Aspergillus flavus in dates
Hajer Aloui, Khaoula Khwaldia, Fabio Licciardello, Agata Mazzaglia, Giuseppe Muratore, Moktar Hamdi, Cristina Restuccia
PII: S0168-1605(13)00489-3
DOI: doi:10.1016/j.ijfoodmicro.2013.10.017 Reference: FOOD 6341
To appear in: International Journal of Food Microbiology
Received date: 31 July 2013 Revised date: 12 October 2013 Accepted date: 24 October 2013
Please cite this article as: Aloui, Hajer, Khwaldia, Khaoula, Licciardello, Fabio, Maz-zaglia, Agata, Muratore, Giuseppe, Hamdi, Moktar, Restuccia, Cristina, Efficacy of the combined application of chitosan and Locust Bean Gum with different citrus essential oils to control postharvest spoilage caused byAspergillus flavusin dates,International Journal of Food Microbiology(2013), doi: 10.1016/j.ijfoodmicro.2013.10.017
ACCEPTED MANUSCRIPT
Efficacy of the combined application of chitosan and Locust Bean Gum with different
citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates
Hajer Alouia,c, Khaoula Khwaldiaa,*, Fabio Licciardellob, Agata Mazzagliab, bGiuseppe Muratoreb, Moktar Hamdic, Cristina Restucciad
aLaboratoire des Substances Naturelles (LSN), Institut National de Recherche et d’Analyse
Physico-chimique (INRAP), Pôle Technologique de Sidi Thabet, 2020 Sidi Thabet, Tunisia
b
Department of Agricultural and Food Productions (DiSPA), University of Catania, via Santa
Sofia 98, 95123 Catania, Italy
cLaboratoire d’Ecologie et de Technologie Microbienne, Institut National des Sciences
Appliquées et de Technologie (INSAT), 2 Boulevard de la terre, BP 676, 1080 Tunis, Tunisia
d
Department of Agri-Food and Environmental Management Systems (DiGeSA), University of
Catania, via Santa Sofia 98, 95123 Catania, Italy
*Corresponding author. Laboratoire des Substances Naturelles (LSN), Institut National de
Recherche et d’Analyse Physico-chimique (INRAP), Pôle Technologique de Sidi Thabet,
2020 Sidi Thabet, Tunisia. Tel.: +216 71 537666; fax: +216 71 537688.
ACCEPTED MANUSCRIPT
ABSTRACT
This study reports the efficacy of the combined application of chitosan (CH) and Locust Bean
Gum (LBG) in combination with different citrus essential oils (EOs) to inhibit Aspergillus
flavus in vitro and on artificially infected dates for a storage period of 12 days. The effect of
these treatments on the fruits’ sensory characteristics was evaluated to verify the complete
absence of off-odours and off-flavours. Bergamot EO was the most effective in reducing
mycelial growth, followed by bitter orange EO. Both bergamot and bitter orange oils
significantly reduced conidial germination and a complete inhibition was obtained at
concentrations higher than 2%. The mixtures based on CH-2% (v/v) bergamot EO or CH-2%
(v/v) bitter orange EO proved to be the most effective coatings to reduce conidial germination
resulting in a 87-90% inhibition compared with the control. In fruit decay assays coatings
based on CH incorporating citrus oils were able to reduce fungal decay in the range of 52-
62% at day 12.
The study results and the complete absence of off-flavours and off-odours demonstrate
the potential of CH coatings carrying citrus EOs at sub-inhibitory concentrations to control
postharvest growth of A. flavus in dates.
ACCEPTED MANUSCRIPT
1. Introduction
Date (Phoenix dactylifera L) is one of the most consumed fruit in North Africa,
Middle East and South-Asian countries, mainly because of its high content of carbohydrates
(70–80%), dietary fibre (6.40–11.50%), minerals (0.10–916 mg/100 g dry weight), vitamins
(C, B1, B2, B3 and A) and antioxidant compounds. During field production, handling,
transportation and storage, dates are susceptible to damage and to colonization by spoilage
fungi (Jowkar et al., 2005), which may result in economic losses, especially for exporting
countries. Tunisia is considered one of the primary date producing countries, and the highest
exporter (Besbes et al., 2009). However, it is estimated that more than 50% of the total
production of dates is lost due to fungal spoilage (Atia, 2011). Aspergillus spp. have been
reported to be the most common fungal species infecting dates (Ahmed et al., 1997). Under
conditions of high humidity and moderate temperature, these postharvest fungi may have the
potential to produce mycotoxins (Shenasi et al., 2002). Among the mycotoxins, aflatoxins
produced by toxigenic strains of Aspergillus flavus and A. parasiticus have been reported to
be the most toxic, being hepatotoxic, teratogenic, mutagenic and immunosuppressive to
human beings and other livestock (Arrus et al., 2005). Since January 2005 methyl bromide,
which was widely used for reducing insect infestation and fungi spores in soil and in stored
commodities, has been banned in countries with developed economies and starting from 2015
it will be banned in developing countries. Hence, other postharvest preservation techniques
such as microwave, heating, ozone (ozonation), controlled atmosphere and modified
atmosphere packaging have been proposed as tools to replace chemical treatments. However,
such treatments have received very little attention, as some of them have been reported to
affect the fruit quality attributes (Dehghan-shoar et al., 2010).
In recent years, considerable attention has been directed toward natural compounds,
ACCEPTED MANUSCRIPT
by aflatoxigenic Aspergillus strains in fruits (Xing et al., 2010; Atia, 2011; Kumar et al.,
2011).
Among a wide variety of EOs, citrus fruit EOs, recognized as safe (GRAS) by the
Food and Drug Administration (2005), appear as promising natural compounds for controlling
postharvest decay in fruits. Such oils, whose major chemical components are volatile (85 to
99%), contain monoterpenes (mainly limonene: from 32 to 98%), sesquiterpene
hydrocarbons, oxygenated derivatives thereof, as well as aliphatic aldehydes, alcohols and
esters (Svoboda et al., 2003). They have been proven to have antifungal properties against
several postharvest phytopathogens, including species of Penicillium and Aspergillus
(Caccioni et al., 1998; Viuda-Martos et al., 2008). The antifungal capacity of citrus oils has
been attributed mainly to the presence of components such as D-limonene, linalool or citral
(Bezic et al., 2005), but also to the amphipathicity of their phenolic compounds which may
facilitate their interaction with both the polar and aliphatic sides of the fungal membrane
(Veldhuizen et al., 2006).
Although EOs have proved to be good antimicrobial agents, their use for maintaining
fruit quality and reducing fungal decay is often limited due to their application costs and other
drawbacks, such as their high volatility, strong flavour and potential toxicity (Bakkali et al.,
2008). The incorporation of these compounds into edible coating formulations can be an
effective approach to solve some of these problems, while at the same time, controlling fruit
postharvest decay, by lowering the diffusion processes and maintaining high concentrations of
active molecules on the surface of the fruit. Among the polysaccharides used in the edible
coating formulations, chitosan (CH) has been documented to possess good film-forming and
bioactive properties either in its polymeric or oligomeric form (Coma et al., 2003).
Additionally, films and coatings based on CH have been shown to act as an effective matrix to
ACCEPTED MANUSCRIPT
with lemon essential oil protected strawberries from gray mould caused by Botrytis cinerea.
Over the last few years, locust bean gum (LBG), extracted from the seeds of Ceratonia siliqua
carob tree has been reported to be another potential coating component due to its good film
forming properties and its ability to form strong gels at relatively low concentrations
(Mikkonen et al., 2007). Recently, an improvement in the postharvest quality of ‘Fortune’
mandarins was noted by Rojas-Argudo et al. (2009) when LBG-lipid edible composite
coatings were applied to their surface.
While the antifungal activity of citrus EOs against many phytopathogenic fungi in “in
vitro” conditions has been well documented in literature, there are only few published data on
their effectiveness in controlling fungal decay of fruits (Perdones et al., 2012;
Sanchez-Gonzalez et al., 2011). However, until recently, there has been no research on the use of
edible coatings enriched with EOs for preserving dates.
This study aimed at screening the antifungal activity of five citrus EOs, namely
bergamot (Citrus bergamia Risso), bitter orange (Citrus aurantium L.), sweet orange (Citrus
sinensis (L.) Osbeck.), lemon (Citrus limon (L.) Burm. f.) and mandarin (Citrus deliciosa
Ten.), against A. flavus, in in vitro conditions, and investigating the efficacy of two different
polysaccharide matrices, CH and LBG, enriched with the most efficient oils in controlling
postharvest decay in inoculated dates. Sensory analysis was carried out to evaluate the effect
of the different coating treatments on the flavour and odour characteristics of the treated
fruits.
2. Materials and methods
2.1. Raw materials
Tunisian dates (Phoenix dactylifera L. variety Deglet Nour) at Rutab stage were
ACCEPTED MANUSCRIPT
ripeness and the absence of mechanical injury or fungal infection. Dates were transported to
the laboratory in polystyrene boxes to avoid mechanical damage, at ambient conditions of
temperature and humidity (18 °C and 75% RH). Coating experiments were carried out on the
same day.
CH (deacetylation degree > 75%, viscosity ≤ 200 mPa s in 1% acetic acid, molecular
weight ~150,000 Da, Sigma Aldrich, Steinheim, Germany) and LBG (molecular weight ~
310,000 Da, Sigma Aldrich, Steinheim, Germany) were used as coating materials.
Bergamot, bitter orange, sweet orange, mandarin and lemon EOs were kindly supplied
by Fratelli di Bartolo (Calatabiano, Catania, Italy). The citrus EOs were produced using a cold
press extraction process. The peel of fresh fruits was cold-pressed and the essential oil was
separated from the crude-extract by centrifugation before being stored in sealed glass vials at
room temperature, prior to use. The density of the different citrus oils, their refraction
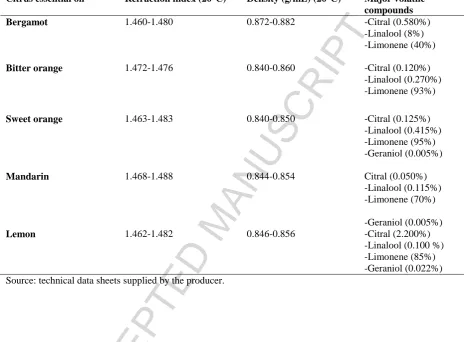
indexes, as well as their major chemical volatiles compounds are summarized in Table 1.
2.2. Fungal strain
Freeze dried culture of A. flavus DSM 1959 was obtained from DSMZ culture
collection (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig,
Germany). The fungus was rehydrated following the supplier’s instructions, inoculated on
potato dextrose agar (PDA) (Oxoid, Basingstoke, Hampshire, England) and incubated at 25
°C until sporulation.
2.3. Screening of antifungal activity of citrus EOs
ACCEPTED MANUSCRIPT
To select the most effective EOs against A. flavus, an in vitro inhibition of mycelial
growth assay was carried out for the 5 citrus oils, using the “poison food” technique (Grover
and Moore, 1962), with some modifications. Tween-80 (Sigma Aldrich, Steinheim, Germany)
was incorporated into the PDA agar medium at a final concentration of 1% (v/v) to enhance
oil solubility. Different concentrations of EOs (0.5– 5% v/v) were then aseptically added to
the sterile molten PDA medium (~ 45 °C) and the resulting media were immediately poured
into Petri plates (9 cm diameter). Plates were dried at room temperature for 30 min prior to
spot inoculation with 10 µL of a conidial suspension of A. flavus at a concentration of 105
conidia/mL. A. flavus conidia were scraped off the surface agar of 14-day-old culture and
suspended in sterile physiological saline solution (0.9% NaCl) containing 0.01% (v/v) Tween
80. The number of conidia was counted using a Thoma counting chamber. PDA with 1% (v/v)
Tween-80 but no EO was used as positive growth control. Inoculated Petri plates were
incubated at 25 °C for 7 days, by which time the growth of the controls had reached the edge
of the plate. Each treatment was replicated three times, and the fungitoxicity of EOs was
measured in terms of mycelial growth inhibition (MGI %) percentage, calculated by the
following formula:
MGI (%) = (dc – dt)/dc × 100, where dc and dt represent mycelial growth diameter in control
and treated Petri plates, respectively (Marandi et al., 2011).
The most promising EOs were selected for all the further experiments.
2.3.2. Determination of selected EOs effective concentration
Based on the previous screening, bergamot and bitter orange EOs were identified to
have the most efficient antifungal activity. In order to determine the minimum effective
concentration of these oils, an in vitro conidial germination inhibition assay was carried out,
ACCEPTED MANUSCRIPT
dextrose broth (Sigma-Aldrich, Steinheim, Germany) containing Tween-80 at a final
concentration of 1% (v/v), and either bergamot or bitter orange EOs at different
concentrations (from 0.5 to 5% v/v), were pipetted on a cavity slide containing 10 µL of a
freshly prepared conidial suspension of A. flavus at a final concentration of 105 conidia/mL.
Cavity slides containing Potato dextrose broth with Tween 80 were used as positive control.
Inoculated slides were placed on moist filter paper in Petri plates and incubated at 25
°C for 10 h. After incubation, 10 µL of a 2% sodium azide solution was added to each slide to
stop further germination. Three replicates were conducted for each treatment and conidial
germination was evaluated by counting 100 conidia using an optical microscope (Olympus,
Hamburg, Germany). A conidium was considered germinated when the length of the germ
tube was at least twice the conidium diameter (Amiri and Bompeix, 2011).
The percent inhibition of germination (IG) was calculated using the following expression:
IG (%) = (Gc– Gt)/Gc × 100, where Gc and Gt represent the number of germinated conidia in
control and treated slides, respectively (Fiori et al., 2000).
Based on this assay, a concentration of 2% (v/v) EO was selected for the evaluation of the in
vitro and the in vivo antifungal activity of the combined treatments based on CH or LBG
incorporating either bergamot or bitter orange EOs.
2.4. Preparation of the film forming dispersions
Pure CH film forming solution was prepared by dispersing CH (1%, w/v) in an
aqueous solution of glacial acetic acid (1%, v/v), at 40 °C for 12 h, whereas that of LBG
(0.5%, w/v) was prepared by dissolving LBG powder in distilled water heated at 70 °C with
constant agitation until all particles were thoroughly dispersed. Composite solutions were
prepared by adding a 2% (v/v) concentration of either bergamot or bitter orange EOs (this
ACCEPTED MANUSCRIPT
solutions, before being homogenized at 13,500 rpm for 4 min, using an Ultra-Turrax T25
(IKA, Labortechnik GmbH., Munich, Germany). All the solutions were then adjusted to pH
5.60 by adding 1 M NaOH.
2.5. In vitro antifungal activity of combined treatments
The in vitro antifungal activity of CH and LBG alone and in combination with either
bergamot or bitter orange EOs at a concentration of 2% (v/v) was evaluated according to the
mycelium growth and conidial germination inhibition assays, using respectively the “poison
food”technique and the “cavity slide” method.
2.6. In vivo antifungal assay: Fruit decay
To evaluate the antifungal activity of the most effective coating treatments, date fruits
previously washed with sodium hypochlorite (0.01%) were dipped into a conidial suspension
of A. flavus at a concentration of 105 conidia/mL for 1 min. After inoculation, fruits were air
dried at room temperature for 2 h. They were then immersed in the different coating solutions
(30 dates per solution) for 1 min and air-dried at room temperature. Thirty uncoated dates
were used as control. The resulting coated dates and the control were stored at simulated
marketing conditions (25 °C, 90% RH) for 12 days and the disease incidence, expressed as
the number of infected berries out of the total number of fruits per treatment, was daily
evaluated. Each experiment was conducted twice.
ACCEPTED MANUSCRIPT
In addition to the postharvest decay control effectiveness study, the effect of pure and
combined EOs based coatings on the sensory properties of dates was evaluated to verify the
complete absence of off-odours and off-flavours, as requested by consumers.
A quantitative estimation of changes in the sensory characters, due to the coating effect, was
completed 24 h after coating, using the sensory profile method (UNI 10957, 2003). The
sensory profile was defined by using a panel of 10 trained judges aged between 28 and 40
years old (UNI EN ISO 8586, 2008), consisting of students and researchers from the
Department of Agricultural and Food Production (DiSPA), University of Catania. In a few
preliminary meetings, by using commercial date samples of variety Deglet Nour, the judges
generated a list of descriptors based on the percentage of citations referring to appearance,
olfactory, gustative and mouthfeel attributes. The final set consisted of 17 descriptors that
were quantified using a nine point intensity scale where the digit 1 indicates the descriptor
absence while the digit 9 the full intensity. Evaluations were carried out in single boxes at the
DISPA sensory analysis laboratory. The order of presentation was randomized between
judges and sessions. Water was provided for cleaning the mouth before tasting the next
sample. All data were acquired by a direct computerized registration system (FIZZ
Byosistemes. ver. 2.00 M, Couternon, France).
2.8 Statistical analysis
The results were analyzed by a multifactor analysis of variance (ANOVA) with a 95%
significance level using Statgraphics® Plus 5.1 (Manugistics Inc., Rockville, MD, USA).
Multiple comparisons were performed through 95% Fisher's LSD intervals.
3. Results and discussion
ACCEPTED MANUSCRIPT
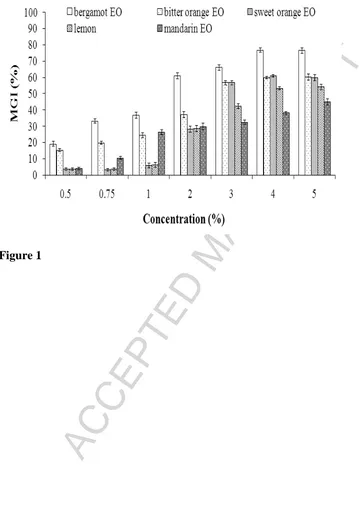
The effects of the different citrus EOs on the mycelial growth of A. flavus after an
incubation period of 7 days are presented in Figure 1. The tested EOs at concentrations
ranging from 0.5 to 5 % (v/v) significantly reduced the mycelial growth of A. flavus when
compared with the control (p < 0.05).
This inhibitory effect increased with increasing concentrations of EOs (from 0.5 to 5%
v/v) and was significantly related to the type of tested EO (p < 0.05). ANOVA results
demonstrated that bergamot oil, resulting in a 76.51% reduction in mycelial growth at a
concentration of 5% (v/v) compared with the control (p < 0.05), was the most effective EO,
followed by bitter orange EO which reduced the mycelial growth in the range of
15.34-60.22% compared with the control. Lemon and mandarin EOs showed the lowest percentage
of mycelial reduction compared with the other EOs (p < 0.05).
The observed difference in antifungal activity among these citrus EOs can be explained on the
basis of the differences in chemical composition of their volatile fractions, as previously
reported by Caccioni et al. (1998), who observed various degrees of growth inhibition when
testing the effect of volatile components of orange, mandarin, citrange and lemon EOs against
P. digitatum and P. italicum. While limonene could be considered as the main inhibitory
component due to its high concentration (93%) in the bitter orange EO, the greater antifungal
activity of bergamot might not be attributed to this volatile component due to the relatively
low concentration (40%) in such oil compared with the other citrus EOs (Table 1). As shown
in Table 1, bergamot was relatively rich in linalool (8%), compared with the other citrus oils.
This monoterpene alcohol, which is reported to have a wide range of antibacterial and
antifungal activity (Pattnaik et al., 1997), might be involved in the higher antifungal activity
of bergamot EO. However, possible synergistic and antagonistic interactions between major
and/or minor components in the EO may also give rise to fungal inhibition (Caccioni et al.,
ACCEPTED MANUSCRIPT
antimicrobial activities in the vapour phase of a wide number of EOs and their main
constituents. They reported a synergistic effect between cinnamaldehyde and cinnamon EO
on A. flavus. The mechanism of action of cinnamon EO on A. flavus and the antifungal
performance of cinnamon-based active packaging materials were elucidated by Manso et al.
(2013). In another study, Goñi et al. (2009) tested the susceptibility of various strains of
microorganisms to the vapour generated by a combination of cinnamon and clove EOs to
detect synergistic, additive or antagonistic effects. Their results revealed a clear
concentration-dependent interaction. Using minimal inhibitory concentrations values, the
mixture of cinnamon and clove exhibited a clear antagonistic effect against E. coli, whereas it
revealed a synergistic effect against Yersinia enterocolitica, Listeria monocytogenes and
Bacillus cereus when the concentrations of maximal inhibition were used.
Due to their interesting effect in reducing the mycelial growth of A. flavus, bergamot and
bitter orange EOs were selected for further experiments, and their most effective
concentrations was determined using the in vitro assay of conidial germination inhibition.
3.2. Screening of the most effective concentrations of the selected oils
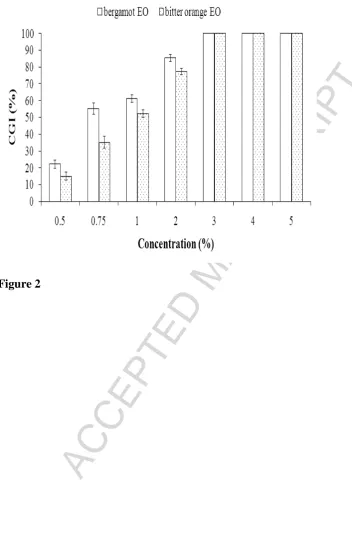
Figure 2 shows the effect of various concentrations of bergamot and bitter orange EOs
on the conidial germination of A. flavus. An ANOVA reveals that both EOs significantly
reduced (p < 0.05) conidial germination when compared with the control. Such reduction was
even more evident increasing the EO concentration (p < 0.05) and a complete inhibition of
conidial germination was obtained at concentrations higher than 2% (v/v). Previous studies
reported the effectiveness of citrus EOs on the inhibition of conidial germination of several
postharvest fungi. In this sense, Sharma and Tripathi (2008) recorded a complete inhibition of
conidial germination of A. niger when using Citrus sinensis oil at a concentration of 1.5
ACCEPTED MANUSCRIPT
observed by Tzortzakis and Economakis (2007) when testing lemongrass EO at a
concentration of 500 ppm (0.5%).
Although concentrations in excess of 2% of the selected EOs were necessary for the
complete inhibition of conidial germination, the use of such high amounts for controlling
postharvest decay in fruits can greatly affect their taste or exceed acceptable flavour
thresholds (Hsieh et al., 2001). For this reason, a concentration of 2%, resulting in,
respectively, 77.33 and 85.36% conidia inhibition for bitter orange and bergamot EOs, was
screened in vitro for the antifungal activity of combined treatments based on CH and LBG
coatings incorporating EOs, and in vivo for the assays on artificially infected dates.
3.3. In vitro antifungal activity of combined treatments
3.3.1. Effect of combined treatments on mycelial growth
The effects of CH and LBG alone, and in combination with either bergamot or bitter
orange EOs (2%, v/v) on mycelial growth of A. flavus, in an incubation period of 7 d at 25 °C,
are presented in Figure 3a. For all treatments, the diameter of the fungus increased gradually
over time (p < 0.05), suggesting its progressive adaptation to the new environment. As it can
be inferred from Figure 3a, pure CH was effective in delaying and reducing the growth of A.
flavus by 24.31% when compared with the control (p < 0.05), while no fungistatic effect
against the tested fungus has been obtained when LBG was used alone (p > 0.05). This result
appeared to be due to the antifungal activity of CH, as previously proved against several
postharvest pathogenic fungi, including A. flavus (Fradjo et al., 1994; Pedro et al., 2013). This
seems to be consistent also with the findings of Ziani et al. (2009) who noted a reduction of
more than 45% in the mycelium growth of A. niger when using pure CH treatment at a
concentration of 3% (w/v). In another study, Ali et al. (2010) found that CH treatment at a
ACCEPTED MANUSCRIPT
by 60%. Many factors such as the molecular weight of CH, the pH as well as the target
pathogen (Yang et al., 2005) may strongly affect the inhibition level by CH, which may
explain the difference in the degree of the MGI. Some authors associated the antifungal effect
of CH to its ability to interact, through its cationic chain, with the negatively charged residues
of the fungal cell surface, affecting the membrane permeability and causing a loss of
intracellular components (Muzzarelli et al. 2001; Yadav and Bhise, 2004). Another
explanation may be the interaction of the diffused hydrolysis products with the microbial
DNA which may affect the mRNA and the protein synthesis and thus the activity of enzymes
responsible for the growth of the fungi (El Ghaouth et al., 1992).
As shown in Figure 3a, the incorporation of either bergamot or bitter orange EOs into
the CH biopolymer matrix led to a delay in the growth of A. flavus. Indeed, fungal colonies
showed a 1-day delay in their growth in the plates having the combined treatments compared
to those coated with pure CH. Moreover, the combined CH-EO treatments caused a further
reduction in the mycelial growth of the tested fungus (p < 0.05) throughout the 7-day
incubation period compared to pure CH. In fact, the growth of A. flavus in the pure CH plates
was respectively 2 and 4 times higher than the growth observed for those coated with CH-2%
bitter orange and CH-2% bergamot at the end of the incubation period (Figure 3b). Such
improvement in antifungal activity caused by the combined effect of CH and EOs has been
also reported by Dos Santos et al. (2012) who noted a strong inhibition of the mycelial growth
of both Rhizopus stolonifer and A. niger when CH and Origanum vulgare L. EO were assayed
in combination. According to this study, the combined treatment was able to induce structural
changes in the mycelia of the tested fungi, including hyphal thinning, surface wrinkling and
the loss of cytoplasmic material characterized by empty hyphae. Such observations suggested
that the antifungal activity of the combined treatment (CH-EO) could include the attack to the
ACCEPTED MANUSCRIPT
With regards to treatments based on LBG, results showed that the incorporation of
bergamot or bitter orange EOs significantly reduced the mycelial growth of A. flavus when
compared with the unmodified control (p < 0.05), while no significant growth reduction was
obtained for the plates treated with pure LBG (p > 0.05). The results seem to be related to the
fungistatic activity of citrus EOs, previously proved against several fungi, including A. flavus
(Viuda-Martos et al., 2008).
3.3.2. Effect of combined treatments on conidial germination
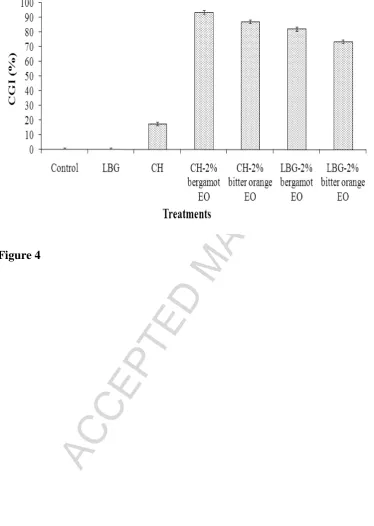
Figure 4 shows the impact of CH and LBG alone and in combination with either
bergamot or bitter orange EO at 2% on conidial germination of A. flavus. ANOVA results
demonstrated that conidial germination was significantly reduced by pure CH and by all the
combined treatments, when compared with the control and the pure LBG treatment, resulting
in 100% conidial germination inhibition (p < 0.05). Overall, combined treatments showed
more significant germination inhibition results compared with pure CH (p < 0.05). The most
effective treatments in reducing conidial germination were those based on CH-2% bergamot
EO and CH-2% bitter orange EO, which inhibited conidial germination in the range of
87-90% compared with the control (p < 0.05), followed by those based on LBG combined with
either 2% bitter orange or 2% bergamot EO, which resulted in 73 and 82% conidial inhibition,
respectively, compared with the control (p < 0.05). In agreement with our findings, previous
studies reported the efficacy of pure CH and natural EOs incorporated in different biopolymer
matrices in reducing conidial germination of several postharvest pathogenic fungi. In this
regard, Li et al. (2009) noted that CH at 1% completely inhibited conidial germination of
Fusarium sulphureum. A similar effect on conidial germination was obtained by Ali et al.
(2010) when CH was assayed at 2% against Colletotrichum gloeosporioides. In another study,
ACCEPTED MANUSCRIPT
and C. gloeosporioides when lemongrass and cinnamon EOs were incorporated in gum
Arabic. Dos Santos et al. (2012) also reported an inhibition by more than 90% of conidial
germination of R. stolonifer and A. niger when CH was assayed in combination with
origanum EO. According to these authors, the application of such combined treatment caused
significant morphological changes in the conidia of the tested fungi including wilting,
disruption, loss of cellular material, and deepening of ridges. These alterations suggest that
CH and origanum EO inhibit germination through an interaction with the cell wall of conidia.
On the basis of the results from the in vitro inhibition of conidial germination assay,
only the combined treatments, which reduced conidial germination of A. flavus by more than
80% (CH-2% bergamot EO, CH-2% bitter orange EO and LBG-2% bergamot EO), were
screened for the in vivo assay in mould-inoculated dates.
3.4. Fruit decay
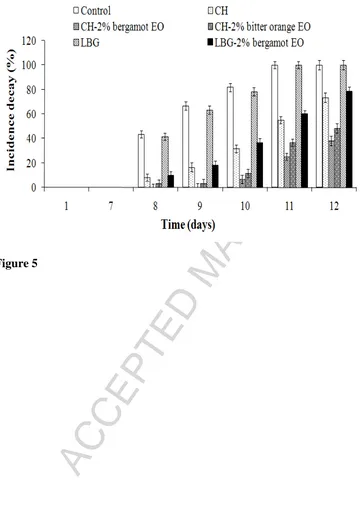
The effects of the different coatings on the decay percentage of inoculated dates,
during storage at 25 °C, are presented in Figure 5.The infection level by A. flavus increased
gradually throughout the storage time (p < 0.05) and was significantly higher for control
fruits and those treated with pure LBG, compared with the other treatments (p < 0.05). A
significant delay in the rate of fungal decay was observed when pure CH-based coatings were
applied to inoculated dates (p < 0.05). Although all of the uncoated dates were infected by
moulds at day 11, the incidence of decay in fruits treated with pure CH was found to be over
half (55%) of the one in control fruits and in those dipped in pure LBG. This result appeared
to be related to the antifungal activity of CH previously proved against several postharvest
fungi including Aspergillus (Hernández-Lauzardo et al., 2008; Li et al., 2009). In agreement
with these findings, Perdonas et al. (2012) observed a significant reduction in the growth of
ACCEPTED MANUSCRIPT
efficacy of pure CH coatings in controlling the incidence of fungal decay in fruits has been
also reported by Ali et al. (2010) and Maqbool et al. (2010) who noted a significant reduction
in the incidence of disease in CH-coated papaya and banana inoculated with C.
gloeosporioides and C. musae, respectively.
The statistical analysis revealed that the most effective coatings to reduce the fungal
decay were those based on CH incorporating either bergamot or bitter orange EO, which
reduced mould in the range of 52- 62% compared with the control (p < 0.05) at day 12. These
coatings were not only effective in controlling fruit decay but also in delaying the onset of
disease symptoms and slowing down mould growth during the storage period (p < 0.05).
After 7 days of storage, symptoms of fungal infection developed rapidly in control fruits
(more than 40 % of dates decayed), however, no signs of fungal decay were detected in fruits
treated with CH-bergamot oil until day 9, and only 3.33% (p > 0.05) of dates coated with
CH-bitter orange oil were infected, at this stage. This result suggests that bergamot and CH-bitter
orange EOs, previously reported to be effective against several postharvest fungi (Caccioni et
al., 1998; Sánchez-González et al., 2010), may enhance the antifungal activity of CH and lead
to a further inhibition of A. flavus in inoculated dates. A similar effect was reported by
Perdonas et al. (2012) who reported a further reduction in the fungal decay of inoculated
strawberries, when lemon EO was added to CH coatings.
Coatings based on LBG and bergamot EO showed lower antifungal activity compared
to coatings formulated with pure CH or CH in combination with citrus EOs (p > 0.05),
resulting in a fungal decay reduction of only 22% at day 12, compared with the control (p <
0.05). Such inhibitory effect appeared to be related to the fungistatic activity of bergamot EO
since no significant effect in the growth of A. flavus was observed in fruits treated with pure
ACCEPTED MANUSCRIPT
3.5. Sensory analysis
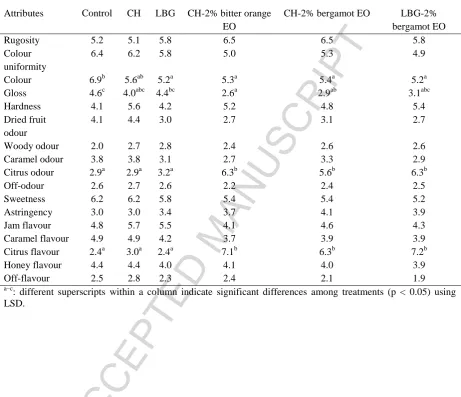
The effects of the different coatings on the sensory characters of dates are summarized
in Table 2. The sensory evaluation showed significant differences (p < 0.05) in four
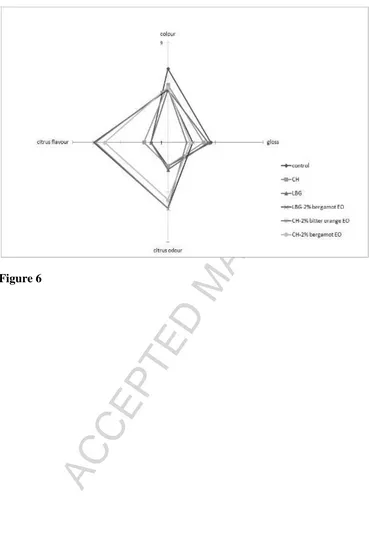
descriptors including colour, gloss, citrus odour and flavour. In the ‘spider’ chart of Figure 6,
the average scores of the only significant attributes that differentiate the samples are reported.
Judges observed a decrease in both the glossiness and colour intensity of dates after the
application of the different coatings (p < 0.05). Such decrease was even more evident in
samples treated with formulations containing citrus EOs (p < 0.05). Such behavior might be
explained by the increase in the opacity of the film, as a result of oil droplet aggregation,
during the drying process, which may reduce absorption of light by the surface of the fruits,
leading to a reduction in their glossiness and colour intensity (Pastor et al., 2011). In
agreement with our findings, Perdones et al. (2012) reported a significant loss of surface
glossiness in CH-coated strawberries, when lemon EO was added to CH coatings. On the
other hand, a high intensity of citrus odour and flavour was detected by the panel in dates
treated with either CH or LBG incorporating citrus EOs. The same observation was reported
in the sensory evaluation of strawberries coated with CH-lemon EO, performed after 24 h
(Perdones et al., 2012). None of the judges found the occurrence of off-flavours and off-odour
attributed to the different coating treatments. Nevertheless, the possibility that the high
intensity of citrus descriptors may affect the typical aroma and flavour of dates and, as a
consequence, the consumer’s acceptance would need further sensory investigations.
4. Conclusion
Combined treatments based on CH or LBG incorporating either bergamot or bitter
orange EOs at 2% (v/v) were proven to be effective in inhibiting A. flavus growth and conidial
ACCEPTED MANUSCRIPT
mycelial growth (by more than 55%) and conidial germination of A. flavus (by more than
85%), and reducing fungal decay in inoculated dates by more than 50% at day 12. These
results and the complete absence of off-flavours and off-odours demonstrate the potential of
the combined application of CH and citrus oils as an effective and promising alternative to
synthetic antifungal agents for controlling postharvest growth of A. flavus in dates.
Acknowledgements
Hajer Aloui was supported by the Tunisian Ministry of Higher Education and Scientific
Research. The authors are grateful to Fratelli di Bartolo (Calatabiano, Catania, Italy) for
providing the citrus essential oils, and to Adonia Samantha (Catania, Italy) for providing
Deglet Nour dates.
References
Ahmed, I.A., Ahmed, A., Robinson, R.K., 1997. Susceptibility of date fruits (Phoenix
dactylifera) to aflatoxin production. Journal of the Science of Food and Agriculture 74, 64–
68.
Ali, A., Muhammad, M.T.M, Sijam, K., Siddiqui, Y., 2010. Potential of chitosan coating in
delaying the postharvest anthracnose (Colletotrichum gloeosporioides Penz.) of Eksotika II
papaya. International Journal of Food Science and Technology 45, 2134–2140.
Amiri, A., Bompeix, G., 2011. Control of Penicillium expansum with potassium phosphite
and heat treatment. Crop Protection 30, 222–227.
Arrus, K., Blank, G., Abramson, D., Clear, R., Holley, R.A., 2005. Aflatoxin production by
ACCEPTED MANUSCRIPT
Atia, M.M.M., 2011. Efficiency of Physical Treatments and Essential Oils in Controlling
Fungi Associated with Some Stored Date Palm Fruits. Australian Journal of Basic and
Applied Sciences 5, 1572–1580.
Bakkali, F., Averbeck, S., Averbeck, D., Idaomar, I., 2008. Biological effects of essential
oils—a review. Food and Chemical Toxicology 46, 446–475.
Besbes, S., Drira, L., Blecker, C., Deroanne, D., Attia, H., 2009. Adding value to hard date
(Phoenix dactylifera L.): compositional, functional and sensory characteristics of date jam.
Food Chemistry 112, 406–411.
Bezic, N., Skocibusic, M., Dunkic, V., 2005. Phytochemical composition and antimicrobial
activity of Satureja montana L. and Satureja cuneifolia Ten. essential oils. Acta Botanica
Croatica 64, 313–322.
Caccioni, D.R.L., Guizzardi, M., Biondi, D.M., Renda, A., Ruberto, G., 1998. Relationship
between volatile components of citrus fruit essential oils and antimicrobial action on
Penicillium digitatum and Penicillium italicum. International Journal of Food Microbiology
43, 73–79.
Coma, V., Deschamps, A., Martial-Gros, A., 2003. Bioactive packaging materials from edible
chitosan polymer. Journal of Food Science 68, 2788–2792.
Cronin, M.J., Yohalem, D.S., Harris, R.F., Andrews, J.H., 1996. Putative mechanism and
dynamics of inhibition of apple scab pathogen Venturia inequalis by compost extracts. Soil
Biology and Biochemistry 28, 1241–1249.
Dehghan-Shoar, Z., Hamidi-Esfahani, Z., Abbasi, S., 2010. Effect of temperature and
modified atmosphere on quality preservation of sayer date fruits (phoenix dactylifera L.).
ACCEPTED MANUSCRIPT
Dos Santos, N.S.T., Aguiar, A.J.A.A., 2012. Efficacy of the application of a coating
composed of chitosan and Origanum vulgare L. essential oil to control Rhizopus stolonifer
and Aspergillus niger in grapes (Vitis labrusca L.). Food Microbiology 32, 345–353.
El Ghaouth, A., Arul, J., Asselin, A., Benhamou, N., 1992. Antifungal activity of chitosan on
postharvest pathogens: induction of morphological and cytological alterations in Rhizopus
stolonifer. Mycological Research 96, 769–779.
Fajardo, J.E., Waniska, R.D., Cuero, R.G., Pettit, R.E., 1994. Phenolic-compounds in peanut
seeds– enhanced elicitation by chitosan and effects on growth and aflatoxin B-1 productionby
Aspergillus flavus. Food Biotechnology 8, 191–211.
Fiori, A.C.G., Schwan-Estrada, K.R.F., Stangarlin, J.R., Vida, J.B., Scapim, A., Cruz, M.E.S.,
Pascholati, S.F., 2000. Antifungal activity of leaf extracts and essential oils of some medicinal
plants against Didymella bryoniae. Journal of Phytopathology 148, 483–487.
Food and Drug Administration (FDA), 2005. GRAS notifications. Available at:
http://www.fda.gov (accessed 28.04.13).
Goñi, P., Lopez, P., Sanchez, C., Gomez-Lus, R., Becerril, R., Nerin, C., 2009. Antimicrobial
activity in the vapour phase of a combination of cinnamon and clove essential oils. Food
Chemistry, 116, 982–989.
Grover, R.K., Moore, J. D., 1962. Toxicometric studies of fungicides against brown rot
organisms Sclerotonia fructicola and S. laxa. Phytopathology 52, 876–880.
Hernández-Lauzardo, A.N., Bautista-Baños, S., Velázquez-del Valle, M.G.,
Méndez-Montealvo, M.G., Sánchez-Rivera, M.M., Bello-Pérez, L.A., 2008. Antifungal effects of
chitosan with different molecular weights on in vitro development of Rhizopus stolonifer
(Ehren.:Fr.) Vuill. Carbohydrate Polymers 73, 541–547.
Hsieh, P.C., Mau, J.L., Huang, S.H., 2001. Antimicrobial effect of various combinations of
ACCEPTED MANUSCRIPT
Jowkar, M.M., Mohammadpour, H., Farshadfar, Z., Jowkar, A., 2005. A look at postharvest
in Iran. Acta Horticulturae 682, 2177–2182.
Kumar, A., Shukla, R., Singh, P., Prakash, B., Dubey, N.K., 2011. Chemical composition of
Ocimum basilicum L. essential oil and its efficacy as a preservative against fungal and
aflatoxin contamination of dry fruits. International Journal of Food Science and Technology
9, 1840–1846.
Li, Y.-C., Sun, X.-J., Bi, Y., Ge, Y.-H., Wang, Y., 2009. Antifungal activity of chitosan on
Fusarium sulphureum in relation to dry rot of potato tuber. Agricultural Sciences in China 8 ,
597–604.
Lopez, P., Sanchez, C., Batlle, R., Nerin, C., 2005. Solid- and vapor-phase antimicrobial
activities of six essential oils: susceptibility of selected foodborne bacterial and fungal strains.
Journal of Agricultural and Food Chemistry 53, 6939–6946.
Lopez, P., Sanchez, C., Batlle, R., Nerin, C., 2007. Vapor-phase activities of cinnamon,
thyme, and oregano essential oils and key constituents against foodborne microorganisms.
Journal of Agricultural and Food Chemistry 55, 4348–4356.
Manso, S., Cacho-Nerin, F., Becerril, R., Nerin, C., 2013. Combined analytical and
microbiological tools to study the effect on Aspergillus Flavus of cinnamon essential oil
contained in food packaging. Food Control 30, 370–378.
Maqbool, M., Ali, A., Ramachandran, S., Smith, D.R., Alderson, P.G., 2010. Control of
postharvest anthracnose of banana using a new edible composite coating. Crop Protection 29
, 1136–1141.
Maqbool, M., Ali, A., Alderson, P.G., Mohamed, M.T.M., Siddiqui, Y., Zahid, N., 2011.
Postharvest application of gum arabic and essential oils for controlling anthracnose and
quality of banana and papaya during cold storage. Postharvest Biology and Technology 62,
ACCEPTED MANUSCRIPT
Marandi, R.J., Hassani, A., Ghosta, Y., Abdollahi, A., Pirzad, A., sefidkon, F., 2011. Control
of Penicillium expansum and Botrytis cinerea on pear with Thymus kotschyanus, Ocimum
basilicum and Rosmarinus officinalis essential oils. Journal of Medicinal Plants Research 5,
626–634.
Mikkonen, K. S., Rita, H., Helén, H., Talja, R. A., Hyvönen, L.,Tenkanen, M., 2007. Effect
of polysaccharide structure on mechanical and thermal properties of galactomannan-based
films. Biomacromolecules 8, 3198–3205.
Muzzarelli, R.A.A., Muzzarelli, C., Tarsi, R., Miliani, M., Gabbanelli, F., Cartolari, M., 2001.
Fungistatic activity of modified chitosans against Saprolegnia parasitica. Biomacromolecules
2, 165–169.
Pastor, C., Sánchez-González, L., Cháfer, M., Chiralt, A., González-Martínez, C., 2010.
Physical and antifungal properties of hydroxypropylmethylcellulose based films containing
propolis as affected by moisture content. Carbohydrate Polymers 82, 1174–1183.
Pastor, C., Sánchez-González, L Marcilla, A., Chiralt, A., Cháfer, M., González-Martínez,
C., 2011. Quality and safety of table grapes coated with hydroxypropylmethylcellulose edible
coatings containing propolis extract. Postharvest Biology and Technology 60, 64–70.
Pattnaik, S., Subramanyam, V.R., Bapaji, M., Kole, C.R., 1997. Antibacterial and antifungal
activity of aromatic constituents of essential oils. Microbios 89(358), 39–46.
Pedro, R.O., Takaki, M., Gorayeb, T.C.C., Del Bianchi, V. L., Thomeo, J.C., Tiera, M.J.,
Tiera, V.A.O., 2013. Synthesis, characterization and antifungal activity of quaternary
derivatives of chitosan on Aspergillus flavus. Microbiological Research 168, 50–55.
Perdones, A., Sánchez-González, L., Chiralt, A., Vargas, M., 2012. Effect of chitosan–lemon
essential oil coatings on storage-keeping quality of strawberry. Postharvest Biology and
ACCEPTED MANUSCRIPT
Rojas-Argudoa, C., del Ríoa, M.A., Pérez-Gago, M.B., 2009. Development and optimization
of locust bean gum (LBG)-based edible coatings for postharvest storage of ‘Fortune’
mandarins. Postharvest Biology and Technology 52, 227–234.
Sánchez-González, L., Cháfer, M., Chiralt, A., González-Martínez, C., 2010. Physical
properties of edible chitosan films containing bergamot essential oil and their inhibitory
action on Penicillium italicum. Carbohydrate Polymers 82, 277–283.
Sánchez-González, L., Pastor, C., Vargas, M., Chiralt, A., González-Martínez, C., Cháfer, M.,
2011. Effect of hydroxypropylmethylcellulose and chitosan coatings with and without
bergamot essential oil on quality and safety of cold-stored grapes. Postharvest Biology and
Technology 60, 57–63.
Sharma, N., Tripathi, A., 2008. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on
growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiological Research
163, 337–344.
Shenasi, M., Candlish, A.A.G., Aidoo, K.O., 2002. The production of aflatoxins in fresh date
fruits and under simulated storage conditions. Journal of Food Science and Agriculture 82,
848–853.
Svoboda, K.P., Greenaway, R.I., 2003. Lemon scented plants. International Journal of
Aromatherapy 13, 23–32.
Tzortzakis, N.J., Economakis, C.D., 2007. Antifungal activity of lemongrass (Cympopogon
citratus L.) essential oilagainst key postharvest pathogens. Innovative Food Science and
Emerging Technologies 8, 253–258.
UNI 10957, 2003. Sensory analysis—method for establishing a sensory profile in foodstuffs
and beverages. UNI, Ente Nazionale Italiano di Unificazione, Milan, Italy.
UNI EN ISO 8586, 2008. Sensory analysis—general guidance for the selection, training and
ACCEPTED MANUSCRIPT
Veldhuizen, E.J., Tjeerdsma-van Bokhoven, J.L., Zweijtzer, C., Burt, S.A., Haagsman, H.P.,
2006. Structural requirements for the antimicrobial activity of carvacrol. Journal of
Agricultural and Food Chemistry 54, 1874–1879.
Viuda-Martos, M., Ruiz-Navajas, I., Fernández-López, J., Pérez-Álvarez, J.A., 2008.
Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit
(Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control 19, 1130–
1138.
Xing, Y., Li, X., Xu, Q., Yun, J., Lu, Y., 2010. Antifungal activities of cinnamon oil against
Rhizopus nigricans, Aspergillus flavus and Penicillium expansum in vitro and in vivo fruit
test. International Journal of Food Science and Technology 45, 1837–1842.
Yadav, A.V., Bhise, S.B., 2004. Chitosan: a potential biomaterial effective against typhoid.
Current Science 87, 1176–1178.
Yang, T.C., Chou, C.C., Li, C.F., 2005. Antibacterial activity of N-alkylated disaccharide
chitosan derivatives. International Journal of Food Microbiology 97, 237–245.
Ziani, K., Fernández-Pan, I., Royo, M., Maté, J.I., 2009. Antifungal activity of films and
ACCEPTED MANUSCRIPT
Figure legends
Figure 1. Effect of citrus EOs on mycelial growth inhibition (MGI) of Aspergillus flavus after
7-day incubation at 25 ± 2 °C. Mean values and LSD intervals.
Figure 2. In vitro conidial germination inhibition (CGI) of Aspergillus flavus by bergamot
and bitter orange EOs. Mean values and LSD intervals.
Figure 3a. Effect of CH and LBG either alone or combined with citrus EOs on mycelial
growth of Aspergillus flavus during a 7 days incubation period. Mean values and LSD
intervals.
Figure 3b. Mycelial growth of Aspergillus flavus, at the end of the incubation period, in
control plates (a), pure CH plates (b) and plates treated with CH incorporating either bergamot
(c) or bitter orange (d) EOs at 2%.
Figure 4. Effect of CH and LBG either alone or combined with citrus EOs on conidial
germination inhibition (CGI) of Aspergillus flavus. Mean values and LSD intervals.
Figure 5. Effect of different treatments on fungal decay of artificially infected dates during
storage at 25 °C. Mean values and LSD intervals.
ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
Table 1. Main physicochemical and compositional data for tested citrus EOs.
Citrus essential oil Refraction index (20°C) Density (g/mL) (20°C) Major volatile compounds
Bergamot 1.460-1.480 0.872-0.882 -Citral (0.580%)
-Linalool (8%) -Limonene (40%)
Bitter orange 1.472-1.476 0.840-0.860 -Citral (0.120%) -Linalool (0.270%) -Limonene (93%)
Sweet orange 1.463-1.483 0.840-0.850 -Citral (0.125%) -Linalool (0.415%) -Limonene (95%) -Geraniol (0.005%)
Mandarin 1.468-1.488 0.844-0.854 Citral (0.050%)
-Linalool (0.115%) -Limonene (70%)
-Geraniol (0.005%)
Lemon 1.462-1.482 0.846-0.856 -Citral (2.200%)
ACCEPTED MANUSCRIPT
Table 2. Sensory profile of coated and uncoated dates.
Mean scores
Attributes Control CH LBG CH-2% bitter orange
EO
ACCEPTED MANUSCRIPT
36
Highlights
Bergamot and bitter orange essential oils strongly inhibited Aspergillus flavus
Coating composed of chitosan and essential oils (EOs) reduced fungal decay on dates
Chitosan and Locust Bean Gum combined with 2% of EOs inhibited conidial germination