties of Polymer-Protected Cobalt Nanoparticles. Materials TransactionsM2002; 43: 406- 409
4. Hosseini H, Asadnia A. Synthesis, Characterization, and Microwave-Absorbing Properties of Polypyrrole/MnFe2O4 Nanocomposite. J Nanomater 2012; 2012: 1-6.
5. Gairola SP, Vivek Verma, Singh A, Purohit LP, Kotnala RK. Modified Composition of Barium ferrite to Act as a Microwave Absorber in X-band Frequencies.
Solid State Commun 2009: 1-5.
7. Guo J, Duan Y, Liu L, Chen L, Liu S. Electromagnetic and Microwave Absorption Properties of Carbonyl-Iron/Fe91Si9 Composites in Gigahertz Range, J
Electromagnetic Analysis Appl 2011; 5: 140-146.
8. Harsroop Kaur, Gagan Deep Aul,; A Review Based on Effects of Change in Thickness and Number of Layers on Microwave Absorbing Materials, Int J Sci Res (IJSR) 2014; 5: 1141-1145.
9. Valko L, Bucek P, Dosoudil R, Usakova M. Magnetic Properties of Ferrite-Polymer Composites. J Electr Eng 2003; 54: 100-103.
10. Hunyek A, Sirisathikul C, Jantaratana P. Magnetic and Dielectic Properties of Natural Rubber and Polyurethane Composites Filled with Cobalt Ferrite, Plastics, Rubber and Composites. 2013; 42.
11. Martin J, Manuel HV, Oscar de A, Carlos L, Angel MM, Manuel V, Carmen M. Fabrication and Characterization of Polymer-Based Magnetic Composite Nanotubes and Nanorods. Euro Polym J 2012; 48:712-719.
12. Guskos N, Maryniak M, Typek J, Pełech I, Narkiewicz U, Rosłaniec Z, Kwiatkowska M.Temperature Dependance Of FMR Field Of Magnetic Nanoparticles/Polymer Composite. Rev Adv Mater Sci 2006; 12: 213-218.
13. Rajshree J, Chetan C, Jyoti J. Magnetic And Dielectric Properties Of Polymer Ceramic Composites Synthesized Using A Melt Compound Technique, Inter J Modern Phy: Conference Series 2013; 22.
14. Pant RP, Dhwan SK, Suri DK, Manju A, Gupta SK, Koneracka M, Kopcansky P, Timko M. Synthesis and Characterization of Ferrofluid-Conducting Polymer Composite, Indian. J Eng Mater Sci, 2004; 11: 267-270.
15. Sitti Ahmiatri S, Azwar M, Budhy Kurniawan. Microwave Absorbing Properties of La0.67Ba0.33Mn1-xTixO3 in The Frequency Range 8-12 GHz, Int J Basic
Appl Sci, IJBAS-IJENS, 2014; 14.
16. Babayan V, Kazantseva Ne,Moučka R, Vilčáková J, Sáha P. Babayan , et.al, Radio Absorbers Based On Polymer Magnetic Composites. Chem. Listy 2014;
108: 4–9.
17. Grujić A, Talijan N, Stojanović D, Stajić-Trošić J, Burzić Z, Balanović LJ, Aleksić R. Mechanical And Magnetic Properties Of Composite Materials With Polymer Matrix. J Min Metall Sect B-Metal, 2010; 46 (1): 25-32.
18. Wang Y, Li T, Zhao L, Hu Z, Gu Y. Research Progress on Nanostructured Radar Absorbing Materials. Energy and Power Engineering 2011; 3: 580-584. 19. Argin M, Karady GG. Characterization of Polyurethane Foam Dielectric Strength, IEEE Transactions and Dielectrics and Electrical Insulation, 2008; 15. 20. Stabik J, Dybowska A, Pluszynki J, Szczepanik M, Suchon L. Magnetic Induction Of Polymer Composites Filled With Ferrite Powders. Archives Mater Sci
Eng 2010; 41: 13-20.
21. Abbas SM, Mahesh Chandra, Verma A, Chatterjee R, Goel TC. Complex Permittivity And Microwave Absorption Properties Of a Composite Dielectric Absorber. Composites: Part A 2006; 37 :2148–2154.
22. Bayrakdar H. Electromagnetic Propagation And Absorbing Property Of Ferrite-Polymer Nanocomposite Structure, Progress In Electomagnetics Research M, 2012; 25.
23. Ramajo LA, Cristóbal AA, Botta PM, Porto López JM, Reboredo MM, Castro MS. Dielectric and Magnetic Response of Fe3O4/epoxy Composites.
Composites: Part A 2009; 40:388–393.
24. Seyed Hossein Hosseini, Asadnia A. Polyaniline/Fe3O4 Coated on MnFe2O4 Nanocomposite: Preparation, Characterization, and Applications in Microwave
Absorption. Academic J 2013; 8.
25. Himel C. Electomagnetic Interference Reflectivity of Nanostructured Manganese Ferrite Reinforced Polypyrrole Composite. Trans Electronic Mater 2013; 14.
26. Shimba K, Furuta K, Morimoto N, Tezuka N, Sugimoto S. Magnetic Properties of Nanoparticles-Polymer Composites Prepared Using surface Modification and Cross-Linking Reaction Mater Transs, 2011; 52:486-490.