CANCER IMMUNOTHERAPY
Amaylia Oehadian
Hematology and Medical Oncology Division , Department of Internal Medicine Hasan Sadikin Hospital Bandung
The realization that human cancers express cancer-associated antigens has stimulated research into the development of immunotherapies to mediate the regression of tumor.1 Cancer immunotherapy or biological therapy refers to antitumor treatment utilizing the actions of natural host-defense mechanisms and/or mammalian-derived substances.2 Research during the past decade has establish immunotherapy as an important anticancer modality. In fact, tumor immunology is now one of the most active areas of cancer research and has prompted the development of several novel therapies currently in use, including cytokine-based therapies, vaccine therapies, and monoclonal antibody therapies. Understanding the scientific rationale behind tumor immunology will better inform practitioners about immunotherapies that are currently available or being studied in ongoing trial.3
This paper will provide a review of immune dysregulation on oncogenesis, followed by an overview of immunotherapy strategies in cancer.
IMMUNE DYSREGULATION IN ONCOGENESIS
Cancer is 100 times more likely to occur in immunosupressed individuals than those with normal immune function. Conversely, heightened immune activity has been postulated as the potential cause of spontaneous tumor regression. On the other hand, development of some tumors is actually associated with chronic imflammation, such as mucosal-associated lymphoid tumor and immunoproliferative small intestinal disease.3
The first recognition that immune system plays an important role in oncogenesis dated back to the 1700s, when it was observed that certain cancer patients who acquired and cleared bacterial infections also experienced remission of their malignancy. In the late 1800s, Dr. Willian B Coley made similar observations about cancer and immune regulation, ultimately leading to the development and use of early forms of cancer immunotherapy. Coley noted that some patients with sarcomas had spontaneous tumor regressions following a bout of erysipelas, a superficial streptococcal skin infection. Coley deliberately infected some of his inoperable patients with erysipelas to stimulate tumor regression. He later refined this approach by using heat-killed Streptococcus pyogenes in combination with heat-killed Serratia marcescens- a mixture that is now commonly known as “Coley’s toxin”. 2,3
Immune surveillance
cells.3 The immune system’s identification of such antigens leads to destruction of the emergent cancer clone. The immune surveillance principle is supported by 2 primary lines of clinical evidence :
The presence of tumor-infiltrating lymphocytes (TILs) within some established tumors.3
The increased incidence of several cancers with immunosuprressant therapy.3
Immunoediting : elimination, equilibrium and escape phases of oncogenesis
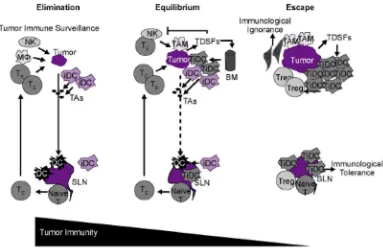
Recent research has shed additional light on the process of immune surveillance in oncogenesis. Immunoediting appears to be an important part of this process and consists of 3 distinct phases : elimination, equilibrium and escape. (Figure 1).3
Figure 1. Immunoediting : elimination, equilibrium, escape.3
BM : bone marrow; iDC : immature dendritic cell; Mj: macrophage, SLN : sentinel lymph node ; TAM : tumor associated antigens ; TDSFs : tumor derived soluble factors ; TE : effector T cell ; TiDC : tumor- associated iDC ; Tregs : regulatory T cells.
Elimination phase
With respect to innate immunity, inflammatory cytokines initially released by growing tumor cells, macrophages , and stromal cells in the tumor microenvirontment are recognized by a number of innate immune cells, including NK cells, NK1.1+ T cells, and T cells, which in turn produce interferon gamma. Interferon gamma promotes the kiliing of tumor cells through its effects on proliferation, angiogenesis and apoptosis. Release of interferon gamma and interleukin (IL)-12 by both tumor-infiltrating NK cells and macrophages leads to additional cell killing through activation of cytotoxic mechanisms involving perforin, tumor necrosis factor-related apoptosis-inducing ligand, as well as reactive oxygen species.3
Infiltrating dendritics cells, recruited to tumor-draining lymph nodes by NK cells, can phagocytose necrotic tumor cells, resulting in the presentation of tumor-associated antigens and the priming of naïve T cells. Bringing in adaptive immunity processes, antigen-specific cytotoxic CD4+ and CD8+ T lymphocytes subsequently migrate to the site of the tumor where they identify and kill tumor cells, producing additional interferon gamma and other cytokines that further enhance tumor cell killing.3
Equilibrium phase
Selection pressure produced by the immune system actually promotes the establishment of less immunogenic tumor variants within the tumor microenvironment. Because the mutation rate of tumor cells is extremely high, genetic changes can develop that permit these cells to acquire resistance to previously establish immune effector cells, thereby enabling them to evade elimination by the immune system. The equilibrium phases of immunoediting is characterized by the persistence of tumor cell population. Given that highly immunogenetic tumor cells are continuously eliminated while resistant tumor cells are spontaneously generated and gradually maintained, it has been hypothesized that the equilibrium phase is likely the longest of the 3 immunoediting phases, evolving over the span of several years.3
Escape phase
The escape phase represents the point at which cancer cells are no longer sufficiently recognized and/or controlled by the immune system to prevent disease progression. Tumor escape can be achieved through several mechanisms, including downregulation of various immunoregulatory components :
- Tumor-derived soluble factors
- Alterations in signal transduction
The CD3-zeta chain functions as an important transmembrane signaling molecule in TILs. Down regulation of zeta chain expression has been associated with increased levels of IL-10 and transforming growth factor-beta and decreased levels of interferon gamma. Low or absence zeta chain expression in immune cells impairs the function of TILs and hence predicts for poor prognosis and unfavorable survival outcomes in cancer patients.3
- Immunologic tolerance
In some cases, antigen-expressing tumor cells are ignored by the immune system because the antigens are masked by surrounding nontumor cells (eg, immature dendritic cells, fibroblast, endothelial cells) that compete with mature dendritic cells for antigen binding. As such, these depressed levels of tumor antigen are not sufficient to produce or sustain a clinically meaningful cytotoxic T-cell response. As a result, these antigen-presenting tumors cells are largely ignored by surveillance T cells. Immature dendritic cells are also capable of stimulating regulatory cells (eg, CD4+ and CD25+ cells), which suppress T-cell activation and further contribute to immunologic tolerance.3
CURRENT IMMUNOTHERAPY STRATEGIES
Current clinical investigation is being directed at stimulating the immune system via cytokines, antibodies, and vaccines to improve tumor recognition, thereby allowing an individual to mount an immune response that can effectively eradicate tumors. It is important to distinguish between cytokine , antibody , and vaccine therapies :
The goal of vaccine therapy is to stimulate active antitumor immunity by priming or expanding immune responses, which can recognize and destroy tumor cells.3
Cytokines and antibody therapies work indirectly by supplying the necessary tools directly to effectuate and antitumor response.3
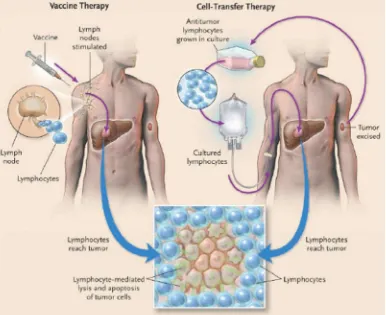
The two main approaches to immunotherapy for cancer are vaccine therapy and cell-transfer therapy.1 ( Figure 2)
There are three requirements for an effective immunotherapy for cancer (Figure 2) :
A sufficient number of avid tumor-reactive lymphocytes must be present in the tumor bearing host.1
These lymphocyte must be capable of reaching and extravasating at the site of the cancer.1
Figure 2. Two approach in immunotherapy.1
1. Cytokines-based therapies
Cytokines are small signaling molecules essential to mediating immune responses. A handful of cytokines are currently approved for cancer treatment, the most common of which are interferon alfa and IL-2 .3,4
Interferon alfa
Interferon alfa was first isolated on 1970 from white blood cells in a search for agent that interfere with viral infection-hence its name.3 Interferon alfa functions on a number of levels including :
- upregulation of genes encoding for the major histocompatibility complex class I molecules, tumor antigens , and adhesion molecules.3
- Promotes the activity of B cells, T cells , macrophages and dendritic cells - Increases the expression of Fc receptors.3
Interleukin-2
Interleukin-2, a T-cell growth factor, is currently approved for the treatment of renal cell carcinoma and malignant melanoma. In metastatic renal cell cancer, response rates approaching 23% have been reported, and these responses have been durable for up to 24 months.3
Sargramostim
Sargramostim , a recombinant human granulocyte macrophage–colony stimulating factor (rhGM—CSF) has been shown to induce the differentiaion of myeloid dendritic cells that promote the development of T-helper type 1 (cellular) immune responses. Sargramostim has been used to augment the activity of rituximab in patients with follicular lymphoma and to induce autologous antitumor immunity in patients with hormone-refractory prostate cancer. The addition of sargramostim to standard vaccines may increase effectiveness by recruiting dendritic cells to the site of vaccination.5
2. Monoclonal antibody therapies
Since their initial clinical development in the late 1970s, monoclonal antibodies directed against antigens expressed on cancer cells have proven to be one of the most promising classes of immunomodulatory agents. Monoclonal antibodies function by :
Directly disrupting cancer cell activity through their affinity for relevant antigens.4,6
Enhancing the immune response againts cancer cell through antibody-dependent cell-mediated or complement-dependent cytotoxicity.4,6
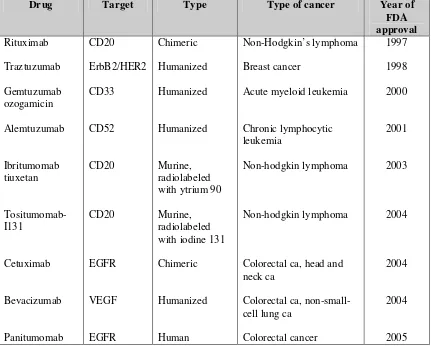
To date, a total of 9 monoclonal antibodies have been approved for a variety of therapeutic indications (table 1)
Table 1. Currently approved monoclonal antibody therapies for cancer.3
Drug Target Type Type of cancer Year of
FDA approval Rituximab Traztuzumab Gemtuzumab ozogamicin Alemtuzumab Ibritumomab tiuxetan Tositumomab-I131 Cetuximab Bevacizumab Panitumomab CD20 ErbB2/HER2 CD33 CD52 CD20 CD20 EGFR VEGF EGFR Chimeric Humanized Humanized Humanized Murine, radiolabeled with ytrium 90
Murine, radiolabeled with iodine 131
Chimeric
Humanized
Human
Non-Hodgkin’s lymphoma
Breast cancer
Acute myeloid leukemia
Chronic lymphocytic leukemia
Non-hodgkin lymphoma
Non-hodgkin lymphoma
Colorectal ca, head and neck ca
Colorectal ca, non-small-cell lung ca
Colorectal cancer 1997 1998 2000 2001 2003 2004 2004 2004 2005
EGFR : epidermal growth factor receptor; HER2 : human epidermal growth factor receptor-2; VEGF : vascular endothelial growth factor
Cytotoxic T lymphocyte antigen 4 (CTLA-4)
CD4+ T cells play key role in the adaptive immune response to foreign antigens. T cell activation depends on a 2-step signaling process :
The first signal is delivered through antigen recognition by the T-cell receptor.3
A second or costimulatory signal is also required for optimal activation of T cells. CD28 ligation by B7 is a potent mediator of positive costimulation. By contrast, B7 ligation of CTLA-4, a homolog of CD28, acts as a critical for many immune responses.3
Because costimulatory molecule interactions are critical for many immune responses, a greater understanding of CTLA-4 function may promise development of immunotherapies where enhancement or inhibition of the immune responses is clinically beneficial. Monoclonal antibodies that exert their antitumor effects indirectly via the immune system are currently being investigated in clinical trial. As of June 2007, there are 2 fully human anti-CTLA-4 monoclonal antibodies in advanced clinical trials : tremelimubab and ipilimumab.3,7
Ipilimumab (MDX-101) is a fully human monoclonal antibody, that overcomes CTLA-4-mediated T-cell suppression to enhance the immune response against tumors.7 Preclinical and early clinical studies of patients with advanced melanoma show that ipilimimab promotes antitumor activity as monotherapy and in combination with treatments such as chemotherapy, vaccines, or cytokines.3,7
3. Vaccine therapies
In cancer therapy, potential use of vaccine include passive or adoptive immunotherapy and active specific immunotherapy. With passive immunotherapy, the goal is to enhance and/or stimulate the immune system using exogenous cytokines, antibodies, immune cells, or growth factors. With active specific immunotherapy, a specific tumor-associated antigen elicits and endogenous immune or antitumor response.6
The goal of therapeutic vaccines in the treatment of cancer is to prime and expand the host’s immune system to identify and destroy tumor cells. Over the past few decades, many clinical trials have tested a variety of cancer vaccines. Although the number of patients achieving objective responses has been small, these studies have always identified a consistent subgroup of patients who achieve significant clinical benefit from such therapies.3 The variety of vaccine approaches has included :
Antigen peptides to known major histocompatibility complex motifs.3
Viral vectors.3
Whole cell vaccines.3
Dendritic cell caccines.3
Anti-idiotype vaccine.3
Despite encouraging phase I and II study results, no therapeutic cancer vaccine has been approved by FDA. Only a few therapeutic vaccine strategies have progressed to phase III testing.3
Viral vectors vaccine
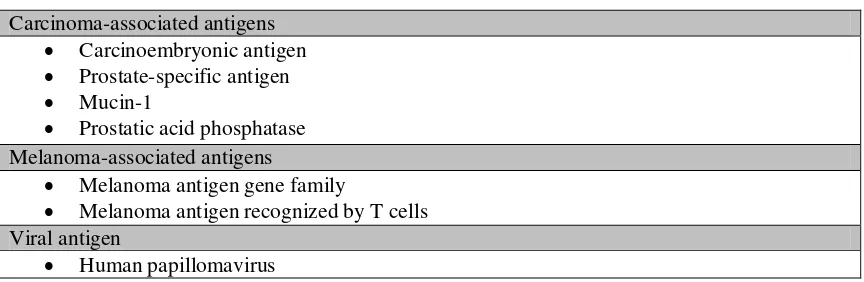
Table 2. Tumor antigens targeted by viral vector vaccines.3
Carcinoma-associated antigens Carcinoembryonic antigen Prostate-specific antigen Mucin-1
Prostatic acid phosphatase Melanoma-associated antigens
Melanoma antigen gene family
Melanoma antigen recognized by T cells Viral antigen
Human papillomavirus
The concept of using replication viruses as anticancer agents is not a new one, but the ability to genetically modifiy this viruses into increasingly potent and tumor-specific vectors is a recent phenomenon. The paradoxical roles of the immune response are addressed with respect to oncolytic viral therapy, as it, on one hand, impedes the spread of viral infection, and on the other, augment tumor cell destruction through the recruitment of T cells “vaccinated” against tumor antigen. The most commonly used oncolytic viruses are adenoviruses, herpes simplex viruses, vaccinia viruses, reovirus and Newcastle disease viruses.8 Oncolytic viruses mediate the destruction of tumor cells by several potential mechanisms (table 3)
Table 3. Mechanism of antitumoral efficacy of oncolytic viruses.8
Mechanism Example
Direct cell lysis due to viral replication
Direct cytotoxicity of viral protein
Induction of antitumoral immunity
Nonspecific (eg. TNF)
Specific (eg. CTL response)
Sensitization to chemotherapy and radiotherapy
Transgene expression
Adenoviruses Herpesviruses
Adenovirus E4ORF4
Adenovirus (EIA) Herpes simpleks virus
Adenovirus (EIA)
Adenovitus (AdTK RC)
Dendritic cell vaccines
Dendritic cell vaccines are one of the most prominent vaccine strategies being tested for stimulating a tumor-specific immune response. These vaccines are typically created by culturing the patient’s own dendritic cells in the presence of tumor peptides and immunogenic adjuvants. Several dendritic vaccine strategies have focused on treating individuals with metastatic melanoma.3
Conclusions
Immunoediting appears to be an important part of immune surveilance in oncogenesis and consists of 3 distinct phase : elimination, equilibrium and escape. Anticancer immune therapies consist of cytokines, monoclonal antibodies and vaccines. The best use of these agents may involve combination therapies with multiple immunologically active agents as well as other treatment modalities including chemotherapy or radiation therapy. In this way, immune therapies may be better able to identify and eradicate tumor cells through the induction of programmed cell death as well as by disrupting the tumor microenvirontment and halting angiogenesis while maintaining and stimulating antitumor immune acitivity.
Reference :
1. Rosenberg SA. Shedding light on immunotherapy for cancer. N Engl J Med 2004;350:1461-3.
2. Davis T, Streicher H, Zwiebel JA, Kim B, Litton GJ. Biological therapy interferons, interleukin-2, monoclonal antibodies, and tumor vaccines. In : Pazdur R, Coia LR, Hoskins WJ, Wagman LD, eds .Cancer management: a multidisciplinary approach.5th ed. New York:PRR;2001.p.905.
3. Kirkwood JM, Hodi FS, King E, Bowser AD, Janssen D, Mortimer J, et al. Immunology for the oncologist : historical review of immune dysregulation and immune strategies. Clinical Care Options Oncology 2007.
4. Burmester GR, Pezzutto A. Wirth J. Atlas of immunology. New York :Thieme NewYork ; 2003.p. 150-6.
5. Waller EK. The role of sargramostim (rhGM-CSF) as immunotherapy. The Oncologist 2007;12:22-6.
6. Estena FJ. Monoclonal antibodies, small molecules , and vaccines in the treatment of breast cancer.The Oncologist 2004;9:4-9.