Post-vitrification survival and in vitro maturation
rate of buffalo (Bubalus bubalis) oocytes: effect of
ethylene glycol concentration and exposure time
A. Dhali, R.S. Manik
∗, S.K. Das, S.K. Singla, P. Palta
Embryo Biotechnology Centre, National Dairy Research Institute, Karnal 132001, India
Received 21 September 1999; received in revised form 14 March 2000; accepted 2 May 2000
Abstract
The present study was undertaken to investigate the effects of ethylene glycol concentration and time of exposure to equilibration solution on the post-thaw morphological appearance and the in vitro maturation rate of buffalo oocytes. Vitrification solution-I (VS-I) consisted of 4.5 M ethylene glycol (EG), 3.4 M dimethyl sulphoxide, 5.56 mM glucose, 0.33 mM sodium pyruvate and 0.4% w/v bovine serum albumin in Dulbecco’s phosphate buffered saline (DPBS), whereas vitrification solution-II (VS-II) contained 3.5 M EG, with other constituents at same concentrations as in VS-I. The equilibration solutions-I and II were prepared by 50% dilution (v/v) of VS-I and VS-II, respectively, in DPBS. Prior to vitrification, the cumulus-oocyte complexes (COCs) were exposed to equilibration solution-I or II for 1 or 3 min at room temperature (25–30◦
C). Groups of four to five oocytes were then placed in 15ml of respective vitrification solution, and immediately loaded
into 0.25 ml French straws, each containing 150ml of 0.5 M sucrose in DPBS. The straws were
placed in liquid nitrogen (LN2) vapour for 2 min, plunged and stored in LN2for at least 7 days. The
straws were thawed by keeping in warm water at 28◦C for 20 s, and the oocytes were equilibrated for
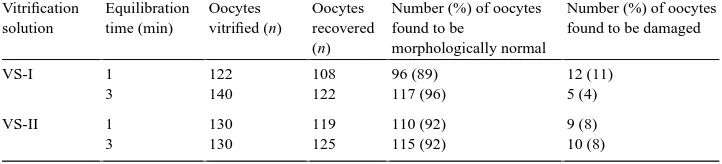
5 min in 0.5 M sucrose for one-step dilution. The percentage of oocytes found to be morphologically normal varied from 89 to 96% for the two equilibration solutions and the two exposure times. Among the damaged oocytes, cracking of zona pellucida was the abnormality observed most frequently. The nuclear maturation rate of oocytes equilibrated in equilibration solutions-I and II for 1 (28 and 24%, respectively) or 3 min (32 and 33%, respectively) did not differ significantly. These results show that it is possible to cryopreserve buffalo oocytes by vitrification using a combination of 3.5 M EG and 3.4 M DMSO with an exposure time of 3 min. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Buffalo; Dimethyl sulphoxide; Ethylene glycol; Oocyte; Vitrification
∗Corresponding author.
E-mail address: [email protected] (R.S. Manik).
1. Introduction
The techniques available for cryopreservation of mammalian embryos and oocytes include controlled freezing and thawing procedures, the one-step procedure, ultrarapid freezing and vitrification (for review see Palasz and Mapletoft, 1996). Although the con-trolled freezing method is still widely used, its field applications are limited by complex freezing procedures and the requirement for expensive freezing machines. Vitrification, which is a relatively recent approach is defined as a physical process by which a highly concentrated solution of cryoprotectants solidifies during cooling, without formation of ice crystals (Niemann, 1991). It offers several advantages over conventional equilibrium meth-ods, e.g. faster and simplified freezing and thawing procedures, high oocyte/embryo survival and no requirement for an expensive freezing machine. Vitrification has been successfully applied for cryopreservation of bovine oocytes and embryos at various developmental stages (Mahmoudzadeh et al., 1993; Bautista and Kanagawa, 1998; Otoi et al., 1998; Le Gal and Massip, 1999), and live offsprings have been obtained from vitrified-thawed cattle oocytes (Fuku et al., 1992). The ability to cryopreserve oocytes could increase their availability for a broad range of reproductive technologies in buffalo, the principal dairy animal in the developing countries in Asia.
The concentration of cryoprotectants required to achieve vitrification is very high which can lead to toxic effects on oocytes (Niemann, 1991). Therefore, the concentration of cryoprotectants and the duration of time for which oocytes are exposed to cryoprotec-tants are of critical importance. Two approaches have been followed to minimise these toxic effects, (1) the very brief exposure of oocytes to high concentrations of cryopro-tectants in the vitrification solution is preceded by equilibration of oocytes in an equi-libration solution containing lower concentrations of cryoprotectants (Kono et al., 1991; Wood et al., 1993), and (2) the exposure time may be shortened since the amount of intracellular cryoprotectant(s) required for successful vitrification has been reported to be rather small (Nagakata, 1989). The duration of exposure to cryoprotectants depends primarily upon their permeability into the oocytes/embryos. EG has the highest perme-ability due to its lowest molecular weight compared with glycerol, DMSO and PG.
The present study was undertaken to investigate the effects of concentration of ethylene glycol and duration of exposure to cryoprotectants on the post-vitrification morphological appearance and the in vitro maturation rate of buffalo oocytes.
2. Materials and methods
All chemicals and media were purchased from Sigma (St. Louis, MO, USA) unless other-wise indicated. The FSH-P was from Schering-Plough (Kenilworth, NJ, USA). Disposable Petri dishes were from Becton, Dickinson and Co. (Lincoln Park, NJ, USA), and the 0.22 and 0.45mm filters were from Millipore Corp. (Bedford, MA, USA). Mineral oil was from
2.1. Collection of oocytes
Buffalo ovaries were collected in an abattoir and were transported within 4 to 5 h to the laboratory in 0.9% saline at 32–37◦C. Follicular oocytes (3–8 mm in diameter) were aspirated with a 19-gauge needle attached to a 5 ml glass syringe. The aspiration medium consisted of TCM-199 supplemented with 5% EBS and Dulbecco’s phosphate buffered saline (DPBS) supplemented with 0.6% bovine serum albumin (BSA) at a 1:1 ratio (v/v). All the aspirated cumulus-oocyte compexes (COCs) with homogenous cytoplasm were used in the study, except those oocytes which were recovered in a denuded form.
2.2. Vitrification of oocytes
The vitrification solution-I (VS-I) consisted of 4.5 M ethylene glycol (EG), 3.4 M DMSO, 5.56 mM glucose, 0.33 mM sodium pyruvate and 0.4% w/v BSA, dissolved in DPBS whereas vitrification solution-II (VS-II) contained 3.5 M EG, with other constituents at same concentrations as in VS-I. The equilibration solutions-I and II were prepared by 50% dilution of VS-I and VS-II, respectively, in DPBS. The aspirated oocytes were washed five to six times in the washing medium (TCM-199+10% EBS) after which these were equilibrated in equilibration solution-I or II for 1 or 3 min. These were then transferred into 15ml droplets of the respective vitrification solution at room temperature (25–30◦C),
and immediately loaded into 0.25 ml French straws (four to five oocytes per straw), each containing 150ml of 0.5 M sucrose in DPBS. The straws were sealed with hot forceps and
were then precooled by keeping them in liquid nitrogen (LN2) vapour at a height of about
5 cm from the level of LN2for 2 min following which these were plunged in LN2. After
storage for at least 7 days, the straws were warmed rapidly by transferring them to a water bath at 28◦C for 20 s. The contents were expelled into an empty plastic dish and the oocytes were allowed a 5 min equilibration in 0.5 M sucrose solution in DPBS for one-step dilution. The oocytes were then transferred to fresh washing medium, and were washed four to five times with it.
2.3. Morphology and maturation rate of oocytes after vitrification-warming
For evaluation of post-thaw morphology, the vitrified-warmed oocytes were examined under an inverted microscope. The criteria used for assessing the post-thaw morphology of vitrified-warmed oocytes were as follows; Normal: oocytes with spherical and symmetrical shape with no sign of lysis, membrane damage, swelling, vacuolization, degeneration or leakage of the cellular content; Abnormal: oocytes with a ruptured zona pellucida or ruptured vitelline membrane, and fragmented cytoplasm with signs of degeneration. The in vitro developmental potential of morphologically normal vitrified-warmed oocytes was examined by in vitro maturation of oocytes in 50ml droplets (10 to 15 oocytes per droplet) of maturation
medium (TCM-199+15% FBS+5mg/ml FSH-P) for 26 h in a CO2incubator (5% CO2in
Table 1
Effect of ethylene glycol concentration and exposure time on the proportion of buffalo oocytes recovered mor-phologically normal after vitrification-warminga
aThe data are from eight trials.
were then stained with 2% Giemsa stain and evaluated for the presence of telophase-I and metaphase-II stages.
2.4. Statistical analysis
The experiment was replicated eight times. The data were analysed by analysis of variance (ANOVA) after arcsin transformation.
3. Results
Following vitrification-warming, the proportion of oocytes recovered morphologically normal or in a damaged form is shown in Table 1. The percentage of oocytes found to be morphologically normal which varied from 89 to 96% for the two equilibration solutions and the two exposure times was not significantly different.
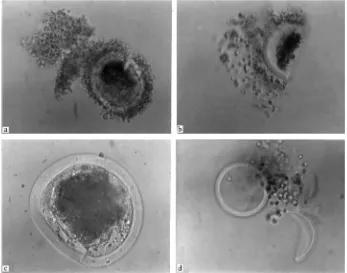
The morphological abnormalities observed in oocytes after vitrification-warming are shown in Table 2 and Fig. 1.
The nuclear maturation rate of vitrified-warmed oocytes did not differ for the oocytes exposed to the two equilibration solutions for 1 or 3 min (Table 3). The nuclear maturation rate of COCs that were run as controls in each trial was 67%.
Table 2
Types of damages observed in buffalo oocytes after vitrification-warminga
Vitrification
Fig. 1. Morphological abnormalities in buffalo oocytes after vitrification-warming (a) crack in Zona Pellucida; (b) oocyte split in two halves; (c) change in shape of oocyte; (d) leakage of oocyte contents.
4. Discussion
The results of the present study show that it is possible to cryopreserve buffalo oocytes by vitrification using 3.4 M DMSO in combination with 3.5 or 4.5 M EG, after exposure of
Table 3
Effect of ethylene glycol concentration and exposure time on the nuclear maturation rate of buffalo oocytes after vitrification-warminga
Vitrification solution
Equilibration time (min)
Number of oocytes taken
Number of oocytes found to be in
Number (%) of oocytes matured
Telophase I Metaphase II
Control – 46 3 28 31 (67)
VS-I 1 82 1 22 23 (28)
3 104 3 30 33 (32)
VS-II 1 85 1 19 20 (24)
3 96 5 27 32 (33)
oocytes to these cryoprotectants at 50% concentration for 3 min. The percentage of oocytes recovered morphologically normal in the present study compares favourably with other reports in which mouse oocytes were vitrified either in DMSO alone (Wood et al., 1993; Bos-Mikich et al., 1995) or in a mixture of DMSO, acetamide, propylene glycol (PG) and polyethylene glycol (Nagakata, 1989; Shaw et al., 1991) or when bovine oocytes were vitrified in DMSO, acetamide and PG (Hamano et al., 1993). This is substantially higher than the survival rate of 36–39% when mouse oocytes were vitrified in a mixture of EG, ficoll-70 and sucrose (Miyake et al., 1993). The nuclear maturation rate of oocytes after vitrification-warming was, however, much lower than that for controls. This reduction in developmental ability could be due to the toxic effects of cryoprotectants and osmotic injury. In addition, the possibility of ultrastructural damages to the oocytes and deleterious effects on chromosomes and other cytoplasmic structures cannot be ruled out since such effects have been demonstrated during cryopreservation of mouse (Van der Elst et al., 1988, 1992) and human oocytes (Pickering et al., 1990; Park et al., 1997). Another reason could be the use of immature rather than mature oocytes for vitrification since freezability of unfertilised oocytes has been reported to be low, and increase as development proceeds to the blastocyst stage after fertilisation (Kasai et al., 1979; Schroeder et al., 1990).
In this study, decreasing the exposure time to 1 min resulted in a reduction in the mat-uration rate for both equilibration solutions. This could probably be due to insufficient permeation of the cryoprotectants into the oocytes. The permeability to EG and DMSO has been reported to be very low in oocytes, and increase after fertilisation and subsequent embryonic development (Leibo, 1988). The presence of compact cumulus mass could have also reduced the rate of passage of cryoprotectants into the oocyte. Although the proportion of oocytes recovered morphologically normal and the post-vitrification nuclear maturation rate were similar for vitrification solutions containing 3.5 and 4.5 M EG for an exposure time of 3 min, use of the lower concentration of EG is recommended in view of its toxic effects, some of which might be manifested during IVF and/or subsequent embryonic development. In conclusion, the results of the present study suggest that it is possible to cryopreserve buffalo oocytes by vitrification using a combination of 3.5 M EG and 3.4 M DMSO with an exposure time of 3 min. Further studies are, however, required to demonstrate the capability of vitrified oocytes to be fertilised and result in birth of calves.
References
Bos-Mikich, A., Wood, M.J., Candy, C.J., Whittingham, D.G., 1995. Cytogenetical analysis and developmental potential of vitrified mouse oocytes. Biol. Reprod. 53, 780–785.
Bautista, J.A., Kanagawa, H., 1998. Current status of vitrification of embryos and oocytes in domestic animals: ethylene glycol as an emerging cryoprotectant of choice. Jpn. J. Vet. Res. 45, 183–191.
Das, S.K., Goswami, S.L., Ramesha, K.P., Datta, T.K., 1994. A technique for chromosome analysis of preimplantation embryos. Ind. J. Dairy Sci. 47, 396–400.
Fuku, E., Kojima, T., Shioya, Y., Marcus, G., Downey, B.R., 1992. In vitro fertilization and development of frozen-thawed bovine oocytes. Cryobiology 29, 485–492.
Hamano, S., Koikeda, A., Kuwayana, M., Nagai, T., 1993. Full term development of in vitro matured and vitrified and fertilized bovine oocytes. Theriogenology 45, 1085–1090.
Kono, T., Kwon, O.Y., Nakahara, T., 1991. Development of vitrified mouse oocytes after in vitro fertilization. Cryobiology 28, 50–54.
Le Gal, F., Massip, A., 1999. Cryopreservation of cattle oocytes: effects of meiotic stage, cycloheximide treatment, and vitrification procedure. Cryobiology 38, 290–300.
Leibo, S.P., 1988. Cryopreservation of embryos. In: Proceedings of the 11th International Congress on Animal Reproduction and AI. 5, pp. 370−377.
Madan, M.L., Chauhan, M.S., Singla, S.K., Manik, R.S., 1994. Pregnancies established from water buffalo (Bubalus bubalis) blastocysts derived from in vitro matured, in vitro fertilized oocytes and co-cultured with cumulus and oviductal cells. Theriogenology 42, 591–600.
Mahmoudzadeh, A.R., Van Soom, A., Van Vlaenderen, I., De Kruif, A., 1993. A comparative study of the effect of one-step addition of different vitrification solutions on in vitro survival of vitrified bovine embryos. Theriogenology 39, 1291–1302.
Miyake, T., Kasai, M., Zhu, S.F., Sakurai, T., Machida, T., 1993. Vitrification of mouse oocytes and embryos at various stages of development in an ethylene glycol-based solution by a simple method. Theriogenology 40, 121–134.
Nagakata, N., 1989. High survival rate of unfertilized mouse oocytes after vitrification. J. Reprod. Fertil. 87, 479–483.
Niemann, H., 1991. Cryopreservation of ova and embryos from livestock: current status and research needs. Theriogenology 35, 109–123.
Otoi, T., Yamamoto, K., Koyama, N., Tachikawa, S., Suzuki, T., 1998. Cryopreservation of mature bovine oocytes by vitrification in straws. Cryobiology 37, 77–85.
Palasz, A.T., Mapletoft, R.J., 1996. Cryopreservation of mammalian embryos and oocytes: recent advances. Biotech. Adv. 14, 127–149.
Park, S., Son, W., Lee, S., Lee, K., Ko, J., Cha, K., 1997. Chromosome and spindle configurations of human oocytes matured in vitro after cryopreservation at the germinal vesicle stage. Fertil. Steril. 68, 920–926. Pickering, S.J., Braude, P.R., Johnson, M.H., Cant, A., Currie, J., 1990. Transient cooling to room temperature
can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertil. Steril. 54, 102–108. Schroeder, A.C., Champlin, A.K., Mobraatin, L.E., Eppig, J.J., 1990. Developmental capacity of mouse oocytes
cryopreserved before and after maturation in vitro. J. Reprod. Fertil. 89, 43–50.
Shaw, P.W., Fuller, B.J., Bernard, A., Shaw, R.W., 1991. Vitrification of mouse oocytes: improved rates of survival, fertilization and development. Mol. Reprod. Dev. 29, 373–378.
Van der Elst, J., Van den Abbeel, E., Jacobs, R., Wisse, E., Van Steirtghem, A., 1988. Effect of 1,2-propanediol and dimethylsulphoxide on the meiotic spindle of the mouse oocyte. Hum. Reprod. 3, 960–967.
Van der Elst, J., Nerinckx, S., Van Steirteghem, A.C., 1992. In vitro maturation of mouse germinal vesicle-stage oocytes following cooling, exposure to cryoprotectants, and ultrarapid freezing: limited effect on the morphology of the second meiotic spindle. Hum. Reprod. 7, 1440–1446.