The effect of carotenoids on the expression of cell surface
adhesion molecules and binding of monocytes to human aortic
endothelial cells
K.R. Martin
1, D. Wu, M. Meydani *
Vascular Biology Program,Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts Uni6ersity,711Washington Street,Boston,
MA02111,USA
Received 19 November 1998; received in revised form 4 June 1999; accepted 20 August 1999
Abstract
Several large epidemiological studies have shown a correlation between elevated plasma carotenoid levels and decreased risk of cardiovascular disease (CVD). One proposed mechanism for the beneficial effect of carotenoids is through functional modulation of potentially atherogenic processes associated with the vascular endothelium. To test this, we incubated confluent human aortic endothelial cell (HAEC) cultures (passages 4 – 8) for 24 h with each of the five most prevalent carotenoids in human plasma, which area-carotene,b-carotene,b-cryptoxanthin, lutein, and lycopene, at an approximate concentration of 1mmol/l. Carotenoids were solubilized in 0.7% (v/v) tetrahydrofuran and incorporated into FBS before adding to cell culture medium. Due to disparate solubilities in aqueous medium, final concentrations ofa-carotene,b-carotene,b-cryptoxanthin, lutein, and lycopene were 1.7, 1.1, 0.7, 0.9, and 0.3mmol/l and monolayers accumulated 647, 158, 7, 113, and 9 pmol/mg protein, respectively. Monolayers were then stimulated with IL-1b (5 ng/ml) for 6 h with subsequent determination of cell surface expression of adhesion molecules as measured by an enzyme-linked immunosorbent assay (ELISA). To assess endothelial cell adhesion to monocytes, IL-1b-stimulated monolayers were incubated for 10 min with51Cr-labeled U937 monocytic cells and adhesion determined by isotope counting.
Pre-incubation of HAEC with b-carotene, lutein and lycopene significantly reduced VCAM-1 expression by 29, 28, and 13%, respectively. Pre-incubation with b-carotene and lutein significantly reduced E-selectin expression by 38 and 34%, respectively. Pre-treatment with b-carotene, lutein and lycopene significantly reduced the expression of ICAM-1 by 11, 14, and 18%, respectively. While other carotenoids were ineffective, lycopene attenuated both IL-1b-stimulated and spontaneous HAEC adhesion to U937 monocytic cells by 20 and 25%, respectively. Thus, among the carotenoids, lycopene appears to be most effective in reducing both HAEC adhesion to monocytes and expression of adhesion molecules on the cell surface. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Carotenoids; Lycopene; Adhesion molecule; Endothelial cell; U937
www.elsevier.com/locate/atherosclerosis
1. Introduction
Cardiovascular disease (CVD) remains a leading cause of morbidity and mortality in the United States and atherosclerosis is a key factor in the pathogenesis of myocardial and cerebral infarction, gangrene, and loss of function in the extremities [1]. Although
numer-ous risk factors contribute to CVD, their presence is only partially responsible for the incidence of CVD. A growing body of literature supports the hypothesis that increased consumption of fruits and vegetables may substantially reduce the incidence of atherogenesis and alleviate its underlying pathologic condition [2,3]. Re-sults of epidemiological studies are generally consistent, showing a protective association between plant-derived antioxidants, such as carotenoids, and CVD [4,5].
Carotenoids are a family of naturally occurring plant pigments that were initially studied due to their role as retinoid precursors. However, carotenoids have now been shown to function in immunomodulation, gap * Corresponding author. Tel.: +1-617-5563126; fax: +
1-617-5563344.
E-mail address:meydani –[email protected] (M. Meydani) 1Present address: Laboratory of Transgenic Carcinogenesis,
Na-tional Institute of Environmental Health Sciences, NaNa-tional Institutes of Health, Research Triangle Park, NC.
junction communication, induction of carcinogen-me-tabolizing enzymes, and photoprotection, in addition to their antioxidant properties [6]. More than 600 distinct carotenoids have been isolated from natural sources and among these approxim. 50 are components of the US diet, with identification of 21 carotenoids in human plasma [7]. There are five well-characterized carotenoids in most blood samples comprising approximately 90% of the total plasma pool of carotenoids. These are
a-carotene, b-carotene, b-cryptoxanthin, lutein and ly-copene [8].
Regulated expression of numerous cell surface adhe-sion molecules is a critical event in the binding of normally non-thrombogenic circulating leukocytes such as the monocyte to the aortic endothelial surface and is one of the earliest detectable events in human and experimental atherosclerosis [9,10]. The subsequent transendothelial migration of these adherent leukocytes, their accumulation in the aortic intima, transformation of monocytes into lipid-engorged foam cells, and secre-tion of cytokines and growth factors are important events in the initiation and progression of atheroscle-rotic plaques [11]. Cell surface adhesion molecules are important regulators of direct cell – cell interactions; inflammatory responses in atherogenesis are directed by regulation and expression of these molecules [12]. Ad-hesion molecule expression in low-density lipoproteins (LDL) receptor-knockout mice fed atherogenic diets showed a significantly reduced incidence of fatty streaks supporting a role for adhesion molecules in atherogene-sis [13]. Constituents of each of the main families of adhesion molecules are involved in the interactions of endothelial cells (EC) and immune cells. Intercellular adhesion molecule (ICAM)-1 and -2 (CD54 and CD102) and vascular cell adhesion molecule (VCAM)-1, (CD106) are members of the immunoglobulin super-family expressed on EC. Of these, ICAM-1 and VCAM-1 increase in response to various inflammatory cytokines. E-Selectin and P-selectin (CD62E and CD62P) on EC also play an early role in adhesion between these two cell types. ICAM-1 and -2 bind to the LFA-1 counterligand; VCAM-1 binds to VLA-4; the selectins recognize certain carbohydrate structures on opposing cells [14,15].
Oxidative stress and expression of adhesion molecules on vascular EC are considered to be impor-tant features in the pathogenesis of atherosclerosis and other inflammatory diseases [16,17]. Studies suggest a molecular linkage between an antioxidant-sensitive transcriptional regulatory mechanism and expression of adhesion molecule genes that expands on the notion of oxidative stress as an important regulatory signal in the pathogenesis of atherosclerosis [18]. In the inflamma-tory response of the arterial wall, leukocyte recruitment to the endothelium is mediated by the interaction of adhesion molecule receptors expressed on the surface of EC and immune cells.
While the pathogenic oxidation of LDL is currently one postulated mechanism for the etiology of atherosclerosis, the role of dietary antioxidants in sup-pressing the deleterious oxidation of LDL has produced conflicting results, suggesting that other factors and possibly alternate mechanisms are involved. The spe-cific contribution of carotenoids in the atherosclerosis process is inconclusive since carotenoids have been shown to protect LDL in some studies but not in others. In fact, the extent of in vitro LDL oxidation and protection by an antioxidant may not indicate in vivo atherosclerotic status as well as was recently em-phasized [19]. However, in one studyb-carotene supple-mentation in rabbits retarded aortic lesion formation [20]. Also, in a more recent study b-carotene supple-mentation in combination with vitamins E and C re-duced aortic valve lesion formation in LDL receptor-knockout mice [21], which may indicate a po-tential effect by carotenoids in the final pathological outcome. Our contention is that carotenoids may act via alternate mechanisms within the endothelial cell to modulate adhesion molecule expression and thus reduce subsequent leukocyte binding. As a result, the focus of this research was to examine in vitro the effect of the five most prevalent plasma carotenoids on expression of key adhesion molecules involved in the atherosclerosis process, and determine the subsequent binding of U937 monocytic cells when carotenoids are incorporated into human aortic endothelial cells (HAEC). Our results indicate that among the test carotenoids, lycopene ap-pears to be the most effective in reducing immune and endothelial cell interaction.
2. Materials and methods
2.1. Human aortic endothelial cell and U937 cell cultures
HAEC were purchased from Clonetics Laboratories (San Diego, CA) and cultured in M-199 medium (Gibco, Grand Island, NY). Culture medium contained 10% fetal bovine serum (FBS) (Sigma, St. Louis, MO), 5 mg/ml EC-derived growth factor, 100 U/ml heparin, 100 U/ml penicillin, 100 U/ml streptomycin, and 1.25
Willebrand factor antigen using immunofluorescent mi-croscopy. Cells were grown to confluence in a humi-dified atmosphere of 95% air and 5% CO2. Following
treatment of HAEC with 0.05% trypsin for 1 – 2 min or until 80% of the cells were detached, cell viability was determined by trypan blue exclusion and expressed as the percent of cells that excluded the dye. The mono-cytic U937 cell line was originally derived from a human histiocytic lymphoma and was procured from American Tissue Type Collection (Rockville, MD). U937 cells were seeded at 5×105
cells/ml, grown in suspension culture in RPMI-1640 medium containing 10% FBS, 100 U/ml penicillin, and 100 U/ml strepto-mycin, and routinely subcultured at a 1:5 ratio two times per week.
Confluent HAEC grown in 24-well plates were sup-plemented with carotenoids by incubating them in medium alone or containing 1 mmol/l a-carotene,
b-carotene, b-cryptoxanthin, lutein or lycopene for 24 h. Based on our pilot dose response studies and the absence of any apparent pro-oxidant effects, we se-lected 1 umol/l as a biologically relevant test concentra-tion. Following supplementation, supernatant was removed, HAEC were washed with phosphate buffered saline (PBS), and medium alone or containing 5 ng/ml human recombinant interleukin (IL)-1b (Pharmingen, San Diego, CA) was added to wells for 6 h to activate cells. Expression of adhesion molecules and adhesion of U937 monocytes were determined (see below).
2.2. Carotenoid supplementation
Stock solutions of carotenoids containing 5 mmol/l were prepared in absolute ethanol and stored under a nitrogen blanket in amber tubes at -70°C. To supple-ment the culture media with carotenoids, the required amount of carotenoid was transferred from stock solu-tion and dried under nitrogen. Carotenoids were redis-solved in tetrahydrofuran (THF) to achieve a final concentration of 0.7% (v/v) in the culture medium. Carotenoids were then mixed with aliquots of FBS and incubated at 37°C for 15 min with gentle mixing by inversion every 5 min. The HAEC were incubated for 24 h in medium containing vehicle or the indicated carotenoid incorporated into the FBS.
2.3. Carotenoid determination
Cellular association of test carotenoids by HAEC and concentrations in media were measured by high performance liquid chromatography (HPLC). Briefly, 2
mg echinenone (gift from Hoffmann-La Roche, Nutley, NJ) was added to homogenates of HAEC cells or media as an internal standard. Aliquots (0.5 ml) of HAEC homogenates were saponified in the presence of 2% pyrogallol (Sigma) at 70oC for 30 min after addition
of 30% KOH (0.1 ml). After extraction of cells or medium with hexane containing 0.02% butylated hy-droxytoluene (BHT), the samples were dried under nitrogen and reconstituted in absolute ethanol (0.04 ml). Carotenoid peaks were separated according to the method of Yeum et al. [22] using a Pecosphere C18 reverse phase column (0.46×8.3 cm×3 mm) protected by a Perkin-Elmer (Norwalk, CT) guard column (0.46×3.3 cm×3mm). The mobile phase was acetoni-trile-tetrahydrofuran-water (50:20:30: v/v/v, solvent A, and 50:44:6, v/v/v, solvent B, with 1% ammonium acetate in water). The gradient procedure at a flow rate of 1 ml/min was as follows: 60% solvent A and 40% solvent B were used for 1 min followed by a 9 min linear gradient to 83% solvent B, a 4 min hold at 83% solvent B, then a 2 min linear gradient to 100% solvent B, a 2 min hold at solvent B, and finally a 2 min gradient back to 60% solvent A and 40% solvent B. The HPLC system was equipped with a Waters 490E pro-grammable multiwavelength detector (Waters Corpora-tion, Milford, MA) with the wavelength set at 450 nm; peaks were integrated using the Waters Millenium chro-matography system.
2.4. Antibodies
Purified mouse anti-human monoclonal antibodies (Pharmingen) were used to quantify the expression of VCAM-1. Mab 51-10C9 VCAM-1 (CD 106; mouse IgG1, k) reacts with the 110 kD glycoprotein of VCAM-1 (CD106), also known as INCAM-110, that is expressed at high levels on the surface of cytokine-stim-ulated endothelium. Mab 68-5H11 reacts with the 97 – 115 kD cell surface glycoprotein E-selectin (CD62E) also known as endothelial-leukocyte adhesion molecule-1 (ELAM-molecule-1) that is expressed on cytokine-stimulated endothelium and is thought to be involved in the early interaction of leukocytes with inflamed endothelium. Mab HA58 reacts with the 85 – 110 kD integral mem-brane glycoprotein ICAM-1 (CD54) that is expressed on EC and both resting and activated lymphocytes and monocytes.
2.5. Enzyme-linked immunosorbent assay (ELISA) for adhesion molecule expression
Endothelial cells were cultured in 96-well plates (3.2×103cells/200ml/well) until confluent, as described
E-se-lectin (2.5mg/ml) or ICAM-1 (1mg/ml) for 2 h at 25°C. After thorough washing, horseradish peroxidase-conju-gated goat anti-mouse antibody (Bio-Rad, Hercules, CA) was diluted and added to the fixed monolayers. After 1 h, cultures were washed and peroxide substrate (Bio-Rad, Hercules, CA) added for 30 min with subse-quent addition of stop solution (48%N,N -dimethylfor-mamide containing 20% SDS). After color development, absorbances were determined at 405 nm to estimate relative adhesion molecule expression.
2.6. Assay for U937 monocytic adhesion to HAEC
Adhesion of U937 cells to HAEC cultures was deter-mined as previously described [23,24]. Briefly, HAEC were plated into 24-well plates and grown to confluence before experimental use. At 100% confluence, M199 alone or containing the indicated concentration of carotenoid was added, and the monolayers were incu-bated for 24 h. After washing to remove unincorpo-rated carotenoid, M199 alone or containing IL-1b (5 ng/ml) was added for 6 h at 37°C. U937 cells (107) were
labeled for 60 min at 37°C with 100mCi51Cr as sodium
chromate (New England Nuclear, Boston, MA) in 1 ml RPMI-1640 containing 10% FBS. The labeled cells were washed by centrifugation, resuspended in RPMI-1640, and 105
viable cells were added per well after washing the HAEC monolayers to remove cytokine. After 10 min at 37°C to allow binding, non-adherent
51Cr-U937 cells were removed by repeated washes with
PBS, and the cells (HAEC and bound U937) lysed by addition of 0.5 ml harvest solution (PBS containing 0.1% SDS and 1 mM EDTA). After mechanical disrup-tion, each solution was transferred to a 5 ml polypropy-lene tube for quantitation of total radiolabel associated with each well by g-ray spectroscopy (Canberra, Syd-ney, Australia). The number of U937 cells bound to endothelial cells was determined from the specific activ-ity (cpm/cell) of the initial cell suspensions and is expressed as the percentage of added cells that are bound to HAEC. Spontaneous release of51
Cr by51
Cr-U937 was less than 5% during the binding phase of the
assay. Experiments were repeated 2 – 3 times with each performed in triplicate or quadruplicate.
2.7. Statistical analysis
Significant differences in the measured parameters between treatment groups were calculated using the unpaired, two-tailed Student’s t-test. Values are pre-sented as means9SEM and P values B0.05 were considered significant.
3. Results
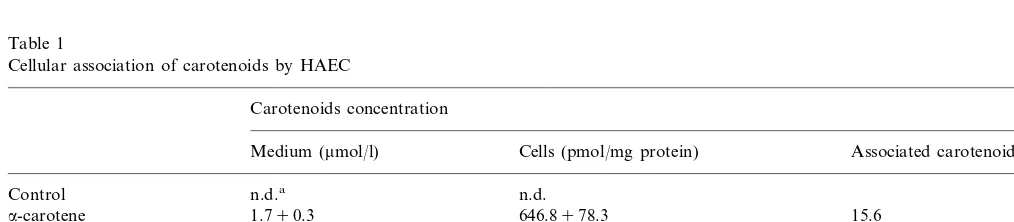
Incubation of HAEC for 24 h with complete cell culture medium containing approx. 1 mmol/l carotenoids increased cellular levels as shown in Table 1. Disparate solubilities due in part to intrinsic chemical characteristics of the carotenoids precluded obtaining equivalent final concentrations of the five test carotenoids in culture medium. However, we were able to incorporate each carotenoid into HAEC to a mea-surable level although there was a 6-fold difference in medium concentrations (Table 1). Cellular association varied over a 74-fold range and from 0.6 – 15.6% of total added carotenoid suggesting in addition to vary-ing solubility, there was concentration-dependent asso-ciation that possibly was also carotenoid- and tissue-specific. In control medium and cell monolayers incubated in unsupplemented medium, carotenoids were not detectable. Pre-incubation with carotenoids did not appear to be cytotoxic to monolayers.
For assessment of the impact of carotenoid incuba-tion on expression of key adhesion molecules, monolay-ers were incubated overnight with the test carotenoids, washed and further incubated with the pro-inflamma-tory cytokine IL-1b (5 ng/ml) for 6 h. Previous studies have shown that IL-1b-stimulated expression of VCAM-1, E-selectin and ICAM-1 is maximal at 6 h and IL-1b at 5 ng/ml is an optimal concentration for inducing expression of most adhesion molecules [25]. Additionally, the cobblestone morphology of quiescent
Table 1
Cellular association of carotenoids by HAEC
Carotenoids concentration
Medium (mmol/l) Cells (pmol/mg protein) Associated carotenoid (%)
n.d. n.d.a
Control
1.790.3
a-carotene 646.8978.3 15.6 1.190.1
b-carotene 153.7916.0 5.6 b-cryptoxanthin 0.790.1 7.491.8 0.6 5.5
Lutein 0.990.2 113.098.5
0.390.1 8.892.1 1.3
Iycopene
Fig. 1. The effect of 24-h incubation witha-carotene (AC),b-carotene (BC),b-cryptoxanthin (CRY), lutein (LUT), and lycopene (LYC) on IL-1b stimulated VCAM-1 adhesion molecule expression. Human aortic endothelial cell (HAEC) cultures were incubated for 24-h in M199 medium alone (CTL) or containing the indicated carotenoid at
1 mmol/l. After washing monolayers with PBS, cells were stimu-lated for 6 h with human recombinant IL-1b(5 ng/ml) and VCAM-1 expression was determined by ELISA as described in Section 2. Values are means9SEM of at least three experiments, each per-formed in triplicate. Asterisks above the bars indicate significant differences between the IL-1bstimulated CTL and each IL-1b stimu-lated carotenoid treatment group. Differences were significant (PB
0.05) between unstimulated control (CTL without cross-bar) and IL-1bstimulated (CTL with cross-bar) although for clarity no aster-isk is presented. There were no significant differences between unstim-ulated carotenoid treatment groups.
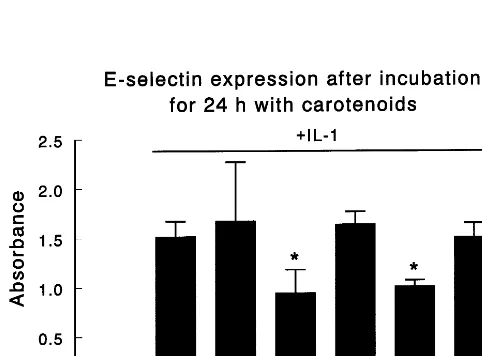
HAEC became spindle-shaped after IL-1b exposure, which is typical of activated endothelium. Basal VCAM-1 expression was low but increased 8.2-fold after incubation with IL-1b (Fig. 1). Pre-incubation withb-carotene, lutein, and lycopene significantly (PB 0.05) reduced VCAM-1 expression by 29, 28 and 13%, respectively. However, neither a-carotene nor b -cryp-toxanthin affected IL-1b-stimulated expression of this molecule. Stimulation of HAEC by IL-1b increased E-selectin expression by 14.7-fold above basal level (Fig. 2). Pre-incubation with b-carotene and lutein sig-nificantly reduced E-selectin expression by 38 and 34%, respectively. However, neither lycopene nor a-carotene or b-cryptoxanthin markedly affected IL-1b-stimulated expression of this adhesion molecule. HAEC have a high constitutive expression of ICAM-1 and IL-1b
treatment further increased expression by 50% (Fig. 3). Similar to VCAM-1, the expression of ICAM-1 was significantly reduced by 11, 14, and 18% in monolayers pre-incubated with b-carotene, lutein and lycopene, re-spectively. Neithera-carotene norb-cryptoxanthin had any impact on ICAM-1 expression. Carotenoids did not affect constitutive expression of adhesion molecules on the surface of HAEC (Fig. 4).
Since upregulated expression of several key adhesion molecules including VCAM-1, E-selectin and ICAM-1 are integral in promoting adhesion of monocytes to the vascular endothelium, we next incubated HAEC with
51Cr-U937 human monocytic cells to determine if
Fig. 3. The effect of 24-h carotenoid pre-treatment on IL-1b stimu-lated ICAM-1 adhesion molecule expression in HAEC. Cultures were incubated and treated as described in Fig. 1 legend using murine anti-human monoclonal antibody to ICAM-1. Values are means9
SEM from at least three experiments, each performed in triplicate. Asterisks above the bars indicate significant differences between the IL-1bstimulated CTL and each IL-1bstimulated carotenoid treat-ment group. Differences were significant (PB0.05) between unstimu-lated control (CTL without cross-bar) and IL-1bstimulated (CTL with cross bar) although for clarity no asterisk is presented. There were no significant differences between unstimulated carotenoid treat-ment groups.
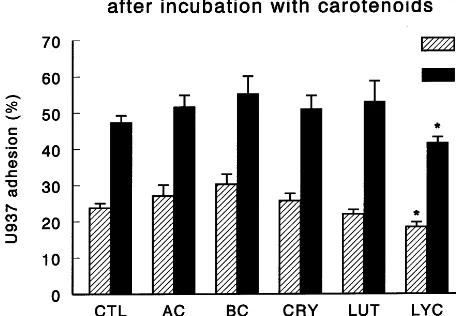
Fig. 4. The effect of 24-h incubation with carotenoids on51Cr-U937
human monocyte adhesion to unstimulated and IL-1b stimulated HAEC monolayers. Cultures were incubated and treated with IL-1b as described in the Fig. 1 legend. After stimulation, monolayers were washed with PBS, and co-incubated with viable 51Cr-labeled U937
cells (105/well) for 10 min at 37°C. Non-adherent cells 51Cr-U937
were removed by repeated washes with PBS, and radioactivity in lysed HAEC-U937 cells measured in each well. Data represent the percentage of initial monocytes added to wells that remained attached with the monolayer. Values are means9SEM from at least two experiments, each performed in triplicate. Asterisks above the bars indicate significant differences (PB0.05) between CTL and groups preincubated with the indicated carotenoid. Differences were signifi-cant (PB0.05) between unstimulated and stimulated control al-though for clarity no asterisk is presented.
prevent LDL oxidation, although studies have shown conflicting results [30,31]. However, one study showed that b-carotene supplementation reduced fatty lesion formation in rabbits [20]. More recently, b-carotene, in combination with vitamins E and C, was shown to reduce aortic valve lesion formation in an LDL recep-tor-knockout mouse model [21]. In our study, we inves-tigated the effect not only ofb-carotene and but also of other major carotenoids commonly present in human plasma on endothelial cell function and the resultant modulation of interactions between immune cells and EC, an event associated with development and progres-sion of atherosclerosis.
To this end, we supplemented HAEC with the five most prevalent carotenoids in human plasma and found that lycopene, relative to the other test carotenoids, was the most effective in reducing adherence of HAEC to U937 cells and suppressing expression of two molecules involved in cellular adhesion. Relative to its molar concentration in EC, lycopene reduced VCAM-1 ex-pression in IL-1b stimulated cells approx. 6-fold more than lutein and 8-fold more thanb-carotene. It was also 16-fold better than lutein and 25-fold better than b -carotene in reducing ICAM-1 expression in HAEC. ICAM-1 mediates the attachment and spreading of monocytes, and VCAM-1 preferentially contributes to firm monocyte adhesion [32,33]. Lycopene was the only test carotenoid that reduced HAEC adherence to U937 cells in both activated and inactivated states. Interest-ingly, the lycopene concentration in HAEC was signifi-cantly lower than other carotenoids, yet it had the greatest impact on both adhesion of monocytes and expression of adhesion molecules.
The final concentration of carotenoids delivered to tissue culture medium after dissolution in THF [34] and incorporation into FBS ranged from 0.3 – 1.7 mmol/l. The a-carotene concentration was 10-fold higher than concentrations of b-carotene, lutein and b -cryptoxan-thin, and was approximately twice the concentration normally found in plasma of healthy adults [35]. The level of lycopene in culture medium, however, was similar to normal plasma levels. Lycopene is the major carotenoid in human plasma and comprises 40% of the total plasma pool with a concentration of 0.2 – 1.1
mmol/l [36,37]. Dietary lycopene occurs predominantly in tomatoes and tomato products. Intake is approx. 3 mg/d in the US [38].
The low concentration of lycopene in HAEC was due in part to insolubility of carotenoids in media and to inherent physical characteristics of carotenoids as sug-gested by Britton [39]. Increasing carotenoid concentra-tions in culture medium required larger volumes of organic solvent vehicle, viz., THF, which caused cytotoxicity.
Endothelial cells recruit leukocytes by selectively ex-pressing three types of adhesion molecules in response carotenoids modulate HAEC adhesion to these cells.
The basal level of 51Cr-U937 binding to unstimulated
HAEC plateaued at 24% and was not markedly af-fected by pre-incubation with b-carotene, a-carotene,
b-cryptoxanthin or lutein. However, pre-incubation of unstimulated cultures with lycopene significantly re-duced 51Cr-U937 binding by 18%. Stimulation of
HAEC with IL-1b significantly increased binding two-fold to 47% and pre-incubation with lycopene signifi-cantly reduced51
Cr-U937 binding to HAEC by 20%. In contrast, pre-incubation of HAEC with b -cryptoxan-thin, a-carotene, b-carotene or lutein did not affect IL-1b-stimulated binding to the HAEC monolayer. Binding of 51Cr-U937 to plastic was less than 6% and
viability of 51Cr-U937 was \95%.
4. Discussion
to inflammatory stimuli such as IL-1b [40]. Pro-inflam-matory cytokines are commonly found in atherogenic lesions, and can induce chemotactic factors, other cy-tokines, and adhesion molecules which collectively con-tribute to the inflammatory process [41]. As a result, we focused on the impact of cell-associated carotenoids and the expression of key adhesion molecules often coupled with atherosclerotic events. We found that three of the five tested carotenoids, viz., b-carotene, lutein, and lycopene, significantly reduced both VCAM-1 and ICAM-VCAM-1 expression in IL-VCAM-1b-stimulated HAEC cultures. Whileb-carotene and lutein significantly sup-pressed E-selectin expression as well, lycopene had no impact on E-selectin.
Upregulation of adhesion molecule expression by cytokine-induced oxygen radical formation results in part from activation of NF-kB [42,43]. NF-kB is a redox-sensitive transcriptional factor that mediates gene expression in inflammatory, immune and acute phase responses [44]. Modulation of adhesion molecule ex-pression by reactive oxygen radicals and antioxidants has been investigated and evidence supports the role of a redox mechanism in the initiation of atherosclerosis and suggests the potential for dietary intervention with antioxidants [45,46]. Experimental data clearly demon-strate that cellular accumulation of antioxidants includ-ingN-acetylcysteine (NAC), pyrroline dithiocarbamate (PDTC), anda-tocopherol modulate adhesion molecule expression and monocyte adhesion to EC models by blocking activation of NF-kB when stimulated by cy-tokines, phorbol esters, hydrogen peroxide and lipo-polysaccharide [18,47,48]. A number of diverse compounds including aspirin, isoflavones, flavonoids, phytoalexins, and antibiotics have also been shown to reduce both endothelial adhesion molecule expression and subsequent leukocyte binding [49 – 51]. Several po-tentially non-antioxidant mechanisms have been pro-posed for these and other compounds including inhibition of protein tyrosine kinases [49], inhibition of proteasomes [52], and decreased degradation of Ikb [53], as well as modulation of cyclic AMP levels [50] and protein kinase C activity [54]. Additionally, it has been reported theVCAM-1, ICAM-1, and E-selectin may share a common regulatory signal immediately after receptor activation by receptor inducers, but ICAM-1 and E-selectin gene expression may be modified by gene-specific signal transduction mecha-nisms [45].
Carotenoids have been reported to function in im-munomodulation, cellular gap junction communication, enzyme induction, and as retinoid precursors [8,39]. In addition to forming potentially bioreactive retinoids, carotenoids may be metabolized to a number of com-pounds through central and/or excentric cleavage to form apocarotenals, as well as forming oxidative metabolites [6,7].
In our studies, cellular association was readily de-tectable in HAEC over a range consistent with values reported in the literature for several mammalian cell types, including hepatoma cells [55], human hepatocytes [56], and buccal mucosal cells [57]. Carotenoids have been detected in lipid-rich atherosclerotic lesions, and in particular b-carotene and lycopene, although their concentrations have been reported to be low [58]. The elevated level of cellulara-carotene suggests concentra-tion-dependent association which may be tissue-specific, and may depend on properties of a-carotene as well. Expression of endothelial adhesion molecules may reflect endothelial activation, although leukocyte adhe-sion may not be substantially altered [59]. Our results indicate that b-carotene and lutein modulate adhesion molecule expression, but these two carotenoids did not reduce binding of monocytes to EC. In contrast, ly-copene, or one of its metabolites, significantly reduced monocyte binding to both unstimulated and IL-1b
stimulated HAEC suggesting a structure-specific mech-anism of action [7]. Numerous studies have demon-strated variable patterns of adhesion molecule expression and differing effects on monocyte binding when antioxidants were incorporated into cells. Faruqi et al. [60] and Vandermeeren et al. [47] found that incubation of EC with a-tocopherol or dimethylfu-marate significantly reduced both adhesion molecule expression, viz., VCAM-1, E-selectin and ICAM-1, and U937 binding. However, Weber et al. [61] demonstrated that PDTC and NAC dose-dependently reduced cy-tokine-induced VCAM-1, but not ICAM-1, expression in HUVEC, although PDTC decreased adhesion of U937 cells to EC. Furthermore, Faruqi et al. [60,62] reported that the antioxidants a-tocopherol, probucol and NAC inhibited binding of U937 to EC which correlated with E-selectin expression, but other antioxi-dants including ascorbic acid, BHT, and desferral did not inhibit binding. Conversely, Marui et al. [18] showed that in IL-1b stimulated EC, VCAM-1 expres-sion was reduced 90% by PDTC and NAC, but ICAM-1 and E-selectin were insensitive to antioxidant inhibition. Erl et al. [63] reported that IL-1b-stimulated expression of VCAM-1 and E-selectin in EC, but not ICAM-1, was inhibited by a-tocopherol succinate in a time- and dose-dependent manner. Vanhee et al. [40] demonstrated that stimulation of EC with cytokine increased expression of ICAM-1 but did not affect VCAM-1 or E-selectin, which resulted in increased binding of U937 to EC presumably through an ICAM-1 adhesion pathway. Thus, cytokine- and cell-specific induction of adhesion molecules and subsequent bind-ing of U937 appear to be modulated in an antioxidant-specific manner.
suggests that carotenoids are located in precise loca-tions and orientaloca-tions in cellular structures, and their physicochemical properties are strongly influenced by neighboring molecules. Lutein and lycopene possess exceptionally high antioxidant activity compared to other carotenoids [7] and in fact lycopene possesses the greatest physical quenching capacity [64]. Woodall et al. [65] have shown that lycopene with its opened rings and extended conjugated diene system reacts rapidly with oxidizing agents suggesting key function as an in vivo antioxidant.
Lycopene is one of the major carotenoids in the diet of North Americans and Europeans. Epidemiological studies have suggested that intake of lycopene, in addi-tion to the prevenaddi-tion of cancers of the prostate or gastrointestinal tract [38,66,67], may be also associated with reduced risk of CVD. The recent European study (EURAMIC multicenter study) evaluated the associa-tion between adipose tissue concentrations of carotenoids and the risk of myocardial infarction [4]. A significant inverse association for lycopene alone and acute myocardial infarction was evident [68]. Most recently, a population-based case-control study in Costa Rica also reported that a lower adipose content of lycopene was associated with increased risk of my-ocardial infarction after adjustment for environmental factors such as smoking, rural area residence, and low socioeconomic status [5]. Although many epidemiologi-cal studies support the hypothesis that diets rich in carotenoid-containing fruits and vegetables are associ-ated with reduced coronary heart disease, minimal fo-cus has been given to lycopene regarding its role in preventing CVD.
Our in vitro data are in concurrence with the findings of these studies in that among the carotenoids tested, only lycopene modulated adhesion molecule expression and reduced binding of U937 monocytes suggesting a pivotal role for lycopene in attenuating atherogenesis. As a result, further elucidation of the metabolic and functional contributions of lycopene and/or its metabo-lites in the vascular endothelium are necessary to assess potential protective roles.
Acknowledgements
This material is based upon work supported by the US Department of Agriculture, under agreement No. 58-1950-9-001. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Department of Agriculture. The authors would like to thank Joanne Meegan for preparation of this manuscript.
References
[1] Ross R. The pathogenesis of atherogenesis: a perspective for the 1990’s. Nature 1993;362:801 – 9.
[2] Hininger I, Chopra M, Thurnham DI, Laporte F, Richard MJ, Favie RA, Roussel AM. Effect of increased fruit and vegetable intake on the susceptibility of lipoprotein to oxidation in smok-ers. Euro J Clin Nutr 1997;51:601 – 6.
[3] O’Keefe JH Jr, Lavie CJ Jr, McCallister BD. Insights into the pathogenesis and prevention of coronary artery disease. Mayo Clin Proc 1995;70:69 – 79.
[4] Kohlmeier L, Hastings SB. Epidemiologic evidence of a role of carotenoids in cardiovascular disease prevention. Am J Clin Nutr 1995;62:1370S – 6S.
[5] Campos H, Siles X. Low b-cryptoxanthin and lycopene carotenoid concentrations in adipose tissue are associated with increased risk of myocardial infarction. Circulation 1998;98:I – 374.
[6] Wang X-D. Absorption and metabolism of b-carotene. J Am Coll Nutr 1994;13:315 – 25.
[7] Khachik F, Beecher GR, Jr JCS. Lutein, lycopene, and their oxidative metabolites in chemoprevention of cancer. J Cell Biochem 1995;22:236 – 46.
[8] Thurnham DI. Carotenoids: functions and fallacies. Proc Nutr Soc 1994;53:77 – 87.
[9] Cybulsky MI, Gimbrone MA Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherosclero-sis. Science 1991;251:788 – 91.
[10] Li H, Cybulsky MI, Gimbrone MA, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononu-clear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb 1993;13:197 – 204.
[11] Campbell JH, Campbell GR. Cell biology of atherosclerosis. J Hypertens 1994;12:S129 – 30.
[12] Walpola PL, Gotlieb AI, Cybulsky MI, Langille BL. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler Thromb Vasc Biol 1995;15:2 – 10.
[13] Nageh M, Sandberg ET, Marotti KR, Lin AH, Melchior EP, Bullard DC, Beaudet AL. Deficiency of inflammatory cell adhe-sion molecules against atherosclerosis in mice. Arterioscler Thromb Vasc Biol 1997;17:1517 – 20.
[14] Cartwright JE, Whitley GSJ, Johnstone AP. The expression and release of adhesion molecules by human endothelial cells and their consequent binding of lymphocytes. Exp Cell Res 1995;217:329 – 35.
[15] Jang Y, Lincoff AM, Plow EF, Topol EJ. Cell adhesion molecules in coronary artery disease. J Am Coll Cardiol 1994;24:1591 – 601.
[16] Hennig B, Toborek M, McClain CJ, Diana JN. Nutritional implications in vascular endothelial cell metabolism. J Am Coll Nutr 1996;15:345 – 58.
[17] Alexander RW. Theodore Cooper Memorial Lecture. Hyperten-sion and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspec-tive. Hypertension 1995;25:155 – 61.
[18] Marui N, Offermann MK, Swerlick R, Kunsch C, Rosen CA, Ahmad M, Alexander RW, Medford RM. Vascular adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest 1993;92:1866 – 74. [19] Fruebis J, Bird DA, Pattison J, Palinski W. Extent of
[20] Shaish A, Daugherty A, O’Sullivan F, Schonfeld G, Heinecke JW. B-caroteneinhibits atherosclerosis in hypercholesterolemic rabbits. J Clin Invest 1995;96:2075 – 82.
[21] Crawford RS, Kirk EA, Rosenfled ME, LeBoeuf RC, Chait A. Dietary antioxidants inhibit development of fatty streak lesion in the LDL receptor-deficient mouse. Artherioscler Thromb Vasc Biol 1998;18:1506 – 13.
[22] Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI, Russell RM. Human plasma carotenoid response to the inges-tion of controlled diets high in fruits and vegetables. Am J Clin Nutr 1996;64:594 – 602.
[23] Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer 1976;17:565 – 77.
[24] DiCorletto PE, De la Motte CA. Characterization of the adhe-sion of the human monocytic cell line U937 to cultured endothe-lial cells. J Clin Invest 1985;75:1153 – 61.
[25] Introna M, Mantovani A. Early activation signals in endothelial cells: Stimulation by cytokines. Arterioscler Thromb Vasc Biol 1997;17:423 – 8.
[26] Hennekens CH, Buring JE, Manson JE. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. New Engl J Med 1996;334:1145 – 9.
[27] Omenn GS, Goodman GE, Thronquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. New Engl J Med 1996;334:1150 – 5.
[28] Serdula MK, Byers T, Mokdad AH, Simoes E, Mendlein JM, Coates RJ. The association between fruit and vegetable intake and chronic disease risk factors. Epidemiol 1996;7:161 – 5. [29] Gillman MW, Cupples LA, Gagnon D, Posner BM, Ellison RC,
Castelli WP, Wolf PA. Protective effect of fruits and vegetables on development of stroke in men. J Am Med Assoc 1995;273:1113 – 7.
[30] Witzum JL. The oxidative hypothesis of atherosclerosis. Lancet 1994;344:793 – 5.
[31] Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherosclerosis. Free Rad Biol Med 1996;20:707 – 27.
[32] Bevilacqua MP, Nelson RM, Mannori G, Cecconi O. Endothe-lial-leukocyte adhesion molecules in human disease. Ann Rev Med 1994;45:361 – 78.
[33] Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 1991;67:1033 – 6. [34] Cooney RV, Kappock TJ, Pung A, Bertram JS. Solubilization,
cellular uptake and activity ofb-carotene and other carotenoids as inhibitors of neoplastic transformation in cultured cells. Meth-ods Enzymol 1993;214:55 – 68.
[35] Fotouhi N, Meydani M, Santos MS, Meydani SN, Hennekens CH, Gaziano JM. Carotenoids and tocopherol concentration in plasma, peripheral blood mononuclear cells and red blood cells following long termb-carotene supplementation in men. Am J Clin Nutr 1996;63:553 – 8.
[36] Johnson E. Human studies on bioavailability and plasma re-sponse of lycopene. Proc Soc Exp Biol Med 1998;218:115 – 20. [37] Stahl W, Sies H. Lycopene : a biologically important carotenoid
for humans? Arch Biochem Biophys 1996;336:1 – 9.
[38] Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev 1998;56:35 – 51.
[39] Britton G. Structure and properties of carotenoids in relation to function. FASEB J 1995;9:1551 – 8.
[40] Vanhee D, Delneste Y, Lasalle P, Gosset P, Joseph M, Tonnel AB. Modulation of endothelial cell adhesion molecule expression in a situation of chronic inflammatory stimulation. Cell Immunol 1994;155:446 – 56.
[41] Akeson AL, Mosher LB, Woods CW, Schroeder KK, Bowlin TL. Human aortic endothelial cells express the type I but not the type II receptor for interleukin-1 (IL-1). J Cell Physiol 1992;153:583 – 8.
[42] Meier B, Radeke HH, Selle S, Habermehl GG, Resch K, Sies H. Human fibroblasts release low amounts of reactive oxygen spe-cies in response to the potent phagocyte stimulants, serum-treated zymosan, N-formyl-methionyl-leucyl-phenylalanine, leukotriene B4 or 12-O-tetradecanoylphorbol 13-acetate. Biochem J 1989;263:539 – 45.
[43] Lenardo MJ, Baltimore D. NF-kB: a pleiotropic mediator of inducible and tissue-specific gene control. Cell 1989;58:227 – 9. [44] Satriano JA, Shuldiner M, Hora K, Xing Y, Schlondorff D.
Oxygen radicals as second messengers for expression of the monocyte chemoattractant protein, JE/MCP-1, and the mono-cyte-colony stimulating factor, CSF-1, in response to tumor necrosis factor-alpha and immunoglobulin G. J Clin Invest 1993;92:1564 – 71.
[45] Cominacini L, Garbin U, Pasini AF, Davoli A, Campagnola M, Contessi GB, Pastorino AM, Cascio VL. Antioxidants inhibit expression of intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 induced by oxidized LDL on human umbilical vein endothelial cells. Free Rad Biol Med 1997;22:117 – 27.
[46] Hennig B, Toborek M, Cader AA. Nutrition, endothelial cell metabolism, atherosclerosis. Crit Rev Food Sci Nutr 1994;1994:253 – 82.
[47] Vandermeeren M, Janssens S, Borgers M, Geysen J. Dimethylfu-marate is an inhibitor of cytokine-induced E-selectin, VCAM-1, and ICAM-1 expression in human endothelial cells. Biochem Biophys Res Comm 1997;234:19 – 23.
[48] Schreck R, Reiber P, Baeurle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kB transcription factor and HIV-1. EMBO J 1991;10:2247 – 58.
[49] Ferrero M, Bertelli A, Fulgenzi A, Pellegatta F, Corsi M, Bonfrate M, Ferrara F, De Caterina R, Giovannini L, Bertelli A. Activity in vitro of resveratrol on granulocyte and monocyte adhesion to endothelium. J Clin Nutr 1998;68:1208 – 14. [50] May MJ, Wheeler-Jones CPD, Pearson JD. Effects of protein
kinase inhibitors on cytokine-induced adhesion molecule expres-sion by human umbilical vein endothelial cells. Br J Pharmacol 1996;118:1761 – 71.
[51] Weber C, Erl W, Pietsch A, Weber PC. Aspirin inhibits nuclear factor-kB mobilization and monocyte adhesion in stimulated human endothelial cells. Circulation 1995;91:1914 – 7.
[52] Xia L, Pan J, Yao L, McEver RP. A proteasome inhibitor, an antioxidant, or a salicylate, but not a glucocorticoid, blocks constitutive and cytokine-inducible expression of P-selectin in human endothelial cells. Blood 1998;91:1625 – 32.
[53] Pierce JW, Read MA, Ding H, Luscinskas FW, Collins T. Salicylates inhibit I kappa B-alpha phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmi-gration. J Immunol 1996;1996:3961 – 9.
[54] Rahman A, Bando M, Kefer J, Anwar KN, Malik AB. Protein kinase C-activated oxidant generation in endothelial cells signals intercellular adhesion molecule-1 gene transcription. Mol Phar-macol 1999;55:575 – 83.
[55] Oarada M, Stahl W, Sies H. Cellular levels of all-trans -b-carotene under the influence of 9-cis-b-carotene in FU-5 rat hepatoma cells. Biol Chem 1993;374:1075 – 81.
[56] Martin KR, Failla ML Jr., J.C.S.b-Carotene and lutein protect HepG2 human liver cells against oxidant-induced damage. J Nutr 1996;126:2098 – 106.
[58] Gerster H. The potential role of lycopene for human health. J Am Coll Nutr 1997;16:109 – 26.
[59] Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev 1991;70:427 – 51.
[60] Faruqi R, Motte CDl, DiCorletto PE. Tocopherol inhibits ago-nist-induced monocyte cell adhesion to cultured endothelial cells. J Clin Invest 1994;94:592 – 600.
[61] Weber C, Erl W, Pietsch A, Strobel M, Ziegler-Heitbrock HWL, Weber PC. Antioxidants inhibit monocyte adhesion by suppress-ing nuclear factor-kB mobilization and induction of vascular adhesion molecule-1 in endothelial cells stimulated to generate radicals. Arterioscler Thromb 1994;14:1665 – 73.
[62] Faruqi RM, Poptic EJ, Faruqi TR, De La Motte C, DiCorleto PE. Distinct mechanisms for N-acetylcysteine inhibition of cy-tokine-induced E-selectin and VCAM-1 expression. Am J Phys-iol 1997;273:H817 – 26.
[63] Erl W, Weber C, Wardemann C, Weber PC. Toco-pheryl succinate inhibits monocytic cell adhesion by
sup-pressing NF-kB mobilization. Am J Physiol 1997;273: H634 – 40.
[64] DiMascio P, Kaiser S, Sies H. Lycopene as the most effective biological carotenoid singlet oxygen quencher. Arch Biochem Biophys 1989;274:532 – 8.
[65] Woodall AA, Lee SW-M, Weesie RJ, Jackson MJ, Britton G. Oxidation of carotenoids by free radicals: relationship between structure and reactivity. Biophys Biophys Acta 1997;1336:33 – 42. [66] Giovannucci E, Clinton SK. Tomatoes, lycopene, and prostate
cancer. Proc Soci Exp Biol Med 1988;218:129 – 39.
[67] Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999;91:317 – 331.
[68] Kohlmeier L, Kark JD, Gomez-Gracia E, Martin BC, Steck SE, Kardinaal AMF, Ringstad J, Thamm M, Masaev V, Riemersma R, Martin-Moreno JM, Kok FJ. Lycopene and myocardial infarction risk in the EURAMIC study. Am J Epidemiol 1997;145:618 – 26.
.