This page
All rights reserved.
No part of this ebook may be reproduced in any form, by photostat, microfilm, xerography, or any other means, or incorporated into any information retrieval system, electronic or mechanical, without the written permission of the publisher. All inquiries should be emailed to [email protected]
PUBLISHING FOR ONE WORLD
NEW AGE INTERNATIONAL (P) LIMITED, PUBLISHERS 4835/24, Ansari Road, Daryaganj, New Delhi - 110002
Material Science has become a very important subject as an interdisciplinary course in almost all univer-sities. Keeping this in view we have developed the subject matter for B.E. (Material Science and Metal-lurgy/Production Engineering/Mechanical Engineering), A.M.I.E., Diploma in engineering, M.Sc. (Material Science/Physics/Chemistry) and B.Sc. (Hons.) courses as per their latest syllabii. The book is also useful for UPSC, GATE, NET, SLET and other entrance examinations.
A reasonably wide coverage in sufficient depth has been attempted, giving the importance to the basic principles, essential theory and experimental details necessary for understanding the nature, properties and applications of materials. All efforts have been made to provide topics which are of great use to the readers, e.g. semiconductors, superconductors, polymers, composites, nanostructured materials, etc. Latest develop-ments, e.g. quantum dots, spinotrics, MOSFET, Microelectronic circuits, MEMS, nanotechnology, etc. are also covered.
To make the text more useful, good number of worked out problems, review questions, problems, short-question answers, typical objective short-questions, suggested readings are provided with each chapter.
We are thankful to M/s New Age International (P) Limited Publishers, for their untiring efforts in bringing out the book with excellent printing and nice get up within the shortest possible time period.
Suggestions for the improvement of the book are most welcome.
Bhilwara S.L. Kakani
This page
Preface v
1. Classification and Selection of Materials 1
1. Introduction 1
2. Engineering Requirements 2
3. Classification of Engineering Materials 5
4. Organic, Inorganic and Biological Materials 7
5. Semiconductors 9
6. Biomaterials 10
7. (a) Current Trends and Advances in Materials 10
7. (b) Advanced Materials 10
7. (c) Smart Materials (Materials of the future) 11
7. (d) Nanostructured Materials and Nanotechnology 12
7. (e) Quantum Dots (QDs) 12
7. (f) Spintronics 12
7. (g) Fermionic Condensate Matter 13
8. Level of Material Structure Examination and Observation 13
9. Material Structure 13
10. Engineering Metallurgy 14
11. Selection of Materials 14 Suggested Readings 17 Review Questions 17 Problems 18
Short Question-Answers 18 Objective Questions 19
2. Atomic Structure, and Electronic Configuration 20
1. Introduction 20
2. The Electron 20
3. Protons 21
4. Neutrons 21
5. Atomic Number (Z) 22
6. Atomic Weight and Mass Number 22
7. Isotopes 23
8. Isobars 23
9. Isotones 23
11. Atomic Nucleus 24
12. Atomic Models 24
13. Vector Atom Model 43
14. Quantum Numbers 44
15. Pauli Exclusion Principle and Electronic Configuration of Atoms 47
16. Wave Mechanical Picture of the Atom 54
17. Periodic Table 56 Suggested Readings 59 Review Questions 59 Problems 60
Short Questions 61 Objective Questions 61 Short Question-Answers 63
3. Crystal Geometry, Structure and Defects 64
1. Introduction 64
2. Crystals 65
3. Single Crystal 65
4. Whiskers 65
5. Lattice Points and Space Lattice 66
5. (a) Basis 66
6. Unit Cell 67
7. Primitive Cell 67
8. Crystal Classes 68
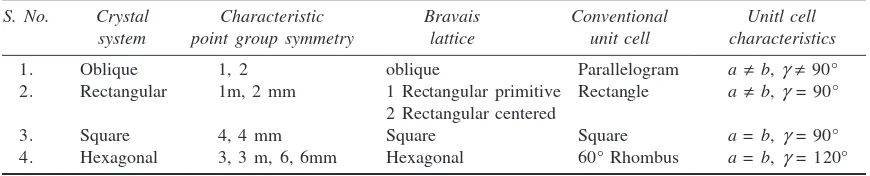
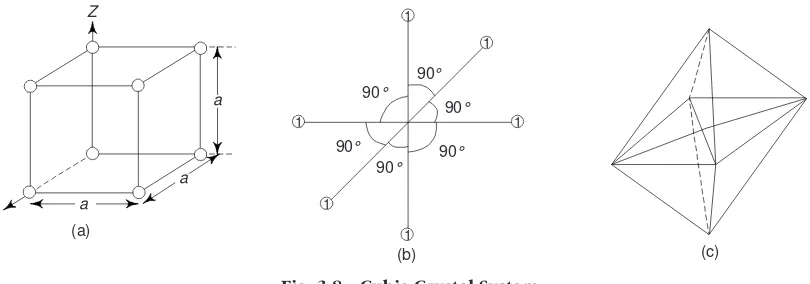
9. Crystal Systems 69
10. Crystal Structure for Metallic Elements 75
11. Atomic Radius 78
12. Density of Crystal 79
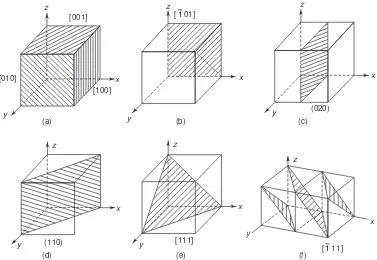
13. Directions, Lattice Planes and Miller Indices 80
14. Interplanar Spacings 83
14. (a) Angle Between Two Planes or Directions 84
15. Representation of Crystal Planes in a Cubic Unit Cell 84
16. Sketching the Plane from the given Miller Indices 86
17. Common Planes in a Simple Cubic Structure 86
18. Co-ordination Number 87
19. Defects or Imperfections in Crystals 95
20. Point Imperfections 96
21. Line Defects or Dislocations 98
22. Surface and Grain Boundary Defects 101
23. Volume Imperfections 104
24. Liquid Crystals 104
25. Anisotropy 105
26. Frank-Read Source 106
27. Theory of Dislocations: Salient Features 107
28. Determination of Crystal Structure by X-Ray Diffraction 110
29. Bragg’s X-ray Spectrometer 112
30. Reciprocal Lattice 115
31. Methods of Determining Crystal Structure 118
33. Crystal Growth 122 Suggested Readings 123 Review Questions 123 Problems 124
Objective Questions 125 Short Questions Answers 127
4. Bonds in Solids 129
1. Introduction 129
2. Types of Bonds 129
3. Forces Between Atoms: Mechanism of Bond Formation and Bond Energy 131
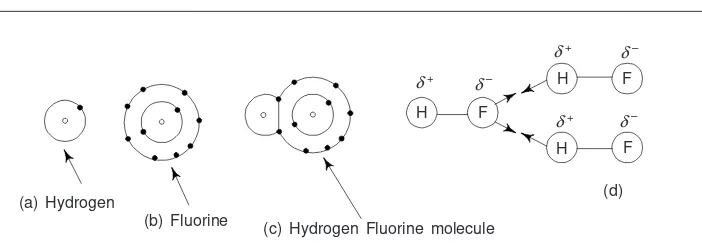
4. Ionic Bonding 134
5. Covalent Bond 140
6. Metallic Bond 142
7. Comparison of Ionic, Covalent and Metallic Bonds 144
8. Secondary Bonds 145
9. Mixed Bonds (More About Secondary Bonds) 147
10. Properties of Solid Materials 148
11. Periodic Table and Chemical Bonding: Electronegativity 150 Suggested Readings 151
Review Questions 151 Problems 152
Objective Questions 153 Short Question Answers 154
5. Electron Theory of Metals 155
1. Introduction 155
2. Metallic Bonding 155
3. Drude-Lorentz Theory 156
4. Sommerfield Free-Electron Theory 156
5. Fermi-Dirac Distribution Function (Electron Energies in a Metal) 159
6. Band Theory of Solids 160
7. Brillouin Zones 162
8. Distinction between Conductors, Insulators and Semiconductors 163
9. Electrical Resistance of Materials 164
10. Energy Bands 165
11. Equation of Motion of an Electron 166
12. Resistivity and Conductivity 167
13. Current Density in Metal 167
14. Mobility 168
15. Mean Free Path 171
16. Thermoelectricity 173
17. Origin of the Thermoelectric Effect 174
18. Magnitude and Direction of Thermo E.M.F. 174
19. Uses of Thermocouples 175 Suggested Readings 175 Review Questions 176 Problems 176
Objective Questions 176 Short Question Answers 177
6. Photoelectric Effect 179 1. Introduction 179
2. Experimental Arrangement to Study the Photo Electric Effect 179
3. Failure of Classical Theory 182
4. Einstein’s Photoelectric Equation 183
5. Millikan’s Verification of Einstein’s Equation 184
6. Photoelectric Cells 186
7. Uses of photoelectric Cells 191 Suggested Readings 194 Review Questions 194 Problems 194
Objective Questions 195 Short Question-Answers 196
7. Diffusion in Solids 197
1. Introduction 197
2. Types of Diffusion 197
3. Diffusion Mechanisms 197
4. Diffusion Coefficient: Fick’s Laws of Diffusion 199
5. Dependence of Diffusion Coefficient on Temperature 202
6. Factors Affecting Diffusion Coefficient (D) 202
7. Self Diffusion 203
8. Inter-Diffusion 203
9. Experimental Determination of D using a Diffusion Couple 203
10. Diffusion with Constant Concentration (Case Hardening) 204
11. The Kirkendall Effect 204
12. Diffusion in Oxides and Ionic Crystals 205
13. Surface Diffusion 205
14. Activation Energy of Diffusion 205
15. Applications of Diffusion 206 Suggested Readings 210 Review Questions 210 Problems 211
Objective Questions 211 Short Question-Answers 212
8. Mechanical Properties of Materials and Mechanical Tests 213 1. Introduction 213
2. Common Terms 214
3. Atomic Model of Elastic Behaviour 226
4. Modulus (Y) as a Parameter of Design 227
5. Fundamental Mechanical Properties 228
Stress-Rupture Test 243
6. Factors affecting Mechanical Properties 244
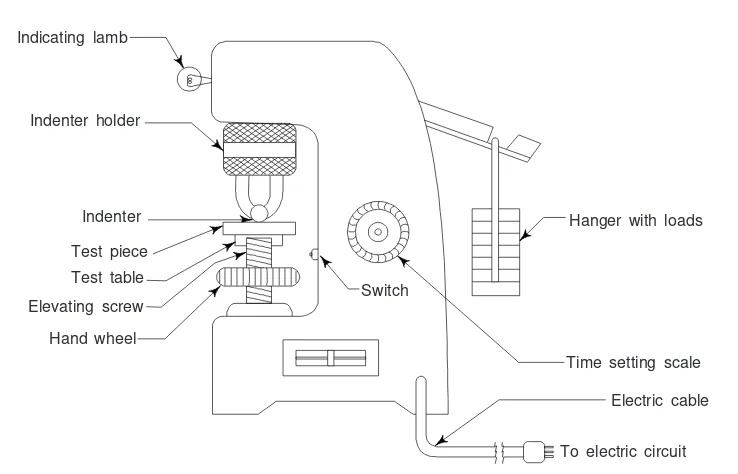
7. Mechanical Tests 246
8. Non-Destructive Testing (NDT) 258
9. Fracture 260
Objective Questions 274 Short Question-Answers 276
9. Alloy Systems, Phase Diagrams and Phase Transformations 279 1. Introduction 279
2. Alloy Systems 280
3. Solid Solutions 281
4. The Families of Engineering Alloys 283
5. Hume-Rothery’s Rules 285
6. Intermediate Phases or Intermediate Compounds (or Intermediate Solid Solutions) 286
7. Phase Diagrams 286
8. The Phase Rule or Gibb’s Phase Rule or Condensed Phase Rule 287
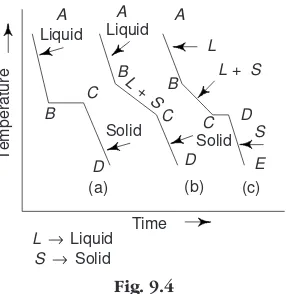
9. Cooling Curves (Time-Temperature Curves) 288
10. Construction of a Phase Diagram or Constitutional Diagram 289
11. The Lever Rule 290
12. Equilibrium Diagrams for Binary Alloys Forming Eutectic 292
13. Ceramic and Ternary phase Diagrams 294
14. Applications of Phase Diagrams 295
15. Coring 295
16. Phase Transformations 296
17. The Kinetics of Solid State Reactions 296
18. Multiphase Transformations 300
19. Applications of Phase Transformations 300
20. Micro-constituents of Fe-C System 302
21. Allotropic forms of Iron 304
21. Iron-carbon System 305
22. Iron-carbon equilibrium or Phase Diagram 305
23. Modified Iron-carbon Phase Diagram 306
24. Formation and Decomposition of Austenite 309
25. Types and Properties of Carbon-Steels 311
26. Isothermal Transformations-TTT Diagram 312
27. Transformation of Austenite upon Continuous Cooling 314
28. Transformation of Austenite to Martensite 315
29. Metals for Nuclear Energy 316 Suggested Readings 318 Review Questions 318 Problems 319
Short Question Answers 319 Objective Questions 320
10. Heat Treatment 321
1. Introduction 321
2. Heat-Treatment Processes 322
3. Annealing 322
4. Annealing Operations 323
5. Mass Effect 336
6. Principal Equipment for Heat Treatment 336
7. Major Defects in Metals or Alloys due to Faulty Heat Treatment 338
8. Surface Finish After Heat Treatment 339
9. Measurement of High Temperatures and Pyrometers 340
Suggested Readings 344 Review Questions 344 Problems 345
Short Question-Answers 346 Objective Questions 346
11. Deformation of Materials 348
1. Introduction 348
2. Elastic Deformation 348
3. Plastic Deformation 349
4. Deformation by Twinning 355
5. Comparison between Slip and Twinning 356
6. Plastic Deformation of Polycrystalline Materials 357
7. Work Hardening or Strain Hardening 357
8. Season Cracking 359
9. Bauschinger Effect 359
10. Anelasticity 359
11. Adiabatic and Isothermal Straining 360
12. Yield Point Phenomenon and Related Effects 361
13. Atomic Diffusion–An Elastic After Effect 363
14. Preferred Orientation 364
15. Recovery, Recrystallization and Grain Growth 365
16. Hot-Working 368 Suggested Readings 371 Review Questions 371 Problems 372
Objective Questions 373 Short Question Answers 373
12. Oxidation and Corrosion 375
1. Introduction 375
2. Corrosion-resistant Materials 375
3. Electrochemical Corrosion 375
4. Galvanic (Two-Metal) Corrosion 380
5. Corrosion Rates 381
6. High Temperature Oxidation or Dry Corrosion 382
7. Passivity 382
8. Environmental Effects 383
9. Specific Forms of Corrosion 383
10. Corrosion Prevention and Control 388
11. Corrosion Monitoring and Management 394 Suggested Readings 396
Review Questions 396 Problems 397
Short Question-Answers 397 Objective Questions 398
13. Thermal and Optical Properties of Materials 400
2. Heat Capacity 400
3. Theoretical Models 403
4. Thermal Expansion 406
5. Thermal Conductivity (K) 409
6. Refractories 414
7. Thermal Stresses 415
8. Thermal Fatigue 416
9. Thermal Shock 416
10. Melting Point (M.P.) 416
Section B: Optical Properties 418 1. Optical Properties 418
2. Interactions of Light with Solids 418
3. Atomic and Electronic Interactions 418
4. Optical Properties of Metals 419
5. Optical Properties of Non metals 420
6. Applications of Optical Phenomena 424 Suggested Readings 426
Review Questions 427 Problems 428
Objective Questions 428 Short Question-Answers 430
14. Electrical and Magnetic Properties of Materials 431
1. Introduction 431
2. Electrical Conduction 431
3. Electrical Conductivity (I) 433 4. Electronic and Ionic Conduction 435
5. Band Structure in Solids 435
6. Conduction in Terms of Band and Atomic Bonding Models 438
7. Electrical Resistivity of Metals 440
8. Electrical Characteristics of Alloys Used for Commercial Purposes 442
9. Mechanisms of Strengthening in Metals 442
10. Insulators 443
11. Dielectrics 445
12. Magnetism 456
Suggested Readings 480 Review Questions 480 Problems 482
Objective Questions 483 Short Question Answers 484
15. Semiconductors 488
1. Introduction 488
2. Intrinsic Semiconductors 489
3. Extrinsic Semiconductors 494
4. Semiconductor Devices 500
5. The Transistor 514
6. Semiconductors in Computers 518
7. Microelectronic Circuits 518
8. Microelectromechanical Systems (MEMS) 519
9. Quantum Dots (QDs) 520
10. Spintronics 520
Suggested Readings 522 Review Questions 523 Problems 523
Objective Questions 524 Short Question Answers 525
16. Superconductivity and Superconducting Materials 526
1. Introduction 526
2. Superconducting Materials 529
3. HTSC Cuprate Materials Characteristics 532
4. Characteristic Properties of Superconductors 535
5. Josephson Effects 540
6. Properties of HTSC Oxides 541
7. Thermodynamics of a Superconductor 542
8. Theory of Superconductivity 544
9. Quantum Tunneling 547
10. Applications of Superconductivity 548 Suggested Readings 551
Review Questions 551 Short Questions 552 Problems 552
Objective Questions 553 Short Question Answers 553
17. Organic Materials: Polymers and Elastomers 555
1. Introduction 555
2. Polymers 555
3. Broad Classifications 558
4. Basic Concepts of Polymer Science 559
5. Molecular Configurations 567
6. Thermoplastic and Thermosetting Polymers 568
7. Copolymers 568
8. Polymer Crystallinity 569
9. Defects in Polymers 570
10. Mechanical Properties of Polymers 570
11. Mechanisms of Deformation 572
12. Crystallization, Melting and Glass Transition Phenomena in Polymers 572
13. Polymer Types 573
14. Miscellaneous Applications of Polymers 578
15. Advanced Polymeric Materials 579
16. Polymer Additives 580
17. Manufacturing Processes Involving Polymers 582
18. Reinforced Polymers 584
19. Behaviour of Polymers 585
20. Fabrication of Fibres and Films 586
21. Wood 587
Problems 590
Objective Questions 590 Short Questions Answers 591
18. Composites 593
1. Introduction 593
2. General Characteristics 594
3. Particle-Reinforced Composites 596
4. Fibre-Reinforces Composites 598
5. Fabrication 609
Suggested Readings 611 Review Questions 611 Problems 611
Objective Questions 612 Short Question Answers 612
19. Nanostructured Materials 614
1. Introduction 614
2. Production Methods for CNTs 621
3. Key Issues in Nanomanufacturing 624
4. Mechanical and Electronic Properties of Carbon Nanotubes 624
5. Nanostructures in Motion 624
6. Nanomaterial Advantage 624 Suggested Readings 626 Review Questions 626
Appendix 1: Units, Conversion Factors, Physical Constants 627 Units 627
Appendix 2: Conversion Factors 630 Appendix 3: Physical Constants 632
Appendix 4: Prefix Names, Symbols and Multiplication Factors 633
Subject Index 634
This page
1
Classification and
Selection of Materials
1. INTRODUCTION
Materials science and engineering plays a vital role in this modern age of science and technology. Various kinds of materials are used in industry, housing, agriculture, transportation, etc. to meet the plant and individual requirements. The rapid developments in the field of quantum theory of solids have opened vast opportunities for better understanding and utilization of various materials. The spectacular success in the field of space is primarily due to the rapid advances in high-temperature and high-strength materials.
The selection of a specific material for a particular use is a very complex process. However, one can simplify the choice if the details about (i) operating parameters, (ii) manufacturing processes, (iii) functional requirements and (iv) cost considerations are known. Factors affecting the selection of materials are sum-marized in Table 1.1.
Table 1.1 Factors affecting selection of materials
(i) (ii) (iii) (iv)
Manufacturing processes Functional requirements Cost considerations Operating parameters
l Plasticity l Strength l Raw material l Pressure
l Malleability l Hardness l Processing l Temperature
l Ductility l Rigidity l Storage l Flow
l Machinability l Toughness l Manpower l Type of material
l Casting properties l Thermal conductivity l Special treatment l Corrosion requirements
l Weldability l Fatigue l Inspection l Environment
l Heat l Electrical treatment l Packaging properties l Protection from fire
l Tooling l Creep l Inventory l Weathering
l Surface finish l Aesthetic look l Taxes and custom duty l Biological effects
There are thousands and thousands of materials available and it is very difficult for an engineer to possess a detailed knowledge of all the materials. However, a good grasp of the fundamental principles which control the properties of various materials help one to make the optimum selection of material. In this respect, materials science and engineering draw heavily from the engineering branches, e.g. metallurgy, ceramics and polymer science.
2. ENGINEERING REQUIREMENTS
While selecting materials for engineering purposes, properties such as impact strength, tensile strength, hardness indicate the suitability for selection but the design engineer will have to make sure that the radiography and other properties of the material are as per the specifications. One can dictate the method of production of the component, service life, cost etc. However, due to the varied demands made metallic materials, one may require special surface treatment, e.g. hardening, normalising to cope with the service requires. Besides, chemical properties of materials, e.g. structure, bonding energy, resistance to environ-mental degradation also effect the selection of materials for engineering purposes.
In recent years polymeric materials or plastics have gained considerable popularity as engineering materials. Though inferior to most metallic materials in strength and temperature resistance, these are being used not only in corrosive environment but also in the places where minimum wear is required, e.g. small gear wheels, originally produced from hardened steels, are now manufactured from nylon or teflon. These materials perform satisfactorily, are quiet and do not require lubrication.
Thus, before selecting a material or designing a component, it is essential for one to understand the requirements of the process thoroughly, operating limitations like hazardous or non-hazardous conditions, continuous or non-continuous operation, availability of raw materials as well as spares, availability of alternate materials vis-a-vis life span of the instrument/equipment, cost etc. Different materials possess different properties to meet the various requirement for engineering purposes. The properties of materials which dictate the selection are as follows:
(a) Mechanical Properties The important mechanical properties affecting the selection of a material are:
(i) Tensile Strength: This enables the material to resist the application of a tensile force. To withstand the tensile force, the internal structure of the material provides the internal resistance.
(ii) Hardness: It is the degree of resistance to indentation or scratching, abrasion and wear. Alloying techniques and heat treatment help to achieve the same.
(iii) Ductility: This is the property of a metal by virtue of which it can be drawn into wires or elongated before rupture takes place. It depends upon the grain size of the metal crystals.
(iv) Impact Strength: It is the energy required per unit cross-sectional area to fracture a specimen, i.e., it is a measure of the response of a material to shock loading.
(v) Wear Resistance: The ability of a material to resist friction wear under particular conditions, i.e. to maintain its physical dimensions when in sliding or rolling contact with a second member.
(vi) Corrosion Resistance: Those metals and alloys which can withstand the corrosive action of a medium, i.e. corrosion processes proceed in them at a relatively low rate are termed corrosion-resistant.
(vii) Density: This is an important factor of a material where weight and thus the mass is critical, i.e. aircraft components.
(b) Thermal Properties The characteristics of a material, which are functions of the temperature, are termed its thermal properties. One can predict the performance of machine components during normal operation, if he has the knowledge of thermal properties. Specific heat, latent heat, thermal conductivity, thermal expansion, thermal stresses, thermal fatigue, etc. are few important thermal properties of materials. These properties play a vital role in selection of material for engineering applications, e.g. when materials are considered for high temperature service. Now, we briefly discuss few of these properties:
(i) Specific Heat (c): It is the heat capacity of a unit mass of a homogeneous substance. For a homogeneous body, c = C/M, where C is the heat capacity and M is the mass of the body. One can also define it as the quantity of heat required to raise the temperature of a unit mass of the substance through 1°C. Its units are cal/g/°C.
Classification and Selection of Materials 3
conducting element is one unit. Truly speaking the capability of the material to transmit heat through it is termed as the thermal conductivity. Higher the value of thermal conductivity, the greater is the rate at which heat will be transferred through a piece of given size. Copper and aluminium are good conductors of heat and therefore extensively used whenever transfer of heat is desired. Bakelite is a poor conductor of heat and hence used as heat insulator.
The heat flow through an area A which is perpendicular to the direction of flow is directly proportional to the area (A) and thermal gradient (dt/dx). Thermal conductivity (K) is given by hot and cold side of the material (°C) and x is the distance between the two faces (m).
The thermal conductivity of a metal can be expressed as
K = initial velocity of the electron. We must note that similar expression is used for electrical conductivity. The ratio of heat and electrical conductivity (k and s respectively) is given by
2
Obviously, the thermal conductivity (K) and electrical conductivity (s) vary in the same fashion from one material to another. The ratio
2 2
3 æ öè ø k e
p is known as Wridemann—Franz ratio. Thermal conductivity
for some of the materials is given in Table 1.1
Table 1.2 Thermal conductivity for some materials
Type of the material Material Thermal conductivity (K) (W/m/k)
(i) Metals Copper 380
(iv) Thermal Resistance (RT): It is the resistance offered by the conductor when heat flow due to tempera-ture difference between two points of a conductor. It is given by
RT = 1- 2
H
q q
second – °C/k Cal
where H® rate of heat flow and q1 and q2 are temperatures at two points (°C).
(v) Thermal Diffusivity (h): It is given by
h= Thermal conductivity ( ) cm /s3
Heat capacity ( p) density ( ) K
C ´ r
= p K
C r represent heat requirement per unit volume
A material having high heat requirement per unit volume possesses a low thermal diffusivity because more heat must be added to or removed from the material for effecting a temperature change.
(vi) Thermal Fatigue: This is the mechanical effect of repeated thermal stresses caused by repeated heating and cooling.
The thermal stresses can be very large, involving considerable plastic flow. We can see that fatigue failures can occur after relatively few cycles. The effect of the high part of the temperature cycle on the strength of material plays an important factor in reducing its life under thermal fatigue.
(c) Electrical Properties Conductivity, resistivity, dielectric strength are few important electrical prop-erties of a material. A material which offers little resistance to the passage of an electric current is said to be a good conductor of electricity.
The electrical resistance of a material depends on its dimensions and is given by
Resistance = Resistivity ´ Length Cross-section area
Usually resistivity of a material is quoted in the literature. Unit of resistivity is Ohm-metre.
On the basis of electrical resistivity materials are divided as: (i) Conductors (ii) Semiconductors and (iii) Insulators. In general metals are good conductors. Insulators have very high resistivity. Ceramic insu-lators are most common examples and are used on automobile spark plugs, Bakelite handles for electric iron, plastic coverings on cables in domestic wiring.
When a large number of metals and alloys are sufficiently cooled below transition temperature, Tc, enter the state of superconductivity in which the dc resistivity goes to zero. The estimates of the resistivity in the super-conducting phase place it at less than 4 ´ 10–25 W-m, which is essentially zero for all practical purposes. The highest value of Tc upto 133 K has been reached for mercury cuprate.
(d) Magnetic Properties Materials in which a state of magnetism can be induced are termed magnetic materials. There are five classes into which magnetic materials may be grouped: (i) diamagnetic (ii) para-magnetic (iii) ferropara-magnetic (iv) antiferropara-magnetic and (v) ferripara-magnetic. Iron, Cobalt, Nickel and some of their alloys and compounds possess spontaneous magnetization. Magnetic oxides like ferrites and garnets could be used at high frequencies. Because of their excellent magnetic properties alongwith their high electrical resistivity these materials today find use in a variety of applications like magnetic recording tapes, inductors and transformers, memory elements, microwave devices, bubble domain devices, recording hard cores, etc. Hysteresis, permeability and coercive forces are some of the magnetic properties of magnetic substances which are to be considered for the manufacture of transformers and other electronic components.
Classification and Selection of Materials 5
(f) Optical Properties The optical properties of materials, e.g. refractive index, reflectivity and absorp-tion coefficient etc. affect the light reflecabsorp-tion and transmission.
(g) Structure of Materials The properties of engineering materials mainly depends on the internal ar-rangement of the atoms on molecules. We must note that in the selection of materials, the awareness regarding differences and similarities between materials is extremely important.
Metals of a single type atom are named pure metals. Metals in actual commercial use are almost exclusively alloys, and not pure metals, since it is possible for the designer to realize an infinite variety of physical properties in the product by varying the metallic composition of the alloy. Alloys are prepared from mixed types of atoms. Alloys are classified as binary alloys, composed of two components, as ternary alloys, composed of three components or as multi component alloys. Most commercial alloys are multicom-ponent. The composition of an alloy is described by giving the percentage (either by weight or by atoms) of each element in it.
The basic atomic arrangement or pattern is not apparent in the final component, e.g. a shaft or a pulley but the properties of the individual crystals within the metallic component, which are controlled by the atomic arrangement, are mainly responsible for their application in industry.
One can determine the strength of a piece of metal by its ability to withstand external loading. The structure of metal or alloy responds internally to the applied load by trying to counteract the magnitude of the applied load and thus tries to keep the constituent atoms in their ordered positions if however the load is higher than the force which holds the atoms in place, the metallic bond becomes ineffective and atoms in the metal are then forced into new displaced positions. The movement of atoms from their original positions in the metal is termed as slip. The ease with which atoms move or slip in a metal is an indication of hardness. We must note that the relative movement of atoms or slip within a material has a direct bearing on the mechanical properties of the material.
3. CLASSIFICATION OF ENGINEERING MATERIALS
The factors which form the basis of various systems of classifications of materials in material science and engineering are: (i) the chemical composition of the material, (ii) the mode of the occurrence of the material in the nature, (iii) the refining and the manufacturing process to which the material is subjected prior it acquires the required properties, (iv) the atomic and crystalline structure of material and (v) the industrial and technical use of the material.
Common engineering materials that falls within the scope of material science and engineering may be classified into one of the following six groups:
(i) Metals (ferrous and non-ferrous) and alloys
(ii) Ceramics
(iii) Organic Polymers
(iv) Composites
(v) Semi-conductors
(vi) Biomaterials
(vii) Advanced Materials
conduc-tivity, malleability, stiffness, the property of magnetism, etc. Metals may be magnetic, non-magnetic in nature. These properties of metallic materials are due to: (i) the atoms of which these metallic materials are composed and (ii) the way in which these atoms are arranged in the space lattice.
Metallic materials are typically classified according to their use in engineering as under:
(i)Pure Metals: Generally it is very difficult to obtain pure metal. Usually, they are obtained by refining the ore. Mostly, pure metals are not of any use to the engineers. However, by specialised and very expensive techniques, one can obtain pure metals (purity ~ 99.99%), e.g. aluminium, copper etc.
(ii)Alloyed Metals: Alloys can be formed by blending two or more metals or atleast one being metal. The properties of an alloy can be totally different from its constituent substances, e.g. 18-8 stainless steel, which contains 18%, chromium and 8% nickle, in low carbon steel, carbon is less than 0.15% and this is extremely tough, exceedingly ductile and highly resistant to corrosion. We must note that these properties are quite different from the behaviour of original carbon steel.
(iii)Ferrous Metals: Iron is the principal constituent of these ferrous metals. Ferrous alloys contain signifi-cant amount of non-ferrous metals. Ferrous alloys are extremely important for engineering purposes. On the basis of the percentage of carbon and their alloying elements present, these can be classified into following groups:
(a) Mild Steels: The percentage of carbon in these materials range from 0.15% to 0.25%. These are moderately strong and have good weldability. The production cost of these materials is also low.
(b) Medium Carbon Steels: These contains carbon between 0.3% to 0.6%. The strength of these materials is high but their weldability is comparatively less.
(c) High Carbon Steels: These contains carbon varying from 0.65% to 1.5%. These materials get hard and tough by heat treatment and their weldability is poor.
The steel formed in which carbon content is upto 1.5%, silica upto 0.5%, and manganese upto 1.5% alongwith traces of other elements is called plain carbon steel.
(d) Cast Irons: The carbon content in these substances vary between 2% to 4%. The cost of production of these substances is quite low and these are used as ferrous casting alloys.
(iv)Non-Ferrous Metals: These substances are composed of metals other than iron. However, these may contain iron in small proportion. Out of several non-ferrous metals only seven are available in sufficient quantity reasonably at low cost and used as common engineering metals. These are aluminium, tin, copper, nickle, zinc and magnesium. Some other non-ferrous metals, about fourteen in number, are produced in relatively small quantities but these are of vital importance in modern industry. These includes, chromium, mercury, cobalt, tungsten, vanadium, molybdenum, antimony, cadmium, zirconium, beryllium, niobium, titanium, tantalum and manganese.
(v)Sintered Metals: These materials possess very different properties and structures as compared to the metals from which these substances have been cast. Powder metallurgy technique is used to produced sintered metals. The metals to be sintered are first obtained in powered form and then mixed in right calculated proportions. After mixing properly, they are put in the die of desired shape and then processed with certain pressure. Finally, one gets them sintered in the furnace. We must note that the mixture so produced is not the true alloy but it possesses some of the properties of typical alloys.
Classification and Selection of Materials 7
4. ORGANIC, INORGANIC AND BIOLOGICAL MATERIALS
Organic materials are carbon compounds and their derivatives. They are solids composed of long molecular chains. The study of organic compounds is very important because all biological systems are composed of carbon compounds. There are also some materials of biological origin which do not possess organic composition, e.g., limestone.
Organic Materials
These materials are carbon compounds in which carbon is chemically bonded with hydrogen, oxygen and other non-metallic substances. The structure of these compounds is complex. Common organic materials are plastics and synthetic rubbers which are termed as organic polymers. Other examples of organic materials are wood, many types of waxes and petroleum derivatives. Organic polymers are prepared by polymeri-sation reactions, in which simple molecules are chemically combined into long chain molecules or three-dimensional structures. Organic polymers are solids composed of long molecular chains. These materials have low specific gravity and good strength. The two important classes of organic polymers are:
(a) Thermoplastics: On heating, these materials become soft and hardened again upon cooling, e.g., nylon, polythene, etc.
(b) Thermosetting plastics: These materials cannot be resoftened after polymerisation, e.g., urea-formalde-hyde, phenol formaldeurea-formalde-hyde, etc. Due to cross-linking, these materials are hard, tough, non-swelling and brittle. These materials are ideal for moulding and casting into components. They have good corrosion resistance.
The excellent resistance to corrosion, ease of fabrication into desired shape and size, fine lusture, light weight, strength, rigidity have established the polymeric materials and these materials are fast replacing many metallic components. PVC (Polyvinyl Chloride) and polycarbonate polymers are widely used for glazing, roofing and cladding of buildings. Plastics are also used for reducing weight of mobile objects, e.g., cars, aircrafts and rockets. Polypropylenes and polyethylene are used in pipes and manufacturing of tanks. Thermo-plastic films are widely used as lining to avoid seepage of water in canals and lagoons.
To protect metal structure from corrosion, plastics are used as surface coatings. Plastics are also used as main ingredients of adhesives. The lower hardness of plastic materials compared with other materials makes them subjective to attack by insects and rodents.
Because of the presence of carbon, plastics are combustible. The maximum service temperature is of the order of 100°C. These materials are used as thermal insulators because of lower thermal conductivity. Plastic materials have low modulus of rigidity, which can be improved by addition of filters, e.g., glass fibres.
Natural rubber, which is an organic material of biological origin, is an thermoplastic material. It is prepared from a fluid, provided by the rubber trees. Rubber materials are widely used for tyres of automo-biles, insulation of metal components, toys and other rubber products.
Inorganic Materials
These materials include metals, clays, sand rocks, gravels, minerals and ceramics and have mineral origin. These materials are formed due to natural growth and development of living organisms and are not biologi-cal materials.
Rocks are the units which form the crust of the earth. The three major groups of rocks are:
(i) Igneous Rocks: These rocks are formed by the consolidation of semi-liquid of liquid material (magma) and are called Plutonic if their consolidation takes place deep within the earth and volcanic if lava or magma solidifies on the earth’s surface. Basalt is igneous volcanic where as granite is igneous plutonic.
(iii) Metamorphic Rocks: These rocks are basically sedimentary rocks which are changed into new rocks by intense heat and pressure, e.g., marble and slates. The structure of these rocks is in between igneous rocks and sedimentary rocks.
Rock materials are widely used for the construction of buildings, houses, bridges, monuments, arches, tombs, etc. The slate, which has got great hardness is still used as roofing material. Basalt, dolerite and rhyolite are crushed into stones and used as concrete aggregate and road construction material.
Another type of materials, i.e. Pozzolanics, are of particular interest to engineers because they are naturally occurring or synthetic silicious materials which hydrate to form cement. Volcanic ash, blast furnace slag, some shales and fly ash are examples of pozzolanic materials. When the cement contains 10-20% ground blast furnace slag, then it is called pozzolans-portland cement, which sets more slowly than ordinary portland cement and has greater resistance to sulphate solutions and sea water.
Rocks, stone, wood, copper, silver, gold etc. are the naturally occurring materials exist in nature in the form in which they are to be used. However, naturally occurring materials are not many in number. Nowadays, most of the materials are manufactured as per requirements. Obviously, the study of engineering materials is also related with the manufacturing process by which the materials are produced to acquire the properties as per requirement.
Copper, silver, gold, etc. metals, which occur in nature, in their free state are mostly chemically inert and highly malleable and ductile as well as extremely corrosion resistant. Alloys of these metals are harder than the basic metals. Carbonates, sulphates and sulphide ores are more reactive metals.
Biological Materials
Leather, limestone, bone, horn, wax, wood etc. are biological materials. Wood is fibrous composition of hydrocarbon, cellulose and lignin and is used for many purposes. Apart from these components a small amount of gum, starch, resins, wax and organic acids are also present in wood. One can classify wood as
soft wood and hard wood. Fresh wood contains high percentage of water and to dry out it, seasoning is done. If proper seasoning is not done, defects such as cracks, twist, wrap etc. may occur.
Leather is obtained from the skin of animals after cleaning and tanning operations. Nowadays, it is used for making belts, boxes, shoes, purses etc. To preserve the leather, tanning is used. Following two tanning techniques are widely used:
(a) Vegetable Tanning: It consist of soaking the skin in tanning liquor for several days and then dried to optimum conditions of leather.
(b) Chrome Tanning: This technique involves pickling the skin in acid solution and then revolving in a drum which contains chromium salt solution. After that the leather is dried and rolled.
Limestone is an important material which is not organic but has biological origin. It mainly consist of calcium carbonate and limestone. It is widely used to manufacture cement. In Iron and Steel Industries, limestone in pure form is used as flux.
In early days bones of animals were used to make tools and weapons. Nowadays bones are used for the manufacture of glue, gelatin etc. Bones are laminate of organic substances and phosphates and carbonates of calcium. These are stronger in compression as compared to tension.
Table 1.3 lists typical examples from each of the four groups of materials.
Table 1.3 Important grouping of materials
Material group Important characteristics Typical examples of engineering use
(1) (2) (3)
1. Metals and Alloys Lusture, hardness, thermal and electrical conductivity, resistance to corrosion, mal-leability, stiffness and the property of magnetism
Iron and steels, aluminium, copper, silver, gold, zinc, magnesium, brasses, bronzes, manganin, invar, super alloy, boron, rare-earth alloys, conductors, etc.
Classification and Selection of Materials 9
Some important properties for different groups of materials are summarized in Table 1.4.
Table 1.4 Important properties for different groupings of materials
Property Metals Ceramics Polymers Composites (wood)
1. Tensile strength (N/mm2) 200–2000 10–400 30–100 20–110
2. Density (10N/mm2) 2–8 ´ 103 2–17 ´ 103 1–2 ´ 103 0.5 ´ 103
3. Hardness medium high low low
4. Tensile modulus (103N/mm2) 100–200 150–450 0.7–3.5 4–20
5. Melting point (°C) 200–3500 2000–4000 70–200 —
6. Thermal expansion medium low high low
7. Thermal conductivity high medium low low
8. Electrical conductivity good conductors insulator insulator insulator
5. SEMICONDUCTORS
These are the materials which have electrical properties that are intermediate between the electrical con-ductors and insulators. The electrical characteristics of semiconcon-ductors are extremely sensitive to the pres-ence of minute concentrations of impurity atoms; these concentrations may be controlled over very small spatial regions. Semiconductors form the backbone of electronic industry. The semiconductors have made possible the advent of integrated circuitary that has totally revolutionized the electronics and computer industries. They affect all walks of life whether it is communications, computers, biomedical, power, aviation, defence, entertainment, etc. The field of semiconductors is rapidly changing and expected to continue in the next decade. Organic semiconductors are expected to play prominent role during this decade. Diamond as semiconductor will also be important. Optoelectronic devices will provide three-dimensional integration of circuits, and optical computing.
(Contd.)
Material group Important characteristics Typical examples of engineering use
(1) (2) (3)
Soft, light in weight, poor conductors of electricity and heat, dimensionally un-stable, ductile, combustible, low thermal resistance
They are better than any of the individual components as regards to their properties like strength, stiffness, heat resistance, etc.
Silica, soda-lime-glass, concrete, cement, refractories, Ferrites and garnets, ceramic superconductors, MgO, CdS, Al2O3, SiC, BaTiO3, etc.
Plastics: PVC, PTFE, polyethylene, poly-carbonate
6. BIOMATERIALS
These are employed in components implanted into the human body for replacement of diseased or damaged body parts. Biomaterials must not produce toxic substances and must be compatible with body tissues (i.e., these materials must not cause adverse biological reactions). All the above materials, i.e., metals, ceramics, polymers, composites, and semiconductors—may be used as biomaterials.
7(A) CURRENT TRENDS AND ADVANCES IN MATERIALS
Timber, steel and cement are the materials which are widely used for engineering applications in huge quantities. The consumption of steel in any country is considered as an indicator of its economic well being. For high temperature applications, e.g. steam and gas turbines the design engineers keep creating the demand for various high steel alloy. However, alloys of chromium, nickel, molybdenum and tungsten alongwith iron are better suited for the said applications. Newer materials for combined resistance to high temperature and corrosion are increasing rapidly and material scientists and engineers are busy in devel-oping such materials. Different kinds of ceramics, though difficult to shape and machine, are finding demand for their use at high temperatures.
Recently prepared new metallic materials in conjunction with new processing techniques as isostatic pressing and isothermal forging are capable of imparting better fatigue properties to aircraft components. Powder metallurgy technique while producing finished surfaces and cutting down metal cutting cost is much capable of imparting improved mechanical properties under different loading conditions. Surprisingly, rapid cooling technology achieving cooling rates in the vicinity of one million degree celcius per second and this is being used to produce metal powders which can be used in such product producing techniques as powder metallurgy and hot isostatic pressing to obtain temperature resistant parts. Nowadays, metallurgists have produced several molybdenum and aluminium alloys as well as alloys of titanium and nickel to meet anticorrosion properties at elevated temperatures.
Polymeric materials are growing at annual rate of 9% and have grown in volume more than any other material. In several applications plastics have replaced metals, wood, glass and paper. A new trend in plastic technology is the production of synergistic plastic alloys which have better properties than individual members producing the alloy. Recent discovery of plastic conductors may have wider impact in near future. Ceramics are mainly used as high temperature low load carrying materials. The major drawback of ceramics is the brittleness and difficulty in cutting and shaping. When mixed with metal powder like molybdenum, ceramic produce cerments, which are expected to be useful cutting materials. Tool bits of cerments are expected to find various applications in attaining high cutting speeds and producing better surface finish. Alumina, a well known ceramic is expected to be successfully reinforced with fibres of molybdenum. Due to microcracking of molybdenum fibres, the attempts to achieve better strength in such composite ceramics have not been successful yet. However, such composites have been found to exhibit better impact and thermal shock resistance.
The advent of solar cells, electronic digital circuits and computers in factory automation and use of robots in several industrial applications is adding to the enormous demand of silicon chips and of such material as silicon. Today, semiconductors form the backbone of electronics and they affect all types of instruments/industries related to e.g. communications, computers, biomedical, power, aviation, defence, entertainment etc.
7(B) ADVANCED MATERIALS
Classification and Selection of Materials 11
and are normally relatively expensive. In subsequent chapters are discussed the properties and applications of a good number of advanced materials—for example, materials that are used for lasers, ICs, magnetic information storage, liquid crystal displays (LCDs), fiber optics, and the thermal projection system for the space shuttle orbiter.
7(C) SMART MATERIALS (MATERIALS OF THE FUTURE)
Smart or intelligent materials form a group of new and state-of-the art materials now being developed that will have a significant influence on many of our technologies. The adjective “smart” implies that these materials are able to sense changes in their environments and then respond to these changes in predeter-mined manners—traits that are also found in living organisms. In addition, the concept of smart materials is being extended to rather sophisticated systems that consist of both smart and traditional materials.
The field of smart materials attempts to combine the sensor (that detects an input signal), actuator (that performs a responsive and adaptive function) and the control circuit on as one integrated unit. Actuators may be called upon to change shape, position, natural frequency, or mechanical characteristics in response to changes in temperature, electric fields, and/or magnetic fields.
Usually, four types of materials are commonly used for actuators: shape memory alloys, piezoelectric ceramics, magnetostrictive materials, and electrorheological/magnetorheological fluids. Shape memory al-loys are metals that, after having been deformed, revert back to their original shapes when temperature is changed. Piezoelectric ceramics expand and contract in response to an applied electric field (or voltage); conversely, these materials also generate an electric field when their dimensions are altered. The behaviour of magnetostrictive materials is analogous to that of the piezoelectric ceramic materials, except that they are responsive to magnetic fields. Also, electrorheological and magnetorheological fluids are liquids that experience dramatic changes in viscosity upon the application of electric and magnetic fields, respectively. The combined system of sensor, actuator and control circuit on as one IC unit, emulates a biological system (Fig. 1.1).
Fig. 1.1 Integrated sensor-actuator systems with controller are analogous to biological systems
Nerves and memory
Sensors Control and
computation
Brain
Actuators and Motors
Muscles
These are known as smart sensors, microsystem technology (MST) or microelectromechanical systems (MEMS). Materials/devices employed as sensors include optical fibers, piezoelectric materials (including some polymers), and MEMS.
For example, one type of smart system is used in helicopters to reduce aero-dynamic cockpit noise that is created by the rotating rotor blades. Piezoelectric sensors inserted into the blades, monitor blade stresses and deformations; feedback signals from these sensors are fed into a computer controlled adaptive device, which generates noise cancelling antidose.
MEMS devices are small in size, light weight, low cost, reliable with large batch fabrication technology. They generally consist of sensors that gather environmental information such as pressure, temperature, acceleration etc., integrated electronics to process the data collected and actuators to influence and control the environment in the desired manner.
7(D) NANOSTRUCTURED MATERIALS AND NANOTECHNOLOGY
Nanostructured materials are those materials whose structural elements—clusters, crystallites or mol-ecules—have dimensions in the range of 1-100 nm. These small groups of atoms, in general, go by different names such as nanoparticles, nanocrystals, quantum dots and quantum boxes. Substantial work is being carried out in the domain of nanostructured materials and nanotubes during the past decade since they were found to have potential for high technology engineering applications. One finds a remarkable variations in fundamental electrical, optical and magnetic properties that occur as one progresses from an ‘infinitely extended’ solid to a particle of material consisting of a countable number of atoms. The various types of nanostructured materials which has been considered for applications in opto-electronic devices and quan-tum-optic devices are nano-sized powders of silicon, silicon-nitride (SiN), silicon-carbide (SiC) and their thin films. Some of these are also used as advanced ceramics with controlled micro structures because their strength and toughness increase when the grain size diminishes. Carbon-based nanomaterials and nanostructures including fullerenes and nanotube plays an increasingly pervasive role in nanoscale science and technology. Today, nanotechnology is being heralded as the next enabling technology that will redesign the future of several technologies, products and markets. A brief account of nanostructured materials, particularly of carbon based nanomaterials and nanostructures is presented in a separate chapter.
7(E) QUANTUM DOTS (QDS)
Rapid progress in the fabrication of semiconductor structures has resulted into the reduction of three-dimensional bulk systems to two-three-dimensional, one-three-dimensional, and ultimately to zero three-dimensional systems. These reduced dimensional systems are used in future applications like semiconductor lasers and microelec-tronics. Quantum dots represent the ultimate reduction in the dimensionality of semiconductor devices. These are three dimensional semiconductor structures only nanometer in size confining electrons and holes. QDs operate at the level of single electron which is certainly the ultimate limit for an electronic device and are used as the gain material in lasers. QDs are used in quantum dot lasers, QD memory devices, QD photo-detectors and even quantum cryptography. The emission wavelength of a quantum dot is a function of its size. So by making dots of different sizes, one can create light of different colours.
7(F) SPINTRONICS
A revolutionary new class of semiconductor electronics based on the spin degree of freedom could be created. The study of electron spin in materials is called spintronics. Spintronics is based on the direction of spin- and spin-coupling. Every appliance ranging from electric bulb to laptop computer works on the principle of transport of electric charge carriers-electrons, which cause electric current to flow through the wires. The electrons have both charge and spin. The spin of the electrons could greatly enhance the particles’ usefulness. Presently, the semiconductor technology is based on the number of charges and their energy. The electronic devices, e.g. transistors work due to flow of charge. The electron can be assumed as tiny rotating bar magnet with two possible orientations: spin-up or spin-down. An applied magnetic field can flip electrons from one state to another. Obviously, spin can be measured and manipulated to represent the 0’s and 1’s of digital programming analogous to the “current on and current off” states in a conventional silicon chip.
The performance of conventional devices is limited in speed and dissipation whereas spintronics devices are capable of much higher speed at very low power. Spintronics transistors may work at a faster speed, are also smaller in size and will consume less power.
Classification and Selection of Materials 13
with two magnetic layers surrounding a non-magnetic metal or insulator. If the two magnetic layers are different, then the magnetization direction of one can be rotated with respect to the other. This leads to the utilization of these structures as sensor elements and for memory elements. Scientists are now trying to use the property of the electron-like spin rather than charge to develop new generation of microelectronic devices which may be more versatile and robust than silicon chips and circuit elements. Spins appear to be remarkably robust and move effectively easily between semiconductors. In case of electron transport from one material to another, the spins do not lose its orientation or scatter from impurities or structural effects.
7(G) FERMIONIC CONDENSATE MATTER
Very recently scientists had created a new form of matter called a fermionic condensate matter and predicted it could lead to the next generation of superconductors for use in electricity generation more efficient trains and countless other applications.
The new matter form is the sixth known form of the matter—after solids, liquids, gases, plasma and Bose-Einstein condensate, created only in 1995.
Fermionic condensate matter is a scientific breakthrough in providing a new type of quantum mechanical behaviour. It is related to Bose-Einstein condensate. However, new state is not a superconductor but it is really something in between these two states that may help us in science link these two interesting behaviours. New state of matter uses fermions—the building blocks of matter.
8. LEVEL OF MATERIAL STRUCTURE EXAMINATION AND OBSERVATION
One can examine and observe the internal structure of a material at various levels of observation. The degree of magnification required to study a material by various methods is called a measure of the level of observation. We may note that the details that are revealed or disclosed at a certain level of magnification are generally different from the details that are disclosed at some other level of magnification.
While considering the structures at various levels we may find it is useful to have some concept of relative sizes, and the so-called “logarithmic scale of universe” provides one method of size comparison (Table 1.5).
Table 1.5 Relative sizes within the universe: Logarithmic scale of the universe
Object Average size Logarithmic scale of the universe
1. Nearest galaxy distance 1 million light year 1021
2. Nearest star distance 1 light year 1016
3. Diameter of the sun 1 million kilometer 109
4. Diameter of the earth 103 kilometer 107
5. Man (average dimension) 1 metre 100
6. Sand grain 1 millimetre 10–3
7. Dust particle 1 micrometre 10–6
8. Molecule 1 nanometre 10–9
9. Atom 10–1 nanometre 10–10
9. MATERIAL STRUCTURE
(a) Macrostructure Macrostructure of a material is examined by low-power magnification or naked eye. It deals with the shape, size and atomic arrangement in a crystalline material. In case of some crystals, e.g., quartz, external form of the crystal may reflect the internal symmetry of atoms. Macrostructure may be observed directly on a fracture surface or on a forging specimen. The individual crystals of a crystalline material can be visible, e.g. in a brass doorknob by the constant polishing and etching action of a human hand and sweat. Macrostructure can reveal flaws, segregations, cracks etc. by using proper techniques and one can save much expenses by rejecting defective materials at an early stage.
(b) Micro Structure This generally refers to the structure of the material observed under optical micro-scope. Optical microscopes can magnify a structure about 1500 to 3000 times linear, without loss of resolution of details of the material structure. We may note that optical microscopes can resolve two lines separately when their difference of separation is 10–7 m (= 0.1 mm). Cracks, porosity, non-metallic insclutions within materials can be revealed by examining them under powerful optical microscope.
(c) Sub Structure When crystal imperfections such as dislocation in a structure are to be examined, a special microscope having higher magnification and resolution than the optical microscope is used. Electron microscope with magnifications 105 are used for this purpose. Another important modern microscope is
field ion microscope, which can produce images of individual atoms as well as defects in atomic arrange-ments.
(d) Crystal Structure This reveals the atomic arrangement within a crystal. X-ray diffraction techniques and electron diffraction method are commonly used for studying crystal structure. It is usually sufficient to study the arrangement of atoms within a unit cell. The crystal is formed by a very large number of unit cells forming regularly repeating patterns in space.
(e) Electronic Structure This refers to the electrons in the outermost shells of individual atoms that form the solid. Spectroscopic techniques are commonly used for determining the electronic structure.
(f) Nuclear Structure This is studied by nuclear spectroscopic techniques, e.g., nuclear magnetic reso-nance (NMR) and Mössbauer spectroscopy.
10. ENGINEERING METALLURGY
This includes the study of metallurgy, which is of special interest to an engineer. At the time of taking final decision regarding the selection of suitable material for a particular job, it is essential for an engineer to have a thorough knowledge of engineering metallurgy. This helps him in deciding the treatment processes and their sequence, which are to be carried out on the finished components and structures. This includes the following processes:
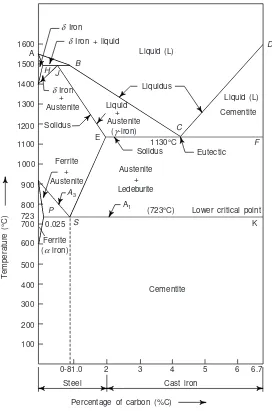
(i) Iron-carbon alloy system: This deals with the structure of iron and steel as well as iron-carbon equi-librium diagrams. This also deals with the transformation of alloys and steels under various sets of condi-tions. A detailed and thorough study of this process helps an engineer to decide the suitability of process and the selection of iron alloy for various types of his jobs.
(ii) Heat Treatment: A thorough knowledge of this helps an engineer in deciding the type of process to be undertaken for the smooth and efficient working of the components and structures.
(iii) Corrosion of Metals: This deals with the colossal problem of corrosion of metals and its prevention. It also deals with the processes which help engineers in improving the life and outward appearance of the metal components and structures.
11. SELECTION OF MATERIALS
Classification and Selection of Materials 15
optimum combination of properties in a material at the lowest possible cost without compromising the quality. The properties and behaviour of a material depends upon the several factors, e.g., composition, crystal structure, conditions during service and the interaction among them. The performance of materials may be found satisfactory within certain limitations or conditions. However, beyond these conditions, the performance of materials may not be found satisfactory.
One can list the major factors affecting the selection of materials as
(i) Component shape (ii) Dimensional tolerance
(iii) Mechanical properties (iv) Fabrication requirements
(v) Service requirements (vi) Cost of the material
(vii) Cost of processing, and (viii) Availability of the material.
All these major factors have a complex effect on the selection of materials. The shape and size of a component has great effect on the choice of the processing unit which ultimately effects the choice of the material. To make it more clear, we consider an example, let the best possible production method is selected, under given conditions, it is die casting, obviously, now the choice of the material becomes limited, i.e. one can only choose materials with lower melting points, e.g. aluminium, zinc, magnesium and thermoplastics.
There are some materials which can be finished to close tolerance while others cannot. Obviously, the required dimensional tolerance for finished components will, influence the choice of materials.
To select a suitable material for specific conditions, all mechanical properties, e.g., hardness, strength, etc. guide us. Method of processing of the material also affects the properties of a component, e.g., forged components can be stronger than the casted components. Different types of working processes may also give different types of fibre structure. However, investment casting can provide precise dimensions at low cost in comparison to machine operations.
Service requirements are dimensional stability, strength, toughness, heat resistance, corrosion resistance, fatigue and creep resistance, electrical and thermal conductivity etc. where as fabrication requirements are castability, i.e., ease in casting a material, weldability-ease in welding the material, machinability-ease to machine a material, formability-ease to form a material, hardenability etc.
In most of the cases, the cost of raw material accounts about 50 per-cent of the finished cost. Obviously, the cost of the material is a major factor which influences the choice of the material or process. We must note that the use of cheaper material will not always reduce the final cost of the component or product. Use of cheaper material may be associated with higher processing cost due to large number of operations to be performed and also more scrap. We can easily see that this sometimes makes the overall cost more than that of expensive raw material in combination with low processing cost due to lesser number of operations and lesser scrap.
The type of material affects the detailed aspect of design and hence the choice of material as well as the process is selected at the early design state, e.g., whether the material is to be joined by spot welding, screws or rivetes, must be decided at the design state.
In most of the industries, the processing cost (labour cost) and other costs such as overhead costs account for about 50% of the production cost. Overhead cost in automatic industries is much more than the other costs. If one can somehow reduce all such costs, the total production cost will automatically reduce. In comparison to conventional processes, sometimes injection moulding process is preferred because the conventional process involves many intermediate stages and several machining processes.
One finds that the cost of production of a component by rolling or forging operations is twice as compared to the production by powered metallurgy process because the rolling or forging are followed by several machining operations for the same finish and tolerances.