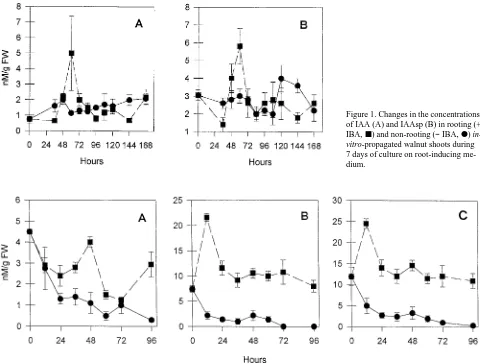
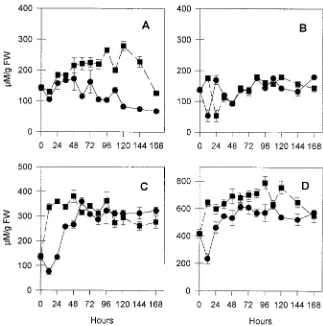
Summary Rooting was induced in in-vitro-propagated wal-nut (Juglans regia L.) shoots by subculturing the shoots on rooting medium containing agar and 3 mg l−1 indole-3-butyric acid (IBA) for 7 days in darkness. Changes in the concentra-tions of endogenous free acetic acid (IAA), indole-3-acetylaspartic acid (IAAsp) and free polyamines were determined during culture on root-inducing medium. In ex-tracts of whole shoots, the concentration of free IAA showed a transient peak at 60 h (around 48 h in extracts from basal shoot portions) and then remained at a relatively low concen-tration for the remainder of the 7-day culture period. The concentration of IAAsp in extracts of whole shoots peaked at about the same time as the concentration of free IAA, whereas the IAAsp concentration in extracts from basal shoot portions peaked earlier, at around 12 h. The concentrations of free polyamines in extracts of whole shoots increased soon after the shoots were transferred to root-inducing medium. The concen-trations of IAA and IAAsp remained stable when the rooted shoots were transferred to a vermiculite/gelrite mixture (with-out auxin) and grown in light.
Keywords: auxin conjugates, Juglans regia, micropropaga-tion, putrescine, spermine.
Introduction
Auxins play a central role in the adventitious rooting process (Davis and Haissig 1994); however, the role of endogenous auxin in rhizogenesis remains unclear (Jarvis 1988, Gaspar and Hofinger 1988, Moncousin 1991). The rooting process comprises a series of interdependent physiological phases (Berthon et al. 1990, De Klerk and Ter Brugge 1992) that are associated with changes in endogenous auxin concentrations (Maldiney et al. 1986, Moncousin et al. 1988, Berthon et al. 1989, Hausman 1993). Blakesley (1994) and Gaspar et al. (1994) postulated that an early and transient increase in the concentration of endogenous free auxin concentration occurs during the inductive phase of rooting (before any cytological event). They suggested that the initiating phase of rooting, i.e., the phase of cell division and cell differentiation, is
charac-terized by a lowering of the free auxin concentration to a minimum, whereas the expressive phase of rooting, i.e., growth and emergence of root primordia, is associated with an increase in free auxin concentration. The present investigation was undertaken to verify the postulated variations in free IAA during the rooting of in-vitro-propagated walnut shoots. Be-cause the role of conjugated auxins in rooting is unclear, we also monitored changes in IAAsp concentrations during root induction of the in-vitro-propagated shoots.
Polyamines are also involved in the rooting process. Corre-lations between polyamine accumulation and the initial stages of adventitious root formation have been observed in some species including Phaseolus (Jarvis et al. 1985), apple (Wang and Faust 1986), tobacco callus culture (Tiburcio et al. 1987), and Prunus avium L. (Biondi et al. 1990). There are also some contradictory results in the literature. For example, Tiburcio et al. (1987) found that α-difluoromethylarginine (DFMA), an inhibitor of putrescine biosynthesis, enhanced the rooting process in tobacco callus culture. In other species there is no effect of polyamines on rooting (e.g., Friedman et al. 1982). To obtain a more detailed understanding of the role of polyamines in root induction, we determined the time course of changes in the concentrations of putrescine, spermidine and spermine during the rooting of in-vitro-propagated walnut shoots.
Materials and methods
Plant material and culture
In-vitro-propagated shoots of Juglans regia L. (clone RG1) were provided by Dr. J.C. Navatel from the Centre Technique Interprofessionel des Fruits et Légumes (CTIFL, Bellegarde, France). Axillary shoot proliferation, with a mean multiplica-tion rate of three, was maintained by subculturing every 3 weeks on DKW medium (Driver and Kuniyuki 1984) con-taining 7.5 g l−1 Roland agar and 1 mg l−1 benzyladenine (BAP) in 600-ml cylindrical glass jars (12 shoots per 100 ml medium). Each glass jar was covered by a glass lid that was kept in place with a sheet of transparent plastic film. The cultures were maintained in a 16-h photoperiod (Sylvania
Changes in the concentrations of auxins and polyamines during
rooting of in-vitro-propagated walnut shoots
MARIE-CLAIRE HELOIR,
1CLAIRE KEVERS,
1JEAN-FRANÇOIS HAUSMAN
1,2and
THOMAS GASPAR
11
Hormonologie fondamentale et appliquée, Institut de Botanique B 22, Université de Liège, Sart Tilman, B- 4000 Liège, Belgium 2
C.R.P., Centre Universitaire de Luxembourg, 162A, avenue de la Faïencerie, L-1511 Luxembourg
Received December 22, 1994
Grolux fluorescent lamps, 312 mol m−2 s−1) at a day/night temperature of 28/25 °C. Almost 100% rooting of isolated shoots was achieved by culturing the in-vitro-propagated shoots on an ‘‘inductive’’ medium composed of MS (Mu-rashige and Skoog 1962) nutrients in Kobe agar with 3 mg l−1 IBA for 7 days in darkness, followed by culture in the light on an ‘‘expressive’’ medium comprising DKW nutrients in a gel-rite/vermiculite mixture without auxin (Jay-Allemand et al. 1992, Ripetti et al. 1994).
Extraction and determination of auxins
Both whole shoots and the basal third of the in-vitro-propa-gated shoots (region where the roots appear) were used for the extraction and analysis of auxins. Non-rooting shoots cultured on MS medium in the absence of IBA were used as controls. The extraction and analytical methods have been described elsewhere (Nordström and Eliasson 1991, Nordström et al. 1991). Briefly, 500 mg fresh weight of either whole shoots or the basal third of shoots was homogenized in liquid nitrogen. The powder was extracted with 10 ml of 5 mM phosphate buffer (pH 6.5) containing [1-14C] IAA as internal standard and butylated hydroxytoluene as antioxidant. After 1 h in darkness, the extract was filtered through a glass-fiber filter which was then rinsed with 5 ml of extraction buffer. The filtrates were passed through Bond-Elut C18 columns activated and condi-tioned to pH 6.5. The eluates were acidified to pH 2.5 with 2.5 M phosphoric acid and then applied to a C18 column, activated and conditioned to pH 2.5. The column was washed with 2 ml of distilled water followed by 2 ml of acidic ethanol (ethanol/glacial acetic acid/water, 20/2/78, v/v). These wash-ings, because they contained a small quantity of auxins, were recovered and applied to another C18 column conditioned to pH 2.5. Auxins were eluted from the two C18 columns (pH 2.5) with two 300-µl aliquots of 80% methanol. Fifty µl of the combined methanol extracts (1.2 ml) was injected in a fully automated Merck-Hitachi HPLC system. The HPLC column was a Lichrosphere 100-RP 18, 12.5 cm long × 4 mm internal diameter, 5 µm particle size, solvent and column temperatures were 30 °C, flow rate was 2 ml min−1, and the mobile phase was acetonitrile/glacial acetic acid/water ( 10/2/88, v/v). The eluate was monitored with a fluorescence detector (excitation at 292 nm, emission at 360 nm). The elution patterns were similar to those shown by Nordström and Eliasson (1991) and Nordström et al. (1991). Fractions were collected and analyzed by FIA (flow injection analysis by mass spectrometry and electrospray in negative ions, Platforn, Fisons) to confirm the identity of IAA and IAAsp. The amounts of IAA in walnut shoots as determined by the HPLC-fluorescence technique were of the same order as those determined by an ELISA technique (Label and Cornu 1988).
Extraction and determination of polyamines
Whole shoots were harvested, weighed and stored at −40 °C until extracted. The extraction, separation, identification and measurement of free polyamines by direct dansylation and HPLC have been described elsewhere (Walter and Geuns 1987). Briefly, 250 mg of frozen shoots was homogenized in
2 ml of 4% HClO4 containing 1,7 diaminoheptane-2HCl as internal standard. After 1 h at 4 °C the homogenate was filtered through glass wool. To 0.2 ml of homogenate, 1 ml of carbon-ate buffer (pH 9) and 1 ml of dansyl chloride solution (10 mg ml−1 acetone) were added. After heating for 1 h at 60 °C, the dansylated polyamines were extracted with 3 ml of toluene. The extract was loaded on a 0.5 g silica gel column and washed with 5 ml of toluol and 5 ml of toluol-triethylamine (10/0.3). The dansylated polyamines were then eluted with 2 × 3 ml of ethyl acetate and the volume reduced under N2. Isocratic HPLC-analysis with acetonitrile/H2O (72/28, v/v) on a 10-cm long × 3 µm octadecyl silica column took 8 min. Solvent flow was 2 ml min−1. Dansylated putrescine, spermidine and sper-mine were injected as references.
The results are the means of measurements of three repli-cates from at least three separate experiments.
Results and discussion
Changes in endogenous IAA and IAAsp concentrations The in-vitro-propagated walnut shoots were completely de-pendent on exogenous auxin for rooting. Shoots cultured in the absence of auxin thus provided a good control for the study of biochemical events preceding root formation by shoots cul-tured in the presence of auxin. Over the 7-day culture period, the concentration of IAA in non-rooting control shoots re-mained constant (Figure 1A). In shoots cultured on the IBA-based rooting medium, the IAA concentration increased up to a transient peak at 60 h of culture. A transient increase in the concentration of free IAA has also been observed in vine cuttings undergoing rooting (Moncousin et al. 1988). After the transient increase, the endogenous free IAA concentration of the shoots decreased to a relatively low concentration for the next 4 days, when the shoots were transferred to the expressive medium. A period of low free auxin concentration preceding visible root formation has also been observed in other cuttings (Moncousin et al. 1988, Berthon et al. 1989, Blakesley et al. 1991, Hausman 1993). These variations in the concentration of free IAA correspond to an inverse variation in peroxidase activity (Ripetti et al. 1994, Gaspar et al. 1994).
The changes in the concentration of IAAsp of the whole shoots closely paralleled the changes in IAA concentration. The concentration of IAAsp remained stable in non-rooting control shoots, except for a small increase between 120 and 144 h (Figure 1B). The IAAsp concentration in shoots cultured on the IBA-based rooting medium exhibited a transient peak around 60 h.
96 h of culture on IBA-based rooting medium.
Several workers have implicated auxin conjugates in the control of adventitious root initiation (see review by Blakesley 1994), but there is no firm evidence that IAAsp triggers adven-titious root formation. It is well known that plant tissues show an increased capacity to accumulate bound auxins following auxin application (Nordström et al. 1991), and this phenome-non likely also occurs in walnut shoot cuttings, because walnut shoots are able to convert IBA into IAA (Van der Krieken et al. 1992, Epstein and Ludwig-Müller 1993). According to Ban-durski (1980), the transient increase in free IAA concentration might result from the hydrolysis of auxin conjugates. Data from Figure 2 do not allow us to determine whether the overall increase in auxins resulted from additional IAA biosynthesis or recovery from IBA. We were unable to identify the mecha-nism underlying the relationship between the transient in-crease in free IAA concentration and the initiation of adventitious roots; however, it has been suggested that cells must achieve a critical peak concentration of endogenous IAA before cell division can occur (Elliott et al. 1987).
Changes in endogenous polyamine concentration
The concentrations of free putrescine, spermidine, spermine and the total amount of free polyamines remained almost constant in non-rooting control shoots during the 7-day culture period (Figure 3). The concentrations of putrescine and sper-mine, but not spermidine, were elevated during the culture of shoots on IBA-based rooting medium. Several studies have reported an increase in the concentration of putrescine during rooting (e.g., Friedman et al. 1985, Tiburcio et al. 1989, Alta-mura et al. 1991). Furthermore, exogenous application of polyamines can increase the number of roots (Jarvis et al. 1985) and enhance rooting (Shyr and Kao 1985), even in the absence of auxin (Hausman et al. 1994).
In several studies, treatment with exogenous auxins in-creased concentrations of endogenous polyamines (Jarvis et al. 1985) before root emergence (Biondi et al. 1990), suggesting that auxins stimulate polyamine biosynthesis (Kyriakidis 1983). Our results provide additional circumstantial evidence that, at an early stage of root induction, increases in auxin concentration favor polyamine accumulation.
Figure 1. Changes in the concentrations of IAA (A) and IAAsp (B) in rooting (+ IBA, j) and non-rooting (− IBA, d)
in-vitro-propagated walnut shoots during
7 days of culture on root-inducing me-dium.
Acknowledgments
This research was supported by the EC research contracts ECLAIR AGRE 0067 and AIR 3CT92-0142. M.C.H. expresses her sincere gratitude for the award of a COMETT fellowship allowing her to work at the University of Liège. J.F.H. thanks the Luxembourg Ministry of National Education for a research grant.
References
Altamura, M.M., P. Torrigiani, F. Capitani, S. Scaramagali and N. Bagni. 1991. De novo root formation in tobacco thin layers is affected by inhibition of polyamine biosynthesis. J. Exp. Bot. 42:1575--1582.
Bandurski, R.S. 1980. Homeostatic control of concentrations of in-dole-3-acetic acid. In Plant Growth Substances. Ed. F. Skoog. Springer-Verlag, Berlin, pp 37--49.
Berthon, J.Y., R. Maldiney, B. Sotta, T. Gaspar and N. Boyer. 1989. Endogenous levels of plant hormones during the course of adventi-tious rooting in cuttings of Sequoiadendron giganteum in vitro. Biochem. Physiol. Pflanzen 184:405--412.
Berthon, J.Y., S. Ben Tahar, T. Gaspar and N. Boyer. 1990. Rooting phases of shoots of Sequoiadendron giganteum in vitro and their requirements. Plant Physiol. Biochem. 28:631--638.
Biondi, S., T. Diaz , M. Iglesias, G. Gamberini and N. Bagni. 1990. Polyamines and ethylene in relation to adventitious root formation in Prunus avium shoot cultures. Physiol. Plant. 78:474--483.
Blakesley, D. 1994. Auxin metabolism and adventitious root initiation.
In Biology of Adventitious Root Formation. Eds. T.D. Davis and
B.E. Haissig. Plenum Press, New York, pp 143--154.
Blakesley, D., G.D. Weston and J.F. Hall. 1991. The role of endo-genous auxin in root initiation. Part I: Evidence from studies on auxin application and analysis of endogenous levels. Plant Growth Regul. 10:341--353.
Davis, T.D. and B.E. Haissig. 1994. Biology of adventitious root formation. Plenum Press, New York, 343 p.
De Klerk, G.J. and J. Ter Brugge. 1992. Factors affecting adventitious root formation in microcuttings of Malus. Agronomie 12:747--755. Driver, J.A. and A.H. Kuniyuki. 1984. In vitro propagation of Paradox
walnut rootstock. HortScience 19:507--509.
Elliot, M.C., A.M. O’Sullivan, J.F. Hall, G.M. Robinson, J.A. Lewis, II, A. Armitage, H.M. Bailey, R.D.J. Barker, K.R. Libbenga and A.M. Mennes. 1987. Plant cell division. The roles of IAA and IAA binding proteins. In Molecular Biology of Plant Growth Control. Eds. J.E. Fox and M. Jacobs. Alan R. Liss, Inc., pp 245--255. Epstein, E. and J. Ludwig-Müller.1993. Indole-3-butyric acid in
plants: occurrence, synthesis, metabolism and transport. Physiol. Plant. 88:382--389.
Friedman, R., A. Altmann and U. Bachrach. 1982. Polyamines and root formation in mung bean hypocotyl cuttings. I. Effects of exogenous compounds and changes in endogenous polyamine con-tent. Plant Physiol. 70:844--848.
Friedman, R., A. Altmann and U. Bachrach. 1985. Polyamines and root formation in mung bean hypocotyl cuttings. II. Incorporation of precursors into polyamines. Plant Physiol. 79:80--83.
Gaspar, T. and M. Hofinger. 1988. Auxin metabolism during adventi-tious rooting. In Adventiadventi-tious Root Formation in Cuttings. Eds. T.D. Davis, B.E. Haissig and N. Sankhla. Dioscorides Press, Portland, OR, pp 117--131.
Gaspar, T., C. Kevers , J.F. Hausman and V. Ripetti. 1994. Peroxidase activity and endogenous free auxin during adventitious root forma-tion. In Physiology, Growth and Development of Plants in Culture. Eds. P.J. Lumsden, J.R. Nicholas and W.J. Davies. Kluwer Acad. Publ., Dordrecht, pp 289--298.
Hausman, J.F. 1993. Changes in peroxidase activity, auxin level and ethylene production during root formation by poplar shoots raised
in vitro. Plant Growth Regul. 13:263--268.
Hausman, J.F., C. Kevers and T. Gaspar. 1994. Involvement of putre-scine in the inductive rooting phase of poplar shoots raised in vitro. Physiol. Plant. 92:201--206.
Jarvis, B.C. 1988. Adventitious root formation with respect to auxin distribution. In Physiology and Biochemistry of Auxins in Plants. Eds. M. Kutacek, R.S. Bandurski and J. Krekule. Academia Praha, pp 295--303.
Jarvis, B.C., S. Yasmin, and M.T. Colema. 1985. RNA and protein metabolism during adventitious root formation in stem cuttings of
Phaseolus aureus cultivar Berkin. Physiol Plant. 64:53--59.
Jay-Allemand, C., P. Capelli and D. Cornu. 1992. Root development of in vitro hybrid walnut microcuttings in a vermiculite-containing gelrite medium. Sci. Hortic. 51:335--342.
Kyriakidis, D.A. 1983. Effect of plant growth hormones and polyami-nes on ornithine decarboxylase activity during the germination of barley seeds. Physiol. Plant. 57:499--504.
Label, P. and D. Cornu. 1988. Determination of plant growth sub-stances in liquid endosperm of immature walnut (Juglans nigra) nuts by an ELISA technique. Plant Growth Regul. 7:209--215. Maldiney, R., F. Pelèse, G. Pilate , B. Sotta, L. Sossountzov and E.
Miginiac. 1986. Endogenous levels of abscisic acid, indole-3-acetic acid, zeatin and zeatin-riboside during the course of adventitious root formation in cuttings of Craigella and Craigella lateral supres-sor tomatoes. Physiol. Plant. 68:426--430.
Moncousin, C. 1991. Rooting in vitro cuttings. In Biotechnology in Agriculture and Forestry, 17. High-Tech and Micropropagation. Ed. Y.P.S. Bajaj. Springer-Verlag, Berlin, pp 231--261.
Moncousin, C., J.M. Favre and T. Gaspar. 1988. Changes in peroxi-dase activity and endogenous IAA levels during adventitious root formation in vine cuttings. In Physiology and Biochemistry of Auxins in Plants. Eds. M. Kutacek, R.S. Bandurski and J. Krekule. Academia Praha, pp 331--337.
Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473--497.
Nordström, A.C. and L. Eliasson. 1991. Levels of endogenous indole-3-acetic acid and indole-3-acetylaspartic acid during adventitious root formation in pea cuttings. Physiol. Plant. 82:599--605. Nordström, A.C., F. A. Jacobs and L. Eliasson. 1991. Effect of
exoge-nous indole-3-acetic acid and indole-3-butyric acid on internal levels of the respective auxins and their conjugation with aspartic acid during adventitious root formation in pea cuttings. Plant Physiol. 96:856--861.
Ripetti, V., C. Kevers and T. Gaspar. 1994. Two successive media for the rooting of walnut shoots in vitro. Changes in peroxidase activity and ethylene production. Adv. Hortic. Sci. 8:29--32.
Shyr, Y.Y. and C.H. Kao. 1985. Polyamines and root formation in mung bean hypocotyl cuttings. Bot. Bull. Acad. Sin. (Taipei) 26:179--184.
Tiburcio, A.F., R. Kaur-Sawhney and A.W. Galston. 1987. Effects of polyamine biosynthetic inhibitors on alkaloids and organogenesis in tobacco callus cultures. Plant Cell Tissue Organ Cult. 9:111--120. Tiburcio, A.F., C.A. Gendy and K. Tran Thanh Van. 1989. Morpho-genesis in tobacco subepidermal cells: Putrescine as a marker of root differentiation. Plant Cell Tissue Organ Cult. 19:43--54. Van der Krieken, W.M., H. Breteler and M.H.M. Visser. 1992. Uptake
and metabolism of indolebutyric acid during root formation on
Malus microcuttings. Acta Bot. Neerl. 41:435--442.
Walter, H. and J. Geuns. 1987. High speed HPLC analysis of polyami-nes in plant tissues. Plant Physiol. 83:232--234.