www.elsevier.com / locate / bres
Interactive report
21
Different stimulatory opioid effects on intracellular Ca
in SH-SY5Y
1
cells
*
Liangyi Chen , Shoubin Zou, Xuelin Lou, Hua-Guang Kang
Institute of Biophysics and Biochemistry, Huazhong University of Science and Technology, Wuhan 430074, PR China Accepted 7 September 2000
Abstract
21 21
Present study revealed the stimulatory effects ofd opioid receptor on intracellular Ca concentration ([Ca ] ) in SH-SY5Y cells.i 21
Fura-2 based single cell fluorescence ratio (F345 / F380) was used to monitor the fluctuation of [Ca ] . Application of the selectivei
2,5
delta-opioid receptor agonist alone, [D-Pen ]-enkephalin (DPDPE), hardly had any effects on cells cultivated for 3–10 days. However, after the cells had been pre-stimulated with cholinoceptor agonist, carbachol, variable calcium elevation was found in 59% of the cultures. The response was naltridole-reversible and dose-dependent, and was abolished completely by thapsigargin (TG) treatment but not by
21 21
administration of CdCl2 or 0-Ca bath solutions. DPDPE-mediated [Ca ] elevation was abolished by pertussis toxin (PTX)i
pretreatment but not cholera toxin (CTX), indicating coupling via G proteins of G / G subfamily. In 17.5% of the responding cells,i o
biphase response was found which may be due to both the stimulatory and the inhibitory effects of opioid. On the other hand, in acutely
21
dissociated cells, DPPDE alone induced [Ca ] increase in 50% of the cultures. The probability and the amplitude of the elevation werei 21
decreased considerably by application of nifedipine or 0-Ca bath solution and was little affected by application of TG. DPDPE
21
activated [Ca ] increase via a PTX-insensitive and CTX-sensitive pathway suggesting coupling through G subunit. All these indicatedi s 21
the opioid modulated the intracellular Ca regulation system through different pathways. SH-SY5Y cell line might be a suitable model for the investigation of the complex mechanism which underlies opioid function. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Opioids: Anatomy, physiology, and behaviour
21
Keywords: DPDPE; Intracellular Ca concentration; SH-SY5Y cells; Stimulatory effects of opioid
1. Introduction reduction of cellular excitability produced by opioid
through G or G subunit.i o
Opioid receptor, as a kind of GTP binding protein- However, from the middle of the 1970s, experiments coupled receptor, modulates various cellular functions. The began to show opioid might also take excitatory roles as best-documented predominant effects of opioid are inhib- well. For example, opiate were found to increase cAMP itory: to block calcium entry through voltage-operated production [8,25], to enhance both L- and N-typed VOC
21 21
Ca (VOC) channel [1,12,18,19,26,33], to suppress basal channel [7,20], to elevate [Ca ]i in NG108-15 cells
21
adenosine 39:59-cyclic monophosphosphate(cAMP) forma- (either through mobilization of Ca store or through tion [6,34], and to promote hyperpolarization by activation membrane depolarization) [10,11,30,36], to prolong action
1
of K channel [32], etc. All these inhibitory actions potential duration of DRG neuron [3,27–29], and finally to underlie the decrease in neurotransmitter release and enhance transmitter release from SK-N-SH and NMB cells [15,16,23]. Some of the stimulatory effects are pertussis toxin (PTX) sensitive while others are cholera toxin (CTX) sensitive [3,28,29]. A CTX-sensitive G subunit
1Published on the World Wide Web on 25 September 2000. s
seems to be involved in the latter case. Which mode of *Corresponding author. Tel.: 186-27-8754-3104; fax: 1
86-27-8754-activities do opiates exerts depends on various parameters, 4375.
E-mail address: [email protected] (L. Chen). such as experimental conditions, age, and receptor types or
L. Chen et al. / Brain Research 882 (2000) 256 –265 257
drugs concentration. Therefore, the aim of present study 2.3. Fura-2 loading was to study both the excitatory and inhibitory effects of
21
opioid on [Ca ] in a rather simple and homogenousi The cell-bearing coverslip were rinsed three times with
preparation. buffer before loading and then incubated with fura-2 /AM
SH-SY5Y cell is a kind of human neuroblastoma cell (2–5mM) for 30 min at 378C. The buffer solution contains line, expressing both m and d opioid receptor [3,13,14]. (mM): NaCl 140, KCl 2.0, CaCl2 2.5, MgCl2 1, 4-(2-Opioid was reported to negatively couple to adenylyl hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)
21
cyclase [21], to inhibit N-type Ca channel [26], to 10, glucose 10, surcose 40 and BSA 0.05%, pH 7.3.
21
increase inositol (1,4,5)-trisphosphate (IP ) accumulations3 Ca -free buffer was the same as the above except that [31]. It also mobilizes calcium store in the presence of MgCl2 was substituted for CaCl , and EGTA (ethylene2
ongoing muscarinic receptor activation [2]. glycol-bis[b-aminoethyl ether]-N,N,N9,N9-tetraacetic acid) Microfluorometric technique was used to detect the (100mM) was added to the buffer.
21
[Ca ] in single SH-SY5Y cells. When the cells werei
21
applied with d opioid receptor agonist, DPDPE, two 2.4. Intracellular Ca measurements different kinds of responses were found. For cells
culti-21
vated for 3–10 days, DPDPE hardly triggered any [Ca ]i The setup of fluorescence measurement was as follows:
response alone. However, by pre-stimulating the cells with the fluorescent microscopy is constructed around a
Ax-21
carbachol, it could elevate intracellular Ca level with iovert 100 (Zeiss, Germany); excitation light at 345 nm or
21
much higher probability through activation of Ca store. 380 nm is provided by a monochromator system (T.I.L.L. On the contrary, DPDPE alone was enough to elevate Photonics, Germany) which is controlled by MacQuadra
21
[Ca ] in acutely dissociated cells (within 48 h). Thei 650 computer (Apple Co., USA). The fluorescence signals 21
response was attributed to the extracellular Ca entry are collected by a photomultiplier and converted to voltage
21
instead of Ca store. PTX but not CTX inhibit the former values, then digitized and sent to the computer. X-CHART responses but failed to block the former ones, while the (Heka, Germany) is used to calculate and monitor the
21
reverse was true for the latter ones. These indicated that intracellular [Ca ] based on the equation provided by
i
opioid may exert the same excitatory effects in the same Grynkiewicz et al. [9]. The fluorescence ratio of F345 / cells via different modulation system. F380 was used in the experiments to reflect the fluctuation
21 of [Ca ] .i
2. Method
3. Results
2.1. Chemicals and drugs
3.1. Response in cells passaged for 3 –10 days
Foetal bovine serum, HEPES, EGTA, carbamylcholine
21
2,5
3.1.1. DPDPE elevate [Ca ] only in the presence of
chloride(carbachol), [D-Pen ]-enkephalin(DPDPE), thap- i
carbachol sigargin, pertussis toxin and cholera toxin are purchased
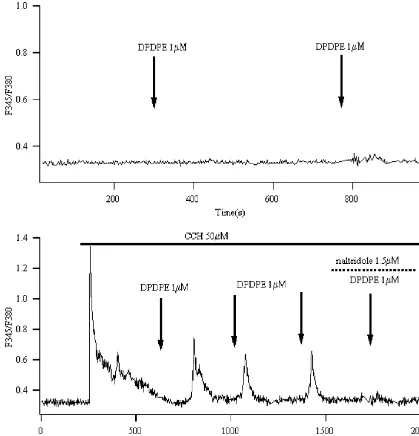
For cells cultivated for 3–10 days, only 9% responded from Sigma, RPMI 1640 from Gibco, fura-2 /AM was from
to the application of selective d opioid agonist DPDPE Molecular Probe. Other ordinary drugs are from local
alone (n5130), while others showed no alternation in chemical drug companies.
fluorescence ratio, as Fig. 1a showed. However, pre-stimu-lated cells with cholinoceptor agonist, carbachol, increased
2.2. Cell culture the probability (59%, n5110) of the cells responding to
DPDPE application (Fig. 1b). Naltrindole, the d receptor The SH-SY5Y cells used here were obtained from antagonist, blocked this effect suggesting that it was Institute of Neuroscience, Beijing Medical University. mediated bydreceptor. To avoid receptor desensitization, They were cultured in RPMI 1640 supplemented with DPDPE were applied for 60 s at intervals of at least 5 min
21 21
penicillin (100 iu ml ), streptomycin (100mg ml ) and [35]. Prolonged application of DPDPE was also tested and foetal bovine serum (15%) in a humidified incubator with no more pronounced increase in the amplitude was found 5% CO2 and 378C in temperature. They were passaged (data not shown).
21
every week and were undifferentiated. Cells fresh disso- The response of [Ca ] to DPDPE was highly variable.i
ciated (within 48 h) or 3–10 days after passage were used Therefore, to investigate the maximum effectiveness of in these experiments, and they were planted onto glass DPDPE, different concentration of DPDPE were adminis-coverslips. Cells pretreated with PTX or CTX were trated to the same cell to decide DPDPE induced con-incubated overnight (12–14 h) in culture medium con- centration-dependent fluorescence ratio increase, where the
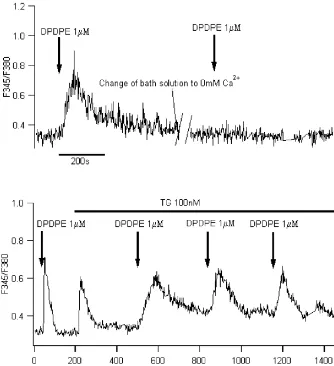
21
Fig. 1. DPDPE elevated in the presence of carbachol. (A) No obvious fluorescence ratio increased in most of the cells tested (91% of 130) whendopioid receptor agonist, DPDPE (1mM) was applied alone. (B) After the cells been stimulated with cholinoceptor receptor agonist, carbachol (CCH, 50mM), DPDPE (1mM) elevated ratio in 59% of cells tested (n5110). The elevation was abolished bydreceptor antagonist, naltridole (1.5mM). Arrows in the
21
figures indicated puffing drugs for 60 s since prolonged time of drug application would not lead to more pronounced alternation of [Ca ] .i
21
nM in the presence of 50mM carbachol (n56) which was the DPDPE-triggered [Ca ] elevation may not be attribu-i 21
in good accordance of the results reported before [2]. ted to extracellular Ca entry.
21 21 Thapsigargin (TG) is an irreversible Ca / Mg
-AT-21
3.1.2. The effects of DPDPE attributed mainly to Ca Pase inhibitor that is located in the sarcoplasmic reticulum. store and were PTX-sensitive Pretreated the cells with it prevents the refilling of the
21 21
Application of Ca channel antagonist, CdCl2 in the Ca store. Exposed the cell with 100 nM TG induced a bath solution, decreased the probability of responding cells gradual increase in fluorescence ratio. Further
administra-21
from 59% to 40% but not to none. Furthermore, in 0 mM tion of opioid elicit no change of [Ca ] (ni 520) even in
21
Ca bath solution, DPDPE still evoked rapid increase of the presence of carbachol (Fig. 3b).
21
L. Chen et al. / Brain Research 882 (2000) 256 –265 259
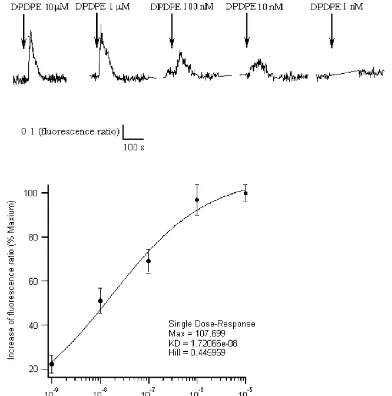
Fig. 2. Concentration dependence of DPDPE-induced ratio increases in single SH-SY5Y cell. (A) Different concentrations of DPDPE were used to stimulate the same cell. Each stimulus was applied for 60 s at intervals of at least 5 minutes to avoid receptor desensitization. CCH (50mM) was always
Hill
presented in the bath solution. (B) The concentration-dependent curve was fitted based on the hill equation y5ymax/(11(K /x)d ) according to the data (n56). The EC50 for DPDPE was about 17 nM (P,0.05).
50), while CTX hardly had any effects on it (Fig. 4). That 3.2. Response in cells acutely dissociated indicated that the effect was mediated via Gi or Go
21
subunit. 3.2.1. DPDPE directly evoked [Ca ] increasei
On the other hand, 1mM DPDPE alone applied for 20s 3.1.3. Stimulatory and inhibitory effects of opioid in the was enough to induce drastic fluorescence ratio increase in same cell 50% of the acutely dissociated cells (n5120), as a typical Of all the cells which responded to the DPDPE stimulus, figure shown in Fig. 6a. No biphase response was found in 17.5% (n570) showed biphase response. Fig. 5 showed a all the cells tested. Prolonged application of DPDPE (from
21 21
typical response. The [Ca ]i first decreased then in- 20 to 100 s) led to augmentation in [Ca ] , as Fig. 6bi
creased after DPDPE application The decay phase was shows.
21
attributed to extracellular Ca entry since replacing the
21
normal buffer with Ca -free buffer (shown in Fig. 5), or 3.2.2. The effects of DPDPE attributed mainly to
21
application of CdCl2 in the buffer (data not show) extracellular Ca entry and were PTX-insensitive
21 21
Fig. 3. DPDPE mobilized calcium store in the presence of CCH. (A) In 0 mM Ca bath solution, DPDPE (1mM) still could elevated [Ca ] in thei
prsence of CCH in 55% of the cells (n550) tested. They were also inhibited by application of 1.5mM naltridole. (B) Calcium store inhibitor, thapsgargin
21
(GG, 100 nM) elevated [Ca ] and further blocked the simulatory effects of DPDPE completely (ni 510).
21
or removal of Ca from bath solution considerably (8% for n530), as data summarized in Fig. 8. This hinted decreased even blocked the response evoked by DPDPE. that the effect might be act via a G subunit other than Gs i
The frequency of DPDPE evoked response was reduced to or G .o
25% (n530) in nifedipine-containing bath solution and
21
even to 10% in Ca -free buffers (n540) (Fig. 8). Also as
Fig. 7a illustrated, the DPDPE triggered response was 4. Discussion
nearly completely abolished by switching the bath solution
21
from normal buffer to Ca -free buffer. However, the Sarne and Gafni suggested that three factors might amplitude of it was hardly affected by administration of determine the stimulatory effects of opioid. They are: (1)
21
L. Chen et al. / Brain Research 882 (2000) 256 –265 261
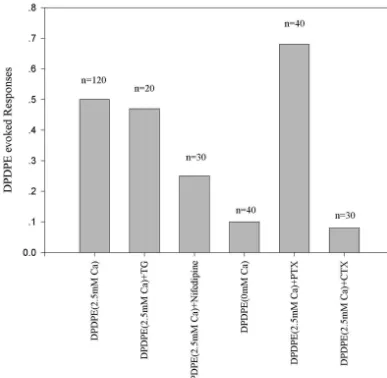
Fig. 4. The frequency of DPDPE evoked responses in different preparation. Summary of results obtained in SH-SY5Y cells. DPDPE alone hardly triggered
21 21
any responses. However, pre-stimulation with CCH increased the frequency greatly. To inhibit the extracellular Ca entry with CdCl or remove the Ca2 21
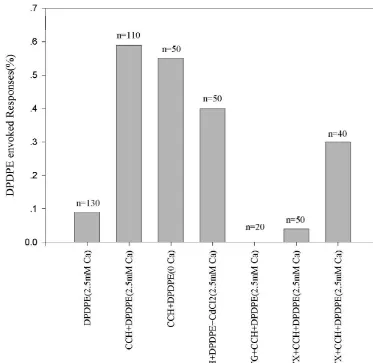
from bath solution hardly decreased the percentage of responding cells. Application of TG to deplete Ca store blocked the effects of DPDPE completely. Pretreated the cells with PTX inhibit the DPDPE evoked response to a great extent, while the reverse was true for CTX pretreatment. This suggested that the effect was mediated through PTX-sensitive G protein.
acutely dissociated cells that had nearly the same position in their division cycle. Both methods enhanced the excitat-ory effects of DPDPE, although the modulation mecha-nisms were different.
The use of a single-cell microfluorometric technique
21
enabled us to detect the intracelluar Ca changes within a single cell. Similar to the work conducted by Conner and Henderson [2], we found that DPDPE application alone
21
hardly triggered any [Ca ] augmentation in cells culti-i
vated for 3–10 days. However, when synergism with stimulatory agent as carbachol, the probability of DPDPE
21 triggered response increased. The elevation of [Ca ]i
21 caused by opioid was attributed mainly to the Ca store,
21
as 0 Ca bath solution would not affect it and administra-tion of TG blocked it completely. The mechanism might Fig. 5. Dual phase of DPDPE-induced ratio increase in single SH-SY5Y be that DPDPE antagonize long time carbachol treatment cell. Biphase curves were found in 17.5% of the DPDPE evoked
induced desensitization of muscarinic receptor, then lead to response. The initial decay phase after DPDPE administration were found 21
21 mobilization of Ca store, as discussed in [2]. PTX but
due to extracellular factors, since removal the Ca from bath solution
21
Fig. 6. DPDPE alone evoked ratio rise in acutely dissociated SH-SY5Y cells. (A) DPDPE (1mM) applied alone could elevate [Ca ] in 50% of acutelyi
dissociated cells tested (n5120). Arrows indicated puffing DPDPE for 20 s. (B) Prolonged administration of DPDPE (1 mM) led to enhanced and prolonged elevation of fluorescence ratio (n55).
suggesting that it be mediated via a PTX-sensitive G ori stimulatory effect was different from former one.
Extracel-21
lular Ca entry, but not the intracellular store played a G protein.o
dominant role this time. TG treatment had little effect on The 17.5% of the culture that exhibited biphase curve
21
the Ca elevation caused by DPDPE. Prolonged applica-after DPDPE stimulus was not reported before. The first
21
21
tion of DPDPE lead to enhanced elevation of [Ca ] .
decay phase of [Ca ] curve was owed to extracellulari i
21 21
Ca influx, while the second climbing phase owed to Previously had been reported that opiate elevated [Ca ]i 21
activation of Ca store. Carbachol had already found to through DHP-sensitive VOC channel in differentiate
45 21
elicited a muscarinic acetylcholine receptors (mAChR)- NG108-15 cells[2;10;20], and basal Ca uptake is at
21
mediated extracellular Ca entry in SK-N-SH human least partly mediated by VOC channel in NMB cells [24]. neuroblastoma [22], and induced a inward store-operated The existence of L-type calcium channel could not be
21
current in gland cells [17]. Thus the possible explanation excluded by the fact that no Ca current was detected in may be that carbachol played an important role in the patch clamp experiment (data not shown). Addition of
21
regulation of [Ca ]i after application, while DPDPE nifedipine into the bath solution both reduced the prob-exerted inhibitory effects on it. It also directly illustrated ability of the responding cells the amplitude of the how opioid coupled to different modulation system con- DPDPE-triggered elevation. Therefore, the increase of
21
temporarily to exerted totally different action on the [Ca ] was at least partially mediated by L type channel.i
intracellular calcium level in the same cell. However, the incomplete blockade by nifedipine compared
21
However, in 50% of acutely dissociated cells tested, to 0-Ca buffer suggested that opiate must evoke some
21
L. Chen et al. / Brain Research 882 (2000) 256 –265 263
21 21
Fig. 7. The stimulatory effect of DPDPE was attributed to extracellular Ca entry. (A) After removal the Ca from bath solution, DPDPE (1mM) hardly
21
triggered fluorescence increase anymore (10% from 40 cells tested). A typical figure was shown here. (B) To deplete the Ca store with TG would not inhibit the stimulatory ability of DPDPE. All arrows indicated puffing DPDPE for 20 s.
VOC channel current. Receptor mediated channel may stimulatory effects may be canceled under normal situa-account for this although further work need to be done. tion. Elevation of GM1 level will lead to the conversion of The excitatory potency of DPDPE was enhanced rather opioid receptor from G –G coupled mode to G coupledi o s
than inhibited by PTX pretreatment, while the reverse was mode, and increase the efficacy of the G -couple receptors
true for CTX application. All these indicated a G me-s to the AC-cAMP transducer system, thus attenuated the diated mechanism existed in acutely dissociated SH-SY5Y inhibitory potency of opioid and uncover the excitatory cells, which is totally different from the previous recorded effects behind them. That might be the explanation why
in normal cultures. PTX enhanced rather than block the excitatory effects of
The different DPDPE-triggered excitatory responses of DPDPE. It might also be the GM1 level that decided the opioid might relate to the endogenous GM1 ganglioside dual activities of opioid in the same cell line although levels on the cell membrane. Gangliosides are abundantly confirmation of the assumption will need other mean of distributed on the surface of most neurons. Recently, a detection.
specific cAMP-PKA-dependent glycolipid, GM1 gan- In conclusion, opioid is a kind of substance that exerts glioside was found to play a important role in the complex regulation activities. Both the stimulatory and the excitatory opioid receptor functions [3–5]. Based on the inhibitory effects of opioid were found and much emphasis result they obtained, Crain and Shen brought forward the had been put on the mechanism of them and how the idea that intercoversion of opioid receptor between inhib- interconversion between the two modes taken place. itory and excitatory modes depends on GM1 level. Opioid However, from our experiment, the function of opioid may was supposed to couple to both G and G simultaneouslyi s be even more complicated than we had thought. DPDPE
21
Fig. 8. The frequency of DPDPE evoked responses in acutely dissociated SH-SY5Y cells. DPDPE alone triggered responses in a large population of the
21
acutely dissociated SH-SY5Y cells. The excitatory effect was abolished by nifedipine or 0-Ca bath solution, but not by application of TG. PTX pretreatment would not block this effect. Instead, they increased the population that responded to DPDPE stimulus. Reversely, CTX pretreatment abolished the effect of DPDPE suggesting coupling of G protein.s
tion of intracellular calcium in SH-SY5Y human neuroblastoma same time to exert opposite effects, and it increased the
21 cells, Br. J. Pharmacol. 117 (1996) 333–340.
basal Ca level also via different mechanisms and
[3] S.M. Crain, K.F. Shen, After chronic opioid exposure sensory different signal transduction paths (different G protein neurons become supersensitive to the excitatory effects of opioid subunit). Therefore, how the cells reacted to the opioid agonists and antagonists as occurs after acute elevation of GM1
ganglioside, Brain Res. 575 (1992) 13–24. activation would depend on the balance of opioid
modula-[4] S.M. Crain, K.F. Shen, Modulatory effects of Gs-coupled excitatory tion networks. The SH-SY5Y cell line may be an ideal
opioid receptor functions on opioid analgesia, tolerance, and depen-model to study how opioid switch between the excitatory dence, Neurochem. Res. 21 (1996) 1347–1351.
and inhibitory mode and further contribute to research [5] S.M. Crain, K.F. Shen, GM1 ganglioside-induced modulation of conducted on the complex molecular mechanism that opioid receptor-mediated functions, Ann. N.Y. Acad. Sci. 845 (1998)
106–125. modulating opioid analgesia, tolerance and dependence.
[6] R.A. Cruciani, B. Dvorkin, S.A. Morris, S.M. Crain, M.H. Makman, Direct coupling of opioid receptors to both stimulatory and inhib-itory guanine nucleotide-binding proteins in F-11 neuroblastoma-sensory neuron hybrid cells, Proc. Natl. Acad. Sci. USA 90 (1993)
References 3019–3023.
[7] P.S. Eriksson, M. Nilsson, M. Wagberg, E. Hansson, L. Ronnback,
21
[1] E. Carpenter, J.P. Gent, C. Peers, Opioid receptor independent Kappa-opioid receptors on astrocytes stimulate L-type Ca
chan-21 1
inhibition of Ca and K currents in NG108-15 cells by the kappa nels, Neuroscience 54 (1993) 401–407.
opioid receptor agonist U50488H, NeuroReport 7 (1996) 1809– [8] A. Fields, Y. Sarne, The stimulatory effect of opioids on cyclic AMP
1812. production in SK-N-SH cells is mediated by calcium ions, Life Sci.
L. Chen et al. / Brain Research 882 (2000) 256 –265 265
21
[9] G. Grynkiewicz, M. Poenie, R.Y. Tsien, A new generation of Ca calmodulin-dependent protein kinase inhibitor 1-[N,O-bis(5-iso-indicators with greatly improved fluorescence properties, J. Biol. quinolinesulfonyl)-N-methyl-L-trosyl]-4-phenylpiperazine (KN-62), Chem. 260 (1985) 3440–3450. Biochem. Pharmacol. 53 (1997) 1107–1114.
[10] W. Jin, N.M. Lee, H.H. Loh, S.A. Thayer, Dual excitatory and [23] Y. Sarne, A. Fields, O. Keren, M. Gafni, Stimulatory effects of inhibitory effects of opioids on intracellular calcium in opioids on transmitter release and possible cellular mechanisms: neuroblastoma3glioma hybrid NG108-15 cells, Mol. Pharmacol. 42 overview and original results, Neurochem. Res. 21 (1996) 1353–
(1992) 1083–1089. 1361.
[11] W. Jin, N.M. Lee, H.H. Loh, S.A. Thayer, Opioids mobilize calcium [24] Y. Sarne, M. Gafni, Determinants of the stimulatory opioid effect on from inositol 1,4,5-trisphosphate-sensitive stores in NG108-15 cells, intracellular calcium in SK-N-SH and NG108-15 neuroblastoma, J. Neurosci. 14 (1994) 1920–1929. Brain Res. 722 (1996) 203–206.
[12] T. Kanemasa, K. Asakura, M. Ninomiya, kappa-opioid agonist [25] Y. Sarne, V. Rubovitch, A. Fields, M. Gafni, Dissociation between
21
U50488 inhibits P-type Ca channels by two mechanisms, Brain the inhibitory and stimulatory effects of opioid peptides on cAMP Res. 702 (1995) 207–212. formation in SK-N-SH neuroblastoma cells, Biochem. Biophys. Res. [13] S.M. Kazmi, R.K. Mishra, Opioid receptors in human neuroblas- Commun. 246 (1998) 128–131.
toma SH-SY5Y cells: evidence for distinct morphine (mu) and [26] E. Seward, C. Hammond, G. Henderson, Mu-opioid-receptor-enkephalin (delta) binding sites, Biochem. Biophys. Res. Commun. mediated inhibition of the N-type calcium-channel current, Proc. R. 137 (1986) 813–820. Soc. Lond. B Biol. Sci. 244 (1991) 129–135.
[14] S.M. Kazmi, R.K. Mishra, Comparative pharmacological properties [27] K.F. Shen, S.M. Crain, Dual opioid modulation of the action and functional coupling of mu and delta opioid receptor sites in potential duration of mouse dorsal root ganglion neurons in culture, human neuroblastoma SH-SY5Y cells, Mol. Pharmacol. 32 (1987) Brain Res. 491 (1989) 227–242.
109–118. [28] K.F. Shen, S.M. Crain, Cholera toxin-A subunit blocks opioid [15] O. Keren, M. Gafni, Y. Sarne, Opioids potentiate transmitter release excitatory effects on sensory neuron action potentials indicating from SK-N-SH human neuroblastoma cells by modulating N-type mediation by Gs-linked opioid receptors, Brain Res. 525 (1990) calcium channels, Brain Res. 764 (1997) 277–282. 225–231.
[16] O. Keren, M. Gafni, Y. Sarne, Potentiation of transmitter release [29] K.F. Shen, S.M. Crain, Cholera toxin-B subunit blocks excitatory from NMB human neuroblastoma cells by kappa-opioids is me- effects of opioids on sensory neuron action potentials indicating that diated by N-type voltage-dependent calcium channels, Brain Res. GM1 ganglioside may regulate Gs-linked opioid receptor functions,
843 (1999) 193–198. Brain Res. 531 (1990) 1–7.
21
[17] X. Liu, A. O’Connell, I.S. Ambudkar, Ca -dependent inactivation [30] D. Smart, D.G. Lambert, delta-Opioids stimulate inositol
1,4,5-21 21
of a store-operated Ca current in human submandibular gland trisphosphate formation, and so mobilize Ca from intracellular cells. Role of a staurosporine-sensitive protein kinase and the stores, in undifferentiated NG108-15 cells, J. Neurochem. 66 (1996)
21
intracellular Ca pump, J. Biol. Chem. 273 (1998) 33295–33304. 1462–1467.
[18] H.C. Moises, K.I. Rusin, R.L. Macdonald, Mu- and kappa-opioid [31] D. Smart, G. Smith, D.G. Lambert, Mu-opioids activate phospholip-receptors selectively reduce the same transient components of high- ase C in SH-SY5Y human neuroblastoma cells via calcium-channel threshold calcium current in rat dorsal root ganglion sensory opening, Biochem. J. 305 (Pt 2) (1995) 577–581 [published erratum neurons, J. Neurosci. 14 (1994) 5903–5916. appears in Biochem J 1995 May 1;307(Pt 3):879].
[19] H. Morikawa, K. Fukuda, S. Kato, K. Mori, H. Higashida, Coupling [32] M. Toselli, P. Tosetti, V. Taglietti, Functional changes in sodium of the cloned mu-opioid receptor with the omega-conotoxin-sensi- conductances in the human neuroblastoma cell line SH-SY5Y during
21
tive Ca current in NG108-15 cells, J. Neurochem. 65 (1995) in vitro differentiation, J. Neurophysiol. 76 (1996) 3920–3927. 1403–1406. [33] M. Toselli, P. Tosetti, V. Taglietti, Mu and delta opioid receptor [20] H. Morikawa, H. Mima, H. Uga, T. Shoda, K. Fukuda, Opioid activation inhibits omega-conotoxin-sensitive calcium channels in a
21
potentiation of N-type Ca channel currents via pertussis-toxin- vol, Pflugers Arch. 433 (1997) 587–596.
sensitive G proteins in NG108-15 cells, Pflugers Arch. 438 (1999) [34] D. Wilkening, S.L. Sabol, M. Nirenberg, Control of opiate receptor-423–426. adenylate cyclase interactions by calcium ions and guanosine-59-trip [21] M. Olasmaa, L. Terenius, Prolonged exposure of human neuro- hosphate, Brain Res. 189 (1980) 459–466.
blastoma SH-SY5Y cell line to morphine and oxotremorine modu- [35] S.H. Yoon, W. Jin, R.J. Spencer, H.H. Loh, S.A. Thayer,
Desensiti-21
lates signal transduction in adenylate cyclase system, Brain Res. 475 zation of delta-opioid-induced mobilization of Ca stores in
(1988) 291–296. NG108-15 cells, Brain Res. 802 (1998) 9–18.
[22] H.L. Puhl, P.S. Raman, C.L. Williams, R.S. Aronstam, Inhibition of [36] S.H. Yoon, T.M. Lo, H.H. Loh, S.A. Thayer, Delta-opioid-induced
21 21
M3 muscarinic acetylcholine receptor-mediated Ca influx and liberation of Gbetagamma mobilizes Ca stores in NG108-15 cells,
21 21
![Fig. 3. DPDPE mobilized calcium store in the presence of CCH. (A) In 0 mM Ca21bath solution, DPDPE (1 mM) still could elevated [Ca21] in theprsence of CCH in 55% of the cells (in550) tested](https://thumb-ap.123doks.com/thumbv2/123dok/3139413.1382769/5.612.130.467.62.499/dpdpe-mobilized-calcium-presence-solution-dpdpe-elevated-theprsence.webp)
![Fig. 6. DPDPE alone evoked ratio rise in acutely dissociated SH-SY5Y cells. (A) DPDPE (11prolonged elevation of fluorescence ratio ( mM) applied alone could elevate [Ca2] in 50% of acutelydissociated cells tested (in5120)](https://thumb-ap.123doks.com/thumbv2/123dok/3139413.1382769/7.612.135.462.63.443/acutely-dissociated-prolonged-elevation-uorescence-applied-elevate-acutelydissociated.webp)