www.elsevier.com / locate / bres
Research report
Dietary restriction attenuates the neuronal loss, induction of heme
oxygenase-1 and blood–brain barrier breakdown induced by impaired
oxidative metabolism
1
*
Noel Y. Calingasan , Gary E. Gibson
Weill Medical College of Cornell University, Burke Medical Research Institute, 785 Mamaroneck Avenue, White Plains, NY 10605, USA Accepted 29 August 2000
Abstract
Experimental thiamine deficiency (TD) is a model of impaired oxidative metabolism associated with region-selective neuronal loss in the brain. Oxidative stress is a prominent feature of TD neuropathology, as evidenced by the accumulation of heme oxygenase-1 (HO-1), ferritin, reactive iron and superoxide dismutase in microglia, nitrotyrosine and 4-hydroxynonenal in neurons, as well as induction of endothelial nitric oxide synthase within the vulnerable areas. Dietary restriction (DR) reduces oxidative stress in several organ systems including the brain. DR increases lifespan and reduces neurodegeneration in a variety of models of neuronal injury. The possibility that DR can protect vulnerable neurons against TD-induced oxidative insults has not been tested. The current studies tested whether approximately 3 months of DR (60% of ad libitum intake) altered the response to TD. Six month-old ad libitum-fed or dietary restricted C57BL / 6 mice received a thiamine-deficient diet either ad libitum, or under a DR regimen respectively for eleven days. The TD mice also received daily injections of the thiamine antagonist pyrithiamine. Control ad libitum-fed or DR mice received an unlimited amount, or 60% of ad libitum intake, respectively, of thiamine-supplemented diet. As in past studies, TD produced region-selective neuronal loss (260%), HO-1 induction, and IgG extravasation in the thalamus of ad libitum-fed mice. DR attenuated the TD-induced neuronal loss (230%), HO-1 induction and IgG extravasation in the thalamus. These studies suggest that oxidative damage is critical to the pathogenesis of TD, and that DR modulates the extent of free radical damage in the brain. Thus, TD is an important model for studying the relationship between aging, oxidative stress and nutrition. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Degenerative disease: other
Keywords: Dietary restriction; Heme oxygenase-1; Neurodegeneration; Oxidative stress; Thiamine
1. Introduction including Alzheimer’s disease [17], Parkinson’s disease [28], and Wernicke–Korsakoff syndrome [5]. As in these Experimental thiamine deficiency (TD) models the human diseases, oxidative stress is a prominent feature of molecular and cellular mechanisms by which chronic TD-induced neurodegeneration in animals. TD induces aberrations in oxidative metabolism associated with well- indicators of oxidative stress including heme oxygenase-1, defined biochemical lesions (i.e., reduction of thiamine- (HO-1) [10], superoxide dismutase [33], ferritin, and dependent enzyme activities) lead to selective neurode- reactive iron [9] in microglia. TD also increases the generation in brain. Reductions in thiamine-dependent nitration product of peroxynitrite, nitrotyrosine [9] and the enzymes also occur in many neurodegenerative disorders lipid peroxidation product, 4-hydroxynonenal [10] in neu-rons within the vulnerable areas. Our recent studies suggest that early stages of TD induce intercellular
adhe-*Corresponding author. Tel.: 11-914-597-2291; fax: 11-914-597- sion molecule-1 (ICAM-1) and endothelial nitric oxide 2757.
synthase (eNOS), indicating that oxidative stress to
mi-E-mail address: [email protected] (G.E. Gibson).
1 crovessels in the thalamus is a critical initial event in the
Present address: Otsuka America Pharmaceutical, Inc., 9900 Medical
Center Drive, Rockville, MD 20850, USA. pathogenesis of TD [8]. Furthermore, TD elevates the
concentration of reactive oxygen species in the thalamus 0.1 ml of saline / 10 g body weight; Sigma, St. Louis, MO). [22]. Thus, although the mechanism underlying the region- Dietary restricted controls (DR / C; n55) received a specific neurodegeneration during TD is unknown, evi- thiamine-supplemented diet and intraperitoneal saline in-dence for the role of oxidative stress is mounting. jection (0.1 ml / 10 g body weight). The thiamine-deficient Dietary restriction (DR) is a well-established means of and thiamine-supplemented diets for the DR / TD and DR / prolonging the lifespan of mammals [3,34]. DR may act by C groups, respectively, were fed at 60% of the ad libitum modulating or reducing oxidative stress in several organ intake.
systems [20]. This hypothesis is further strengthened by For the ad libitum group, TD was induced in 5 animals recent investigations suggesting that DR increases the (AL / TD) as in the DR group except that the thiamine-resistance of neurons to a variety of oxidative insults. For deficient diet was not restricted. Ad libitum controls (AL / example, DR protects neurons against MPTP-induced C; n55) received an unlimited amount of thiamine-sup-toxicity [14], excitothiamine-sup-toxicity, and metabolic injury [4]. DR plemented diet, and intraperitoneal saline injection. also protects hippocampal neurons in mice against the
deleterious presenilin-1 mutation that is linked to early 2.3. Tissue preparation and immunocytochemistry onset of Alzheimer’s disease [36].
If increased oxidative damage is a key underlying Mice were euthanized with halothane after 11 days of mechanism for the sensitivity of selective neurons and the TD, and perfused transcardially with 0.9% NaCl solution blood–brain barrier to TD, then it would be predicted that followed by 4% paraformaldehyde in 0.1 M sodium the dietary restricted, thiamine-deficient mice would be phosphate buffer (PB; pH 7.4). Brains were removed and less sensitive than the ad libitum fed, thiamine-deficient sectioned (35mm thick).
group. The current studies tested whether DR affords Free-floating sections to be analyzed for neuronal loss protection against selective neurodegeneration and blood– were immunostained with an antibody against an excellent
brain barrier breakdown during TD. neuronal marker, neuron-specific nuclear protein, NeuN
[29]. This method eliminates the problem of distinguishing between small interneurons and glial cells, which is
2. Materials and methods encountered in routine hematoxylin-and-eosin or cresyl violet staining. As in our previous studies [8,10], sections
2.1. Animals were incubated in 0.05 M potassium phosphate buffered
saline (KPBS) containing 1% NaOH and 3% H O for 302 2 Six month-old dietary restricted or ad libitum fed min. After rinsing in KPBS 3 times for 10 min each, C57BL / 6NNia mice were obtained from Harlan Sprague– sections were treated with 0.4% Triton X-100 and 1% Dawley (Indianapolis, IN) through the National Institute bovine serum albumin (BSA) in KPBS for 30 min. The on Aging. C57BL / 6NNia mice were used because the TD sections were incubated in NeuN antiserum (Chemicon, pathology in this strain has been extensively studied in our Temecula, CA; 1:1000 in KPBS / 1%BSA / 0.4%Triton) for previous reports [7–10], and the DR paradigm has also 18 h. After rinsing in KPBS containing 0.25% BSA and been characterized in this strain [11]. A stepwise increase 0.02% Triton X-100, sections were incubated in in DR regimen was implemented at the Harlan Sprague– biotinylated anti-mouse IgG (1:200 in KPBS / 0.25% BSA / Dawley facility, from 90% of the ad libitum intake at 12 0.02% Triton; Vector Laboratories, Burlingame, CA) for 1 weeks of age, 80% at 13 weeks, and 60% at 14 weeks and h, followed by avidin–biotin–peroxidase complex (1:200 beyond. Upon arrival, the animals were housed individual- in KPBS; Vector) for 1 h. After rinsing in 0.05 M KPBS, ly and were maintained under constant temperature (708F), the reaction was developed in 0.05% DAB and 0.003% humidity (50%) and 12-h light–dark cycle. The mice were H O in KPBS and stopped with 3 washes of KPBS.2 2 fed a pelleted diet (AIN 76; ICN Nutritional Biochemicals, NeuN-stained sections were also used for the assessment Cleveland, OH) with the dietary restricted group receiving of the blood–brain barrier integrity. Since the monoclonal 60% (2.1 g / day) of the total intake of the ad libitum-fed NeuN antibody requires the use of anti-mouse IgG as mice. The animals were allowed to acclimatize to the secondary antibody, extravasation of IgG, a measure of environment for 5 days before induction of TD. All animal blood–brain barrier breakdown [6,32] was immunohistoch-procedures were approved by the Institutional Animal Care emically detectable in the same sections stained for NeuN. and Use Committee of the Weill Medical College of The pattern of IgG immunoreactivity in NeuN-stained
Cornell University. sections was identical to that of semiadjacent sections that
were stained specifically for mouse IgG following the
2.2. Induction of thiamine deficiency standard method for detection of blood–brain barrier
in 0.1 M sodium phosphate buffered saline (PBS) for 30 For each animal, three sections 175 mm apart were min. The sections were incubated sequentially in (a) 1% examined. Only the intensely stained cells that were bovine serum albumin (BSA) and 0.2% Triton X-100 in distinctly above the background (constitutive) level of PBS for 30 min, (b) HO-1 antibody (1:4000; StressGen staining were counted.
Biotechnologies Corp., British Columbia, Canada) in 0.1
M PBS / 0.5% BSA for 18 h, (c) biotinylated anti-rabbit 2.6. Statistical analysis IgG (Vector) diluted 1:200 in PBS / 0.5% BSA for 1 h, and
(d) avidin–biotin–peroxidase complex (Vector) diluted All values for neuronal and HO-1-labeled cell counts are 1:200 in PBS for 1 h. The chromogen used was 0.05% expressed as means6standard error of the mean (S.E.M.). 3,39-diaminobenzidine tetrahydrochloride dihydrate (DAB; Statistical significance of group differences was tested by
Sigma) containing 0.003% H O in PB.2 2 one-way analysis of variance (ANOVA) followed by the
The specificity of HO-1 antibody binding was confirmed Bonferroni post hoc test. by preadsorption experiments. HO-1 antiserum was
incu-bated with 20mg / ml purified HO-1 protein (StressGen) for
3 h at 378C. Adjacent sections from TD brains were 3. Results
immunostained with the preadsorbed antiserum in parallel
with the non-preadsorbed antibody. Methodological con- 3.1. Behavioral characteristics trol experiments consisted of incubating the sections in
PBS without the primary antibody. Before the initiation of TD induction, the body weight
(g) of the DR mice was 19.662 while that of the AL mice
2.4. Quantitative analysis of neuronal loss was 25.964. TD normally reduces food intake, as
evi-denced by body weight loss. TD mice began to lose weight Areal density nuclear profile counts were determined as after 8 (n53 out of 5) or 9 (2 out of 5) days of TD. The indicators of neuronal cell numbers following the criteria difference between the body weight change of the TD ad of Coggeshall and Lekan [12]. As described in our lib group and the dietary restricted group was not statisti-previous studies [8,10], this strategy was used for several cally significant on days 8 (P50.857) and 9 (P50.335), reasons: (a) Estimates from nuclear profile counts deviate but was significant on days 10 (P50.016) and 11 (P5
less from true numbers compared to total profile counts 0.004). The AL / TD mice showed motor deficits by day 10 which include cytoplasmic edges, since the diameter of the (n55 / 5) while the DR / TD mice exhibited these deficits neuronal nuclei in the submedial thalamic nucleus is small; on day 11 (n55 / 5).
(b) TD did not alter the size of individual neuronal nuclei
in the submedial nucleus; and (c) TD did not alter the 3.2. Macroscopic neuropathological lesions overall size of the submedial nucleus.
NeuN-immunostained sections were used for quantify- No apparent macroscopic differences were observed ing neuronal loss by an investigator who had no knowl- between the brains of AL / C and DR / C mice. As in our edge of the feeding regimen or the experimental treatment. past studies [7–10], the brains of AL / TD mice exhibited Neuronal counts were obtained for an area covering the pinpoint hemorrhages in the thalamus, mammillary body, submedial nucleus (previously called gelatinosus nucleus) inferior colliculus, dorsal lateral and medial geniculate where TD-induced neuronal loss is first detected within the nucleus, superior and inferior olives and some periven-thalamus in mice [10], as in rats [35]. Using the mouse tricular regions. The lateral ventricles showed edematous brain atlas of Franklin and Paxinos [16] as a guide, enlargement. Our previous work revealed that the subme-sections through three rostrocaudal levels (175mm apart) dial thalamic nucleus is the initial site of neuronal loss, of the submedial thalamic nucleus were analyzed under the which spreads to other nuclei until the whole thalamus is 103 objective. Total neuronal cell counts were made affected [8,10]. This consistent pattern makes it convenient
2
within a 0.46 mm area for each side of the brain. This to study the temporal relationships between neuronal loss area encompasses the submedial nucleus and part of and the region-selective neuropathological changes. Thus, adjacent subnuclei. Care was taken to position a calibrated the current studies focused on the thalamus, particularly eyepiece grid by using the mammillothalamic tract as a the submedial nucleus.
landmark. Results are presented as means of three total
counts from different rostrocaudal levels. 3.3. Neuronal loss in thiamine-deficient ad libitum-fed (AL /TD) and dietary restricted (DR /TD) mice
2.5. Quantitative analysis of HO-1 induction
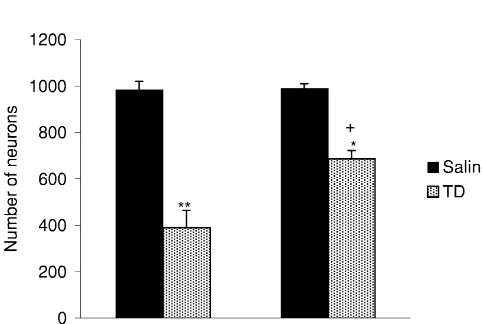
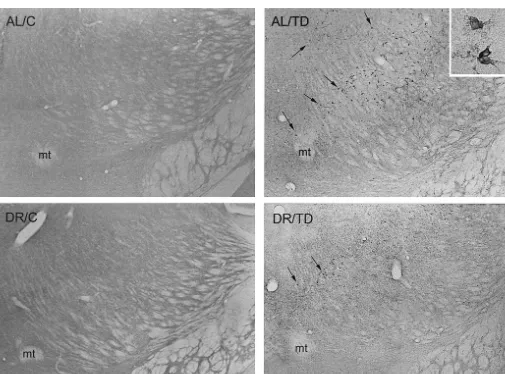
Fig. 1. Attenuation of TD-induced neuronal loss in the submedial thalamic nucleus of DR mice. NeuN immunoreactivities in representative sections of the thalamus of AL / C (top left), AL / TD (top right), DR / C (bottom left) and DR / TD (bottom right) mice show the neuroprotective effect of DR. Diffuse staining in AL / TD and DR / TD sections represents IgG accumulation. mt, mammillothalamic tract. Scale bar5250mm.
The number of NeuN-immunoreactive neurons in the TD also significantly reduced the number of
NeuN-submedial nucleus of AL / C mice was 984636, while that immunoreactive neurons in the submedial nucleus of DR /
of AL / TD was 390674 (Fig. 2). TD compared to dietary restricted controls (DR / C; P,
0.01) (Figs. 1, 2). However, only a 31% reduction occurred in the DR / TD mice (687635) compared to DR / C (990619). (Fig. 2). Thus, DR attenuated the TD-induced neuronal loss in the submedial nucleus (P,0.01, DR / TD vs. AL / TD).
3.4. Heme oxygenase-1 (HO-1) induction in
thiamine-deficient ad libitum-fed (AL /TD) and dietary restricted (DR /TD) mice
HO-1 (EC 1.14.99.3, also known as HSP32) is a marker for both nitrosative and oxidative stress [31]. Our previous studies demonstrate that HO-1 is induced in microglia in the thalamus during TD, and that the pattern of HO-1 induction overlaps with neuronal loss [8,10]. The severity of neuronal loss is directly proportional to HO-1 induction.
Fig. 2. Neuronal loss in the thalamus of AL and DR mice following TD. To determine whether the attenuation of TD-induced The numbers of NeuN-immunoreactive neurons in the submedial thalamic neuronal loss by DR is associated with a reduction of nucleus are shown for the AL / C, AL / TD, DR / C and DR / TD mice.
oxidative damage, HO-1 induction was measured in the
Values represent the mean6S.E.M. of the total cell counts within two
2 thalamus of DR / TD and AL / TD mice.
0.46 mm areas of submedial thalamic nucleus. *P,0.01 (vs. DR / C);
brain in all mice (Fig. 3). No significant differences in the background level of HO-1 immunoreactivity were ob-served between the AL / C and DR / C groups. TD induced HO-1 in microglia within the thalamus of both AL / TD and DR / TD (Figs. 3, 4). However, DR attenuated the microgli-al HO-1 induction in the thmicrogli-alamus during TD. The number of HO-1-labeled microglia in the DR / TD group (2762) was significantly lower than that of the AL / TD mice (7566; P,0.001; Fig. 4). As in past studies [10], the specificity of the HO-1 staining was confirmed by the lack of staining when sections were incubated in preadsorbed antibodies (data not shown).
3.5. IgG extravasation in thiamine-deficient ad
libitum-fed (AL /TD) and dietary restricted (DR /TD) mice
Fig. 4. HO-1 induction in AL / TD and DR / TD mice. Values represent the
Consistent with our previous reports [7–10], IgG
im-mean6S.E.M. of HO-1 labeled microglia in three rostrocaudal levels of
munoreactivity did not accumulate in any brain region of the thalamus. *P,0.001 (vs. AL / TD). either the AL / C or DR / C mice. However, varying degrees
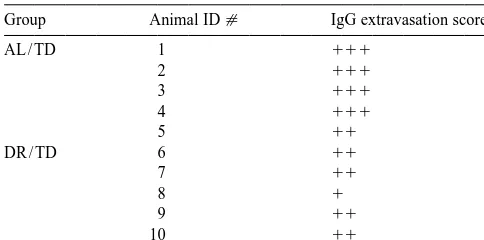
Table 1 DR upregulates several antioxidants including catalase, Dietary restriction and the region-specific increase in IgG immuno- glutathione reductase, glutathione S-transferase, and reactivity in the thalamus after TD
superoxide dismutase [19,21]. During TD in ad libitum-fed
Group Animal ID[ IgG extravasation score*
animals, superoxide dismutase increases in microglia in
AL / TD 1 111 vulnerable regions [33] as an antioxidant response. DR
2 111 may enhance this normal neuroprotective mechanism.
3 111
Thus, a DR-induced elevation of basal levels of superoxide
4 111
dismutase may explain the attenuation of neuronal loss
5 11
during TD.
DR / TD 6 11
7 11 The reduction of blood–brain barrier breakdown by DR
8 1 in the TD thalamus may be due to the ability of DR to
9 11
modulate vascular factors. We previously reported that TD
10 11
induces intercellular adhesion molecule-1 (ICAM-1) and
*
Three sections 175mm apart were scored based on the extent of IgG endothelial nitric oxide synthase (eNOS) in the vulnerable immunoreactivity.
regions, and that gene-targeted disruption of ICAM-1 or
1
Laterodorsal thalamic nucleus and dorsal lateral geniculate nucleus.
11 eNOS attenuates neuronal dropout and HO-1 induction
Laterodorsal thalamic nucleus, dorsal lateral geniculate nucleus and
during TD [8]. These findings suggest that ICAM-1 and
ventrolateral and ventral posteromedial thalamic nucleus.
111
Laterodorsal thalamic nucleus, dorsal lateral geniculate nucleus, eNOS induction are critical events that lead to the TD-ventrolateral, ventral posteromedial and reticular thalamic nucleus. induced neurodegeneration. A recent human study
docu-mented that a 12-week caloric restriction reduces the levels of increases in IgG immunoreactivity occurred in the of circulating endothelial adhesion molecules including thalamus of both AL / TD and DR / TD mice. IgG accumu- ICAM-1 [15]. Thus, although the link between vascular lated more extensively in the thalamus of four out of five factors and neurodegeneration is not well understood, AL / TD mice compared to DR / TD mice (Table 1). modulation of endothelial oxidative stress may explain the Overall, DR reduced the severity of IgG accumulation in neuroprotective effect of DR in the TD model.
the thalamus. Evidence for the beneficial effect of DR in a variety of
models of neurodegeneration is accumulating. DR reduces neurodegeneration in the
1-methyl-4-phenyl-1,2,3,6-tetra-4. Discussion hydropyridine (MPTP) model of Parkinson’s disease [14], excitotoxic and metabolic injury [4], and in mice with A large body of evidence suggests that DR modulates presenilin-1 mutation that is linked to early onset of free radical metabolism ([20] for review). The rationale Alzheimer’s disease [36]. N-methyl-D-aspartate-mediated behind testing the ability of DR to attenuate the TD- excitotoxicity has been implicated in cell death in late induced pathology hinges on the assumption that if oxida- stages of TD [23]. DR induces stress proteins, such as the tive stress plays a key role in selective neurodegeneration heat shock protein (HSP)-70 and glucose-regulated protein during TD, then DR should reduce neuronal loss in this (GRP)-78, that can protect neurons against excitotoxic model. Indeed, the current data demonstrate a neuroprotec- injury [1,2,14,26]. However, DR had no beneficial effect in tive effect of DR in TD. The attenuation of TD-induced transgenic mice expressing amyotrophic lateral sclerosis-loss of NeuN-immunoreactive neurons accompanied a linked Cu / Zn-superoxide dismutase mutations [30]. Thus, dramatic reduction of HO-1 induction and IgG extravasa- DR affords neuroprotection against the death-promoting tion in the thalamus of DR / TD compared to AL / TD mice. action of oxidative stress in many, but not all, models of Our previous work demonstrates that TD-induced increases neurodegeneration. This suggests that the type of reactive in microglial HO-1, an indication of oxidative stress, oxygen species that is involved in different neurodegenera-correlate with the neuronal dropout [10]. In addition to tive disorders, and the type of reactive oxygen species that reactive iron and ferritin elevation in microglia, the is suppressed by DR may differ.
nitration product of peroxynitrite, nitrotyrosine [9], and the The results from studies using animal models provide lipid peroxidation product, 4-hydroxynonenal [10] ac- strong support for the beneficial effects of DR in human cumulate in neurons within the thalamus during late stages neurodegenerative disorders. Case studies reveal that a low of TD. Interestingly, DR suppresses the age-related ac- caloric intake reduces the risk for Alzheimer’s disease [27] cumulation of the pro-oxidant iron [13], reduces kainate- and Parkinson’s disease [25]. DR may be a significant induced 4-hydroxynonenal induction in hippocampal neu- approach for mitigating the neurodegenerative process that rons in mice [36], and attenuates lipid peroxidation in lead to diseases where oxidative stress plays a prominent mitochondrial and microsomal membranes [24]. role, such as Alzheimer’s and Parkinson’s diseases.
[11] K.E. Cheney, R.K. Liu, G.S. Smith, R.E. Leung, M.R. Mickey, R.L.
the brain. Thus, TD is an important tool for testing aspects
Walford, Survival and disease patterns in C57BL / 6J mice subjected
of the relationship between aging, oxidative stress and
to undernutrition, Exp. Gerontol. 15 (1980) 237–258.
nutrition. [12] R.E. Coggeshall, H.A. Lekan, Methods for determining numbers of
cells and synapses: a case for more uniform standards of review, J. Comp. Neurol. 364 (1996) 6–15.
[13] C.I. Cook, B.P. Yu, Iron accumulation in aging: modulation by
Acknowledgements dietary restriction, Mech. Aging Dev. 102 (1998) 1–13.
[14] W. Duan, M.P. Mattson, Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration
The authors appreciate the help of Dr. Bruce Kristal for
of dopaminergic neurons in models of Parkinson’s disease, J.
his suggestions in setting up the dietary restriction
proto-Neurosci. Res. 57 (1999) 195–206.
cols, Preetam Samnarain for technical assistance, and Dr.
[15] C. Ferri, G. Desideri, M. Valenti, C. Bellini, M. Pasin, A. Santucci,
Larry C.H. Park and Hui Zhang for helping with prepara- G. De Mattia, Early upregulation of endothelial adhesion molecules tion of the photomicrographs. Sources of support: Ameri- in obese hypertensive men, Hypertension 34 (1999) 568–573.
[16] K.B.J. Franklin, G. Paxinos, The Mouse Brain in Stereotaxic
can Federation for Aging Research (Dr. Noel Y.
Caling-Coordinates, Academic Press, San Diego, 1996.
asan) and National Institutes of Health grant AG-14600
[17] G.E. Gibson, K.-F.R. Sheu, J.P. Blass, A.C. Baker, K.C. Carlson, B.
and AG-11921 (Dr. Gary E. Gibson)
Harding, P. Perrino, Reduced activities of thiamine-dependent enzymes in brains and peripheral tissues of Alzheimer’s patients, Arch. Neurol. 45 (1988) 836–840.
[18] S. Hsu, L. Raine, H. Fanger, Use of avidin–biotin–peroxidase
References techniques: a comparison between ABC and unlabeled antibody
(PAP) procedures, J. Histochem. Cytochem. 29 (1981) 577–580. [19] A. Koizumi, R. Weindruch, R.L. Walford, Influences of dietary [1] K.B. Aly, J.L. Pipkin, W.G. Hinson, R.J. Feuers, P.H. Duffy, L.
restriction and age on liver enzyme activities and lipid peroxidation Lyn-Cook, R.W. Hart, Chronic caloric restriction induces stress
in mice, J. Nutr. 117 (1987) 361–367. proteins in the hypothalamus of rats, Mech. Aging Dev. 76 (1994)
[20] B.S. Kristal, B.P. Yu, Aging and Its Modulation By Dietary 11–23.
Restriction, in: B.P. Yu (Ed.), Modulation of Aging Processes By [2] V. Amin, D.V. Cumming, R.S. Coffin, D.S. Latchman, The degree of
Dietary Restriction, CRC Press, Boca Raton, 1994, pp. 1–35. protection provided to neuronal cells by a pre-conditioning stress
[21] S. Laganiere, B.P. Yu, Effect of chronic food restriction in aging correlates with the amount of heat shock protein 70 it induces and
rats. II. Liver cytosolic antioxidants and related enzymes, Mech. not with the similarity of the subsequent stress, Neurosci. Lett. 200
Aging Dev. 48 (1989) 221–230. (1995) 85–88.
[22] P.J. Langlais, G. Anderson, S.X. Guo, S.C. Bondy, Increased [3] B.N. Blackwell, T.J. Bucci, R.W. Hartand, A. Turturro, Longevity,
cerebral free radical production during thiamine deficiency, Metab. body weight, and neoplasia in ad libitum-fed and diet-restricted
Brain Dis. 12 (1997) 137–143. C57BL6 mice fed NIH-31 open formula diet, Toxicol. Pathol. 23
[23] P.J. Langlais, R.K. Mair, Protective effects of the glutamate antago-(1995) 570–582.
nist MK-801 on pyrithiamine-induced lesions and amino acid [4] A.J. Bruce-Keller, G. Umberger, R. McFall, M.P. Mattson, Food
changes in rat brain, J. Neurosci. 10 (1990) 1664–1674. restriction reduces brain damage and improves behavioral outcome
[24] D.W. Lee, B.P. Yu, Modulation of free radicals and superoxide following excitotoxic and metabolic insults, Ann. Neurol. 45 (1999)
dismutase by age and dietary restriction, Aging 2 (1990) 357–362. 8–15.
[25] G. Logroscino, K. Marder, L. Cote, M.X. Tang, S. Shea, R. Mayeux, [5] R.F. Butterworth, J.J. Kril, C.G. Harper, Thiamine-dependent
en-Dietary lipids and antioxidants in Parkinson’s disease: a population-zyme changes in the brains of alcoholics: relationship to the
based, case-control study, Ann. Neurol. 39 (1996) 89–94. Wernicke–Korsakoff syndrome, Alcohol Clin. Exp. Res. 71 (1993)
[26] D.H. Lowenstein, P. Chan, M. Miles, The stress protein response in 1084–1088.
cultured neurons:characterization and evidence for a protective role [6] N.Y. Calingasan, H. Baker, K.-F.R. Sheu, G.E. Gibson, Blood–brain
in excitotoxicity, Neuron 7 (1991) 1053–1060. barrier abnormalities in vulnerable brain regions during thiamine
[27] R. Mayeux, R. Costa, K. Bell, C. Merchant, M.X. Tung, D. Jacobs, deficiency, Exp. Neurol. 134 (1995) 64–72.
Reduced risk of Alzheimer’s disease among individuals with low [7] N.Y. Calingasan, S.E. Gandy, H. Baker, K.-F.R. Sheu, J.D. Smith,
calorie intake, Neurology 59 (1999) S296–S297. J.L. Breslow, B.T. Lamb, F.D. Gearhart, J.D. Buxbaum, C. Harper,
[28] Y. Mizuno, S. Matuda, H. Yoshino, H. Mori, N. Hattori, S.I. Ikebe, D.J. Selkoe, D.L. Price, S.S. Sisodia, G.E. Gibson, Novel neuritic
An immunohistochemical study on a-ketoglutarate dehydrogenase clusters with accumulations of APP/APLP2-immunoreactivity in
complex in Parkinson’s disease, Ann. Neurol. 35 (1994) 204–210. brain regions damaged by thiamine deficiency, Am. J. Pathol. 149
(1996) 1063–1071. [29] R.J. Mullen, C.R. Buck, A.M. Smith, NeuN, a neuronal specific nuclear protein in vertebrates, Development 116 (1992) 201–211. [8] N.Y. Calingasan, P.L. Huang, H.S. Chun, A. Fabian, G.E. Gibson,
[30] W.A. Pedersen, M.P. Mattson, No benefit of dietary restriction on Vascular factors are critical in selective neuronal loss in an animal
disease onset or progression in amyotrophic lateral sclerosis Cu / Zn-model of impaired oxidative metabolism, J. Neuropathol. Exp.
superoxide dismutase mutant mice, Brain Res. 833 (1999) 117–120. Neurol. 59 (2000) 207–217.
[31] K. Sandau, J. Pfeilschifter, B. Brune, Nitrosative and oxidative [9] N.Y. Calingasan, L.C.H. Park, L.L. Calo, R.R. Trifiletti, S.E. Gandy,
stress induced heme oxygenase-1 accumulation in rat mesangial G.E. Gibson, Induction of nitric oxide synthase and microglial
cells, Eur. J. Pharmacol. 342 (1998) 77–84. responses precede selective cell death induced by chronic
impair-ment of oxidative metabolism, Am. J. Pathol. 153 (1998) 1381– [32] R. Schmidt-Kastner, D. Meller, B.-M. Bellander, I. Stromberg, L.
1398. Olson, M. Ingvar, A one-step immunohistochemical method for
detection of blood–brain barrier disturbances for immunoglobulins [10] N.Y. Calingasan, L.C.H. Park, K. Uchida, G.E. Gibson, Oxidative
in lesioned rat brain with special reference to false positive labelling stress is associated with region-specific neuronal death during
in immunohistochemistry, J. Neurosci. Methods 46 (1993) 121–132. thiamine deficiency, J. Neuropathol. Exp. Neurol 58 (1999) 946–
role in neuronal cell death due to thiamine deficiency, J. Neurochem. reversibility of thiamine deficiency-induced diencephalic lesions, J.
69 (1997) S136A. Neuropathol. Exp. Neurol. 54 (1995) 255–267.
[34] R. Weindruch, R.L. Walford, The Retardation of Aging and Disease [36] H. Zhu, Q. Guo, M.P. Mattson, Dietary restriction protects hip-By Dietary Restriction, Thomas, Springfield, IL, 1988. pocampal neurons against the death-promoting action of a [35] S.X. Zhang, G.S. Weilersbacher, S.W. Henderson, T. Corso, J.W. presenilin-1 mutation, Brain Res. 842 (1999) 224–229.