Supplemental Materials Yin and MacKerell
Combined Ab initio/Empirical Approach for the Optimzation of Lennard-Jones Parameters
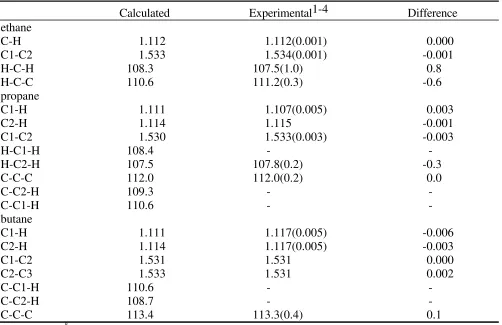
Table S1. Internal geometries for the selected alkanes
Calculated Experimental1-4 Difference
ethane
C-H 1.112 1.112(0.001) 0.000
C1-C2 1.533 1.534(0.001) -0.001
H-C-H 108.3 107.5(1.0) 0.8
H-C-C 110.6 111.2(0.3) -0.6
propane
C1-H 1.111 1.107(0.005) 0.003
C2-H 1.114 1.115 -0.001
C1-C2 1.530 1.533(0.003) -0.003
H-C1-H 108.4 -
-H-C2-H 107.5 107.8(0.2) -0.3
C-C-C 112.0 112.0(0.2) 0.0
C-C2-H 109.3 -
-C-C1-H 110.6 -
-butane
C1-H 1.111 1.117(0.005) -0.006
C2-H 1.114 1.117(0.005) -0.003
C1-C2 1.531 1.531 0.000
C2-C3 1.533 1.531 0.002
C-C1-H 110.6 -
-C-C2-H 108.7 -
-C-C-C 113.4 113.3(0.4) 0.1
Length unit: Å; angle unit: degree.
Table S2. Comparison of calculated and experimental frequencies for ethane
# Assignment Calc. Exp.5-7 %difference* aver. %diff.
1 C-C tor 306 279 9.6
2 CH3 rocking 922 822 12.2
3 CH3 rocking 922 822 12.2
4 C-C str 1002 995 0.7
5 CH3 rocking 1113 1190 6.5
6 CH3 rocking 1113 1190 6.5
7 CH3 asym def 1416 1370 3.4
8 CH3 asym def 1416 1388 2.1
9 CH3 asym def 1437 1469 2.2
10 CH3 asym def 1437 1469 2.2
11 CH3 sym def 1437 1460 1.6
12 CH3 sym def 1439 1460 1.5 5.1**
13 CH3 sym str 2895 2915 0.7
14 CH3 sym str 2909 2915 0.2
15 CH3 asym str 2953 2950 0.1
16 CH3 asym str 2953 2950 0.1
17 CH3 asym str 2964 2974 0.3
18 CH3 asym str 2964 2974 0.3 0.3***
Total average %difference 3.5
* %difference= ((calc.-exp.)/exp)*100
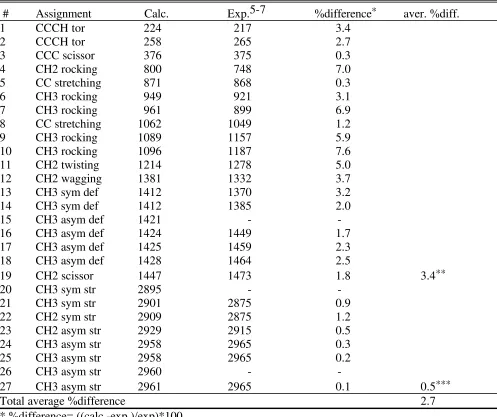
Table S3. Comparison of calculated and experimental frequencies for propane
# Assignment Calc. Exp.5-7 %difference* aver. %diff.
1 CCCH tor 224 217 3.4
2 CCCH tor 258 265 2.7
3 CCC scissor 376 375 0.3
4 CH2 rocking 800 748 7.0
5 CC stretching 871 868 0.3
6 CH3 rocking 949 921 3.1
7 CH3 rocking 961 899 6.9
8 CC stretching 1062 1049 1.2
9 CH3 rocking 1089 1157 5.9
10 CH3 rocking 1096 1187 7.6
11 CH2 twisting 1214 1278 5.0
12 CH2 wagging 1381 1332 3.7
13 CH3 sym def 1412 1370 3.2
14 CH3 sym def 1412 1385 2.0
15 CH3 asym def 1421 -
-16 CH3 asym def 1424 1449 1.7
17 CH3 asym def 1425 1459 2.3
18 CH3 asym def 1428 1464 2.5
19 CH2 scissor 1447 1473 1.8 3.4**
20 CH3 sym str 2895 -
-21 CH3 sym str 2901 2875 0.9
22 CH2 sym str 2909 2875 1.2
23 CH2 asym str 2929 2915 0.5
24 CH3 asym str 2958 2965 0.3
25 CH3 asym str 2958 2965 0.2
26 CH3 asym str 2960 -
-27 CH3 asym str 2961 2965 0.1 0.5***
Total average %difference 2.7
* %difference= ((calc.-exp.)/exp)*100
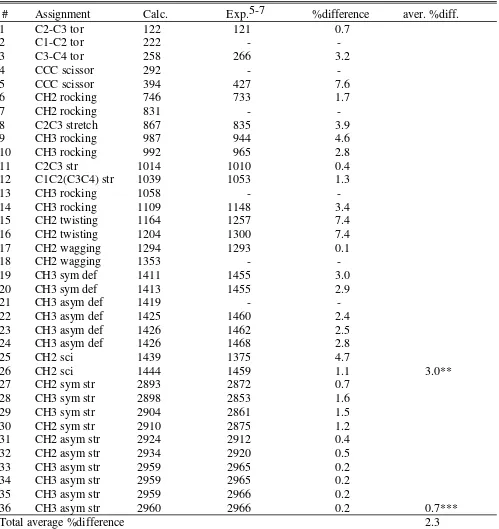
Table S4. Comparison of calculated and reference vibrational freqencies for butane
# Assignment Calc. Exp.5-7 %difference aver. %diff.
1 C2-C3 tor 122 121 0.7
2 C1-C2 tor 222 -
-3 C3-C4 tor 258 266 3.2
4 CCC scissor 292 -
-5 CCC scissor 394 427 7.6
6 CH2 rocking 746 733 1.7
7 CH2 rocking 831 -
-8 C2C3 stretch 867 835 3.9
9 CH3 rocking 987 944 4.6
10 CH3 rocking 992 965 2.8
11 C2C3 str 1014 1010 0.4
12 C1C2(C3C4) str 1039 1053 1.3
13 CH3 rocking 1058 -
-14 CH3 rocking 1109 1148 3.4
15 CH2 twisting 1164 1257 7.4
16 CH2 twisting 1204 1300 7.4
17 CH2 wagging 1294 1293 0.1
18 CH2 wagging 1353 -
-19 CH3 sym def 1411 1455 3.0
20 CH3 sym def 1413 1455 2.9
21 CH3 asym def 1419 -
-22 CH3 asym def 1425 1460 2.4
23 CH3 asym def 1426 1462 2.5
24 CH3 asym def 1426 1468 2.8
25 CH2 sci 1439 1375 4.7
26 CH2 sci 1444 1459 1.1 3.0**
27 CH2 sym str 2893 2872 0.7
28 CH3 sym str 2898 2853 1.6
29 CH3 sym str 2904 2861 1.5
30 CH2 sym str 2910 2875 1.2
31 CH2 asym str 2924 2912 0.4
32 CH2 asym str 2934 2920 0.5
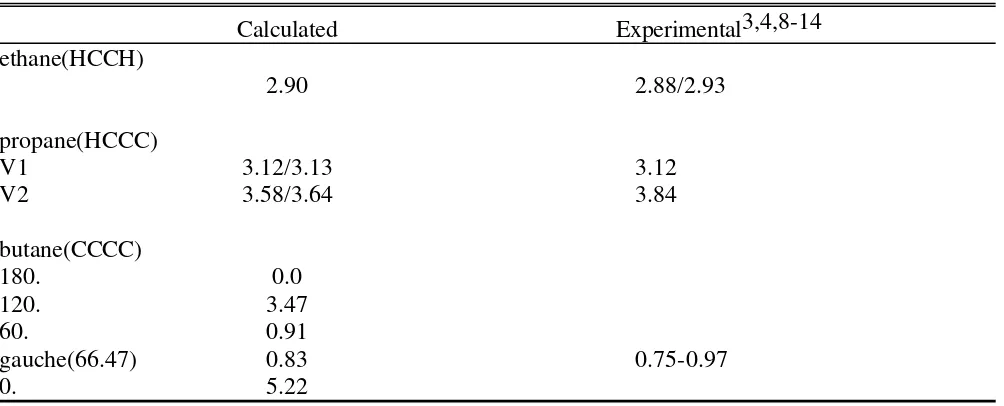
Table S5. Conformational energies in the selected alkanes
Calculated Experimental3,4,8-14
ethane(HCCH)
2.90 2.88/2.93
propane(HCCC)
V1 3.12/3.13 3.12
V2 3.58/3.64 3.84
butane(CCCC)
180. 0.0
120. 3.47
60. 0.91
gauche(66.47) 0.83 0.75-0.97
0. 5.22
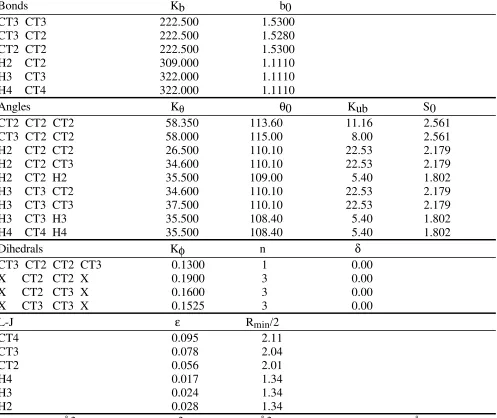
Table S7. Alkane internal parameters
Bonds Kb b0
CT3 CT3 222.500 1.5300
CT3 CT2 222.500 1.5280
CT2 CT2 222.500 1.5300
H2 CT2 309.000 1.1110
H3 CT3 322.000 1.1110
H4 CT4 322.000 1.1110
Angles Kθ θ0 Kub S0
CT2 CT2 CT2 58.350 113.60 11.16 2.561
CT3 CT2 CT2 58.000 115.00 8.00 2.561
H2 CT2 CT2 26.500 110.10 22.53 2.179
H2 CT2 CT3 34.600 110.10 22.53 2.179
H2 CT2 H2 35.500 109.00 5.40 1.802
H3 CT3 CT2 34.600 110.10 22.53 2.179
H3 CT3 CT3 37.500 110.10 22.53 2.179
H3 CT3 H3 35.500 108.40 5.40 1.802
H4 CT4 H4 35.500 108.40 5.40 1.802
Dihedrals Kφ n δ
CT3 CT2 CT2 CT3 0.1300 1 0.00
X CT2 CT2 X 0.1900 3 0.00
X CT2 CT3 X 0.1600 3 0.00
X CT3 CT3 X 0.1525 3 0.00
L-J ε Rmin/2
CT4 0.095 2.11
CT3 0.078 2.04
CT2 0.056 2.01
H4 0.017 1.34
H3 0.024 1.34
H2 0.028 1.34
Kb: kcal/mol/Å2; Kθ: kcal/mol/degree2; Kub: kcal/mol/Å2; Kφ, ε: kcal/mol; length unit: Å; angle unit:
H4
0.09 0.09
0.09
CT3 CT2
CT3 H3
CT3 CT2
CT2 CT3
H3
B
Potential Energy, kcal/mol
Dihedral Angle CCCC
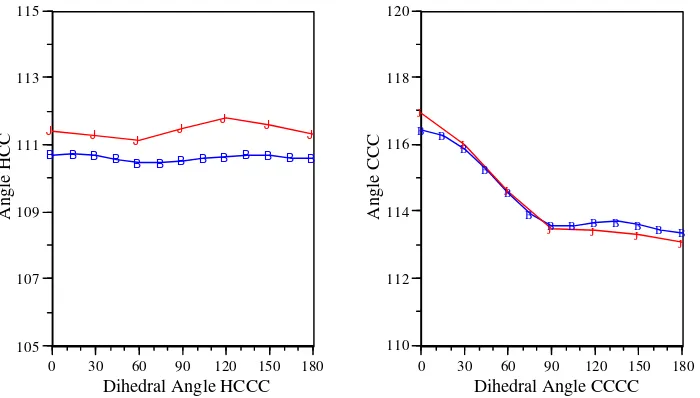
Figure S2. Empirical and ab initio adiabatic energy surfacs for rotation about the CCCC dihedral in butane. (B): ab initio HF/6-31G(d) (J): total empirical energy (H): electrostatic
B B B B B B B B B B B B B
J J J J J J J
110 112 114 116 118 120
0 30 60 90 120 150 180
Angle CCC
Dihedral Angle CCCC
B B B B B B B B B B B B B
J J J J J J J
105 107 109 111 113 115
0 30 60 90 120 150 180
Angle HCC
Dihedral Angle HCCC
Reference:
(1) Hoyland, J. R. I. Chem. Phys.1969, 50, 2775.
(2) Wiberg, K.; Boyd, R. H. J. Am. Chem. Soc. 1972, 94, 5236.
(3) Heenan, P. K.; Bartell, L. S. J. Chem. Phys.1983, 78, 1270.
(4) Bradford, W. F.; Fitzwater, S.; Bartell, L. S. J. Mol. Struct.1977, 38, 185.
(5) Schachtschneider, J. H.; Snyder, R. G. Spectrochim. Acta1963, 19, 117.
(6) Snyder, R. G.; Schachtschneider, J. H. Spectrochim. Acta1965, 21, 169.
(7) Lifson, S.; Warshel, A. J. J. Chem. Phys.1968, 49, 5116.
(8) Aufderheide, K. Croat. Chem. Acta1984, 57, 811.
(9) Hirota, E.; Endo, Y.; Saito, S.; Duncan, J. L. J. Mol. Spectrosc.1981, 89, 285.
(10) Hirota, E.; Matsumara, C.; Morino, Y. Bull. Chem. Soc. Jpn.1967, 40, 1124.
(11) Hoyland, J. R. J. Chem. Phys.1968, 49, 1908.
(12) Compton, D. A.; Montrero, S.; Murphy, W. F. J. Phy. Chem.1980, 84, 3587.
(13) Rosenthal, L.; Robolt, J. F.; Hummel, J. J. Chem. Phys.1982, 76, 617.
(14) Banon, A.; Serrano Adan, F.; Santamaris, J. J. Chem. Phys.1985, 83, 297.