Dietary squalene increases cholesterol synthesis measured with
serum non-cholesterol sterols after a single oral dose in humans
Heikki Relas, Helena Gylling, Tatu A. Miettinen *
Department of Medicine,Di6ision of Internal Medicine,Uni6ersity of Helsinki,PO Box340,00029HYKS,Helsinki,Finland Received 25 June 1999; received in revised form 14 October 1999; accepted 2 November 1999
Abstract
Studies considering long-term squalene consumption have revealed no consistent effects on serum cholesterol levels but the immediate effect of dietary squalene on cholesterol synthesis has not been studied. Thus, the effect of a single dose of dietary squalene on postprandial lipid metabolism was studied in 16 male volunteeers aged 22 – 79 years. Two oral fat meals a week apart were administered to every subject, one without (control) and the other with 500 mg of squalene. Lipids, retinyl palmitate, squalene and non-cholesterol sterols were measured at baseline and after 3, 4, 6, 9, 12 and 24 h postprandially in plasma, chylomicron, VLDL and VLDL bottom and, in six randomly chosen subjects, also in IDL, LDL and HDL. In the fasting samples, squalene was mainly transported in LDL and HDL, whereas in squalene-supplemented postprandium most of squalene was carried in the triglyceride-rich lipoproteins. Postprandial squalene and retinyl palmitate curves closely resembled each other. After the squalene-enriched dietary fat load, squalene was significantly increased compared to control fat loads in plasma, chylomicrons, VLDL and IDL. Squalene addition increased significantly lathosterol/campesterol ratio in chylomicrons and VLDL at 12 h and in VLDL bottom at 9 – 12 h, and increased significantly VLDL lanosterol/campesterol ratio at 12 h, indicating enhanced cholesterol synthesis caused by squalene. Plasma plant sterol levels remained unchanged. In conclusion, a single oral dose of squalene representing a potential daily dietary amount increases cholesterol synthesis within 9 – 12 h detected in chylomicrons, VLDL and VLDL bottom. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Squalene; Cholesterol synthesis; Triglycerides; Lathosterol; Methyl sterols; Plant sterols; Postprandial lipids
www.elsevier.com/locate/atherosclerosis
1. Introduction
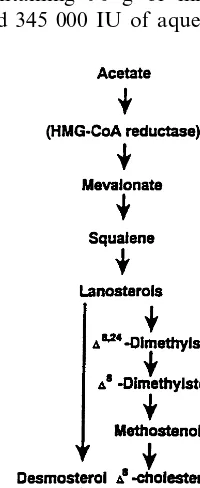
Squalene is a 30-carbon and 50-hydrogen non-steroid intermediate of cholesterol synthesis preceding the for-mation of steroid nucleus. Skin and adipose tissue are rich in squalene in humans, whereas serum contains relatively low levels of squalene [1]. Serum squalene is rapidly equilibrated with the squalene pool in liver [2], and kinetic analyses have shown that serum squalene has a rapid turnover to cholesterol [2 – 6]. In fact, serum squalene concentration has been considered as an indi-cator of cholesterol synthesis rate [7,8] even though not as consistent as the other sterol precursors of choles-terol, i.e. D8-cholestenol, desmosterol and lathosterol [9]. Dietary squalene is readily absorbed such that the
serum concentration reflects also its dietary intake [1,10,11]. Squalene is transported postprandially in chy-lomicrons and chylomicron remnants so that its post-prandial serum concentration can be used to elucidate the rate of fat clearance [12,13]. It has been approxi-mated that, e.g. in the USA, squalene intake averages 100 mg/d [1], but in populations consuming large amounts of olive oil the daily intake can be much higher [1]. High dietary intake of squalene, ranging from 850 to 1000 mg/day, has variable effects on serum cholesterol level ranging from lowered [14] through unchanged [10] to increased values [11]. However, long-term squalene intake increases cholesterol synthesis rate measured with serum cholesterol precursor sterols [10,11] and also methyl sterols [11]. The question now arises what is the effect of a single oral dose of squalene combined with fat on postprandial cholesterol metabolism. Accordingly, we examined changes in cholesterol precursor sterols, indicators of cholesterol
* Corresponding author. Tel.: +358-9-47172220; fax: + 358-9-47174013.
E-mail address:[email protected] (T.A. Miettinen).
synthesis [9], and plant sterols, indicators of cholesterol absorption efficiency [9], during 24 h after an oral fat load with and without squalene.
2. Patients and methods
2.1. Patients
The study group consisted of 16 volunteer males aged 22 – 79 years (56.296.6 years, mean9SE) and body mass index 24.590.5 kg/m2
. One subject had apo-protein (apo) E phenotype 2/3, nine had apo E 3/3, and six subjects apo E 4/3 phenotype. The different apo E phenotype distribution was not a confounding factor, because the comparisons were made intraindividually. The subjects did not have renal, liver or gastrointestinal diseases or any permanent medication except one sub-ject, who had type 2 diabetes well-controlled with glibenclamide over several years.
The study protocol had been accepted by the Ethics Committee of our Department.
2.2. Methods
2.2.1. Postprandial fat clearance
Each subject participated in two oral fat loads in random order, one with and the other without squalene supplement. After a 12-h fast, the subjects were given a fatty meal containing 90 g of milk fat, 432 mg of cholesterol and 345 000 IU of aqueous vitamin A as a
postprandial marker. For the squalene-supplemented test, 500 mg of squalene was added to the fatty meal. The meal was given as a cream-eggshake containing 1200 kcal. After the test meal, the subjects fasted for 9 h, having at 17:00 h their first actual daily meal, which was a low fat, low cholesterol standard hospital meal. Blood samples were drawn before the test meal and after 3, 4, 6, 9, 12 and 24 h. The blood samples were taken into dark heparin-containing tubes.
2.2.2. Analytical procedures
Commercial kits were used to enzymatically analyze serum total and lipoprotein cholesterol and triglycerides (Boehringer Mannheim Diagnostica, Germany). Chy-lomicrons were separated from plasma after carefully overlayering with 1.0063 g/ml NaCl salt solution and by ultracentrifugation in a fixed-angle Beckman® rotor for 30 min, followed by density gradient separation of very low density lipoproteins (VLDL, B1.006 g/ml), intermediate density lipoproteins (IDL, 1.006 – 1.019 g/ ml), low density lipoproteins (LDL, 1.019 – 1.063 g/ml) and high density lipoproteins (HDL, 1.063 – 1.210 g/ml) [15]. Postprandial plasma samples of all subjects were separated into chylomicrons, VLDL and VLDL bottom (\1.006 g/ml), while in six random subjects VLDL bottom was separated into IDL, LDL and HDL.
Serum squalene and noncholesterol sterols D8 -cholestenol, desmosterol, lathosterol and plant sterols, campesterol and sitosterol, and cholestanol, a metabo-lite of cholesterol, were quantitated with gas – liquid chromatography on a 50 m long SE-30 capillary column (Hewlett Packard® Ultra-1) from nonsaponifi-able lipids [16,17]. The quantitations were performed from total plasma, chylomicrons, VLDL and VLDL bottom. Lanosterol, the largest methyl sterol peak, and other methyl sterols of Fig. 1 were quantitated in chylomicrons and VLDL.
Apo E phenotypes were determined with isoelectric focusing from plasma [18]. The analysis of retinyl palmitate was carried out with high pressure liquid chromatography [19]. All procedures were completed in subdued light.
2.2.3. Calculations
Postprandial cholesterol, triglyceride and retinyl palmitate concentrations are given as incremental val-ues calculated by subtracting the respective basal fast-ing value from each postprandial value. Postprandial responses of cholesterol, triglycerides, squalene and retinyl palmitate were also quantitated by calculating the area under the 9-h concentration curve (AUC) for cholesterol and triglycerides and 24-h AUC for squalene and retinyl palmitate for each subject. Areas between the zero level and the respective 9 or 24-h concentration curves were also calculated (AUIC). Serum cholesterol precursor sterols are given as
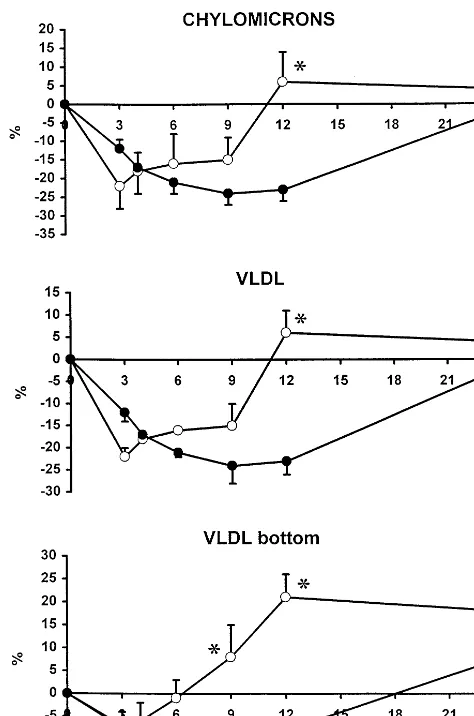
Fig. 2. Postprandial squalene in different lipoprotein fractions. Mean values., squalene in test meal; , no squalene in test meal. (N=16 in chylomicrons and VLDL;N=6 in IDL, LDL and HDL). *PB
0.05 from non-squalene values, analysis of variance and covariance with repeated measures,t-test.
3. Results
Baseline serum lipids were as follows: serum choles-terol 5.1890.23 mmol/l, LDL cholesterol 3.0590.19 mmol/l, HDL cholesterol 1.2690.07 mmol/l and serum triglycerides 1.1890.09 mmol/l. Fasting serum squalene concentration was 163911 mg/dl and was distributed in lipoproteins as follows: 9.592.0% in chylomicrons, 11.891.9% in VLDL, 8.691.2% in IDL, 39.894.2% in LDL and 30.392.2% in HDL. On the contrary, two-thirds of postprandial squalene was carried in triglyceride-rich lipoproteins of density B 1.006 g/ml (Fig. 2).
After supplementation, postprandial squalene AUC (calculated from values in Fig. 2) was significantly increased by 92% to 868% in chylomicrons, VLDL and VLDL bottom compared with the fat load without squalene supplementation (PB0.05 or less for all, Table 1, Fig. 2). In LDL and HDL, assayed in six random subjects, squalene only tended to increase. Postprandial peak times in chylomicrons and VLDL were 4.390.3 and 4.890.6 h for triglycerides, 8.19 0.8 and 8.890.7 h for retinyl palmitate (PB0.05 from triglycerides), and 8.590.7 h and 9.390.6 h for squalene (PB0.05 from triglycerides), respectively. The differences of the peak times were not significant be-tween chylomicrons and VLDL.
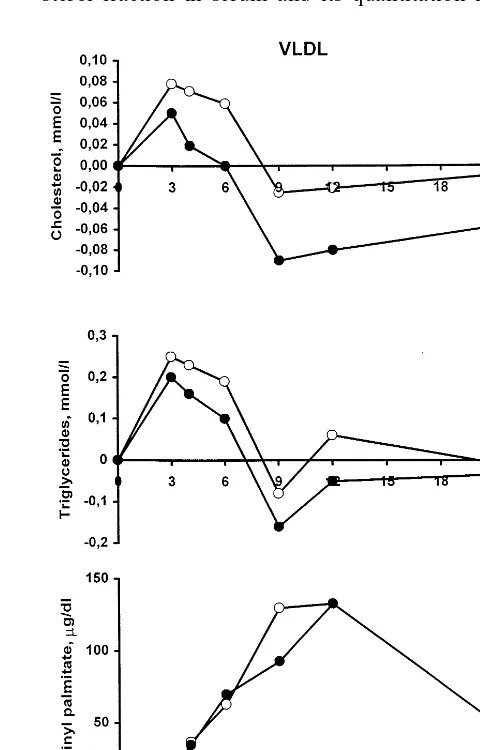
Addition of the single squalene dose to the test meal had no effect on the postprandial cholesterol, triglyce-ride and retinyl palmitate values in chylomicrons (Fig. 3), VLDL (Fig. 4) and VLDL bottom (data not shown).
Cholesterol synthesis expressed by lathosterol/ campesterol ratios in chylomicrons, VLDL and VLDL bottom were diminished after the fat load without squalene up to 12 h, after which the ratio returned to the baseline at 24 h or significantly above it in VLDL bottom (Fig. 5). D8-cholestenol/campesterol ratio was
Table 1
Twenty-four hour AUCs for squalene without (AUC 1) and with (AUC2) squalene supplementation in 16 subjectsa
Chlomi 1918+199 3150+1833 1331+2150 868+113 AUC 2
AUC 1 %
Paramete Difference
rs Difference
80959578* 55939599* 25029206
Plasma 247932*
25629318*
Chylomi- 291931 22719317* 8689113* cron
453957 23839293* 19309288* 5309103* VLDL
18199199 31509182* 13329215* 92917* VLDL
bottom
aMean9SE. AUC, area under curve;mg/dl per h.
*PB0.05.
tions to serum plant sterols in order to elucidate de novo cholesterol synthesis.
2.2.4. Statistical significance
Fig. 3. Postprandial incremental chylomicron cholesterol, triglyce-rides and retinyl palmitate. Mean values., squalene in test meal; , no squalene in test meal, N=16. All curves: no significant difference between without and with squalene in the test meal.
enced by squalene supplementation. In addition, VLDL but not chylomicron lanosterol/campesterol ratio at 12 h was 0.06990.012 without and 0.14190.028 with squalene addition (PB0.05), while the changes of other methyl sterols remained inconsistent (data not shown). Using sitosterol instead of campesterol in calculations gave equal results to campesterol in VLDL and VLDL bottom. Postprandial serum plant sterol levels remained unchanged after the test meal.
4. Discussion
This study shows for the first time that a single 500 mg dose of orally administered squalene increases cholesterol synthesis in humans within 9 – 24 h, mea-sured by increased lanosterol or lathosterol/plant sterol ratios. Lathosterol is the largest cholesterol precursor sterol fraction in serum and its quantitation is simple
Fig. 4. Postprandial incremental VLDL cholesterol, triglycerides and retinyl palmitate. Mean values. , squalene in test meal; , no squalene in test meal, N=16. All curves: no significant difference between without and with squalene in the test meal.
not changed consistently, while desmosterol/ campes-terol curve resembled that of lathoscampes-terol/campesterol (data not shown).
influ-Fig. 5. Postprandial change (%) of lathosterol to campesterol ratio in chylomicrons, VLDL and VLDL bottom. Mean9SE., squalene in test meal; , no squalene in test meal, N=16. *PB0.05 from non-squalene values, analysis of variance and covariance with re-peated measures,t-test.
Increases in precursor sterol/plant sterol ratios in chylomicrons, which are secreted by the intestine, sug-gest that squalene is partly converted to cholesterol in intestinal mucosa, which has a significant capacity to synthesize cholesterol [24]. It is suggested that the newly absorbed squalene was converted to precursor sterols and further to cholesterol also in the liver due to the high postabsorptive squalene concentrations in VLDL bottom. It is, however, not possible to distinguish spe-cifically proportions of intestinal and hepatic choles-terol synthesis by precursor scholes-terol/plant sterol ratios. Postprandial increase in lipoprotein squalene has ear-lier been described 3-4 hours after a fat meal containing squalene [25]. More recently, postprandial squalene in healthy subjects has been shown to reach maximum concentration much later, at 8 – 10 h after oral squalene administration [12,13], which is in agreement with the present study and very similar to the respective peak times of retinyl palmitate. Thus, it could be assumed that the effect of squalene on cholesterol metabolism cannot be seen earlier than 6 – 9 h after oral administra-tion. In the present study, in concordance with earlier observations, about one-third of fasting squalene is transported in triglyceride-rich lipoproteins (density B 1.019 g/ml), whereas of the postprandial squalene about two-thirds can be recovered from these lipoprotein fractions [12,13]. Intestinal absorption of squalene has been estimated to be 60% [10]. Squalene could theoreti-cally interfere with the absorption of other lipid-soluble components which are absorbed in micelles [26]. The present data, however, suggest that 500 mg of squalene given with cream-eggshake meal does not interfere with postprandial cholesterol, triglyceride or retinyl palmi-tate concentrations.
Diurnal variation of cholesterol synthesis has been shown in experimental animals [27 – 29] and in human subjects [30]. Decrease of cholesterol synthesis is re-vealed by lowered precursor sterols in serum at noon and afternoon hours, followed by their rise towards the evening reaching the maximum at night at 02:00 – 04:00 h [30]. Similarly, in this study cholesterol synthesis was decreased, indicated by lowered lathosterol/campesterol ratio during the day time, and it did not reach baseline levels until the next morning. However, when squalene is added to the test meal, cholesterol synthesis is upreg-ulated at 9 – 12 h, indicated by increased lathosterol/ campesterol ratio in chylomicrons, VLDL and VLDL bottom.
This study helps us to understand mechanisms of the effect of cholesterol precursor feeding, squalene in the present case, on serum cholesterol levels. Previous ob-servations on daily squalene consumption have revealed inconsistent results. Chronic intake of squalene, (1 g/ day) mimicking a diet rich in squalene, led to increased cholesterol precursor sterols and raised cholesterol con-centrations, but a smaller dose of squalene (0.5 g/day) simultaneously with D8-cholestenol, desmosterol and
plant sterols by gas – liquid chromatography [17]. Lath-osterol can be used to monitor changes in cholesterol synthesis [8,9,20 – 23], (Fig. 5). Lathosterol/campesterol ratio rather than lathosterol concentration [13] or lath-osterol to cholesterol ratio has been considered even a more reliable indicator of cholesterol synthesis [9]. Ac-cordingly, we used lathosterol/campesterol ratio as an indicator of cholesterol synthesis in this study.
normalized the situation [11]. On the other hand, squalene consumption of 0.86 g/day has been found even to reduce serum cholesterol [14]. This study showed that a single dose of 0.5 g of squalene was enough to increase cholesterol synthesis indicated by precursor sterols from 9 to 12 h after ingestion. The previous observation that daily consumption of 0.5 g of squalene does not increase serum cholesterol levels [11] and the present observation that the same amount as a single dose acutely increases cholesterol synthesis needs explanation. Possible compensatory mechanisms to pre-vent the increase of serum cholesterol during a moder-ate long-term squalene consumption are reduced HMG-CoA reductase activity and increased fecal elimi-nation of cholesterol as cholesterol itself and bile acids [10].
Increased serum squalene concentrations have re-cently been described in postmenopausal women with coronary artery disease [31]. There is, however, a confl-ict considering the lack of association between high squalene intake and coronary artery disease in Mediter-ranean populations consuming lots of olive oil [32]. The range of squalene in olive oil is wide (1.5 – 9.5 mg squalene in 1 g oil) [1,11]. Daily doses that elevate serum cholesterol levels, i.e. 1 g/day, can be reached in individuals consuming large amounts (\100 g/day) of olive oil. On the other hand, squalene has been re-ported to exhibit cellular radioprotective effects proba-bly due to antioxidant properties [33]. Thus, the role of squalene in relation to coronary artery disease is com-plicated, and remains to be further studied.
Acknowledgements
This study was supported by grants from the search Institute of Orion Corporation, Clinical Re-search Institute of the Helsinki University Central Hospital, the Helsinki University Central Hospital and the Finnish Academy, Council of Medical Sciences. The excellent technical assistance of Ms Leena Kaipiainen, Pia Hoffstro¨m, Orvokki Ahlroos, Ritva Nissila¨ and Mr Antti Laine, M.Sc., is greatly acknowledged.
References
[1] Liu GCK, Ahrens EH Jr., Schreibman PH, Crouse JR. Measure-ment of squalene in human tissues and plasma: validation and application. J Lipid Res 1976;17:38 – 45.
[2] Goodman DWS. Squalene in human and rat blood plasma. J Clin Invest 1964;43:1480 – 5.
[3] Liu GCK, Ahrens EH Jr., Schreibman PH, Samuel P, McNa-mara DJ, Crouse JR. Measurement of cholesterol synthesis in man by isotope kinetics of squalene. Proc Natl Acad Sci USA 1975;72:4612 – 6.
[4] Tilvis RS, Miettinen TA. Dietary squalene increases tissue sterols and fecal bile acids in the rat. Lipids 1983;18:32 – 6.
[5] McNamara DJ, Ahrens EHJr, Samuel P, Crouse JR. Measure-ment of daily cholesterol synthesis rates in man by assay of the fractional conversion of mevalonic acid to cholesterol. Proc Natl Acad Sci USA 1977;74:3043 – 6.
[6] Saudek CD, Frier BM, Liu GCK. Plasma squalene: lipoprotein distribution and kinetic analysis. J Lipid Res 1978;19:827 – 35. [7] Miettinen TA. Serum squalene and methyl sterols as indicators
of cholesterol synthesis in vivo. Life Sci 1969;8:713 – 21. [8] Miettinen TA. Detection of changes in human cholesterol
metabolism, Ann Clin Res 1970;2:300 – 320.
[9] Miettinen TA, Tilvis RS, Kesa¨niemi YA. Serum plant sterols and cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol 1990;131:20 – 31.
[10] Strandberg TE, Tilvis RS, Miettinen TA. Metabolic variables of cholesterol during squalene feeding in humans: comparison with cholestyramine treatment. J Lipid Res 1990;31:1637 – 43. [11] Miettinen TA, Vanhanen H. Serum concentration and
metabolism of cholesterol during rapeseed oil and squalene feeding. Am J Clin Nutr 1994;59:356 – 63.
[12] Gylling H, Miettinen TA. Postabsorptive metabolism of dietary squalene. Atherosclerosis 1994;106:169 – 78.
[13] Gylling H, Relas H, Miettinen TA. Postprandial vitamin A and squalene clearances and cholesterol synthesis off and on lovas-tatin treatment in type III hyperlipoproteinemia. Atherosclerosis 1995;115:17 – 26.
[14] Chan P, Tomlinson B, Lee C-B, Lee Y-S. Effectiveness and safety of low-dose pravastatin and squalene, alone and in combi-nation, in elderly patients with hypercholesterolemia. J Clin Pharmacol 1996;36:422 – 7.
[15] Warnick RG, Alberts JJ. Quantitation of lipoproteins. In: Hain-line A, Karon J, Lippel K, editors. Manual of Laboratory Operations, Lipid Research Clinics Program, Lipid and Lipo-protein Analysis. Bethesda, MD: National Institutes of Health, 1982:63 – 77.
[16] Miettinen TA, Koivisto P. Non-cholesterol sterols and bile acid production in hypercholesterolaemic patients with ileal bypass. In: Paumgartner G, Stiehl A, Gerok W, editors. Bile Acids and Cholesterol in Health and Disease. Lancaster: MTP Press, 1983:183 – 7.
[17] Miettinen TA. Cholesterol metabolism during ketoconazole treatment in man. J Lipid Res 1988;29:43 – 51.
[18] Havekes LM, de Knijff P, Beisiegel U, Havinga J, Smit M, Klasen E. A rapid micromethod for apolipoprotein E phenotyp-ing directly in serum. J Lipid Res 1987;28:455 – 63.
[19] Ruotolo G, Zhang H, Bentsianov V, Le N-A. Protocol for the study of the metabolism of retinyl esters in plasma lipoproteins during postprandial lipemia. J Lipid Res 1992;33:1541 – 9. [20] Tilvis RS, Miettinen TA. Serum plant sterols and their relation
to cholesterol absorption. Am J Clin Nutr 1986;43:92 – 7. [21] Miettinen TA. Biosynthesis of cholesterol. Curr Opin Lipidol
1990;1:244 – 7.
[22] Uusitupa MIJ, Miettinen TA, Happonen P, Ebeling T, Turtola H, Voutilainen E, Pyo¨ra¨la¨ K. Lathosterol and other noncholes-terol snoncholes-terols during treatment of hypercholesnoncholes-terolemia with lo-vastatin alone and with cholestyramine or guar gum. Arterioscler Thromb 1992;12:807 – 13.
[23] Bjo¨rkhem I, Miettinen TA, Reihner E, Ewerth S, Angelin B, Einarsson K. Correlation between serum levels of some choles-terol precursors and activity of HMG-CoA reductase in human liver. J Lipid Res 1987;28:1137 – 43.
[25] Lewis RW. Effect of alimentation on human serum squalene levels. Lipids 1975;11:430 – 3.
[26] Carey MC, Small DM, Bliss CM. Lipid digestion and absorption. Annu Rev Physiol 1983;45:651 – 77.
[27] Kandutsch AA, Saucier SE. Prevention of cyclic and triton-in-duced increases in hydroxymethylglutaryl coenzyme A reductase and sterol synthesis by puromycin. J Biol Chem 1969;244:2299 – 305.
[28] Edwards PA, Muroya H, Gould RG. In vivo demonstration of the circadian rhythm of cholesterol biosynthesis in the liver and intestine of the rat. J Lipid Res 1972;13:396 – 401.
[29] Strandberg TE, Tilvis RS, Miettinen TA. Diurnal variation of plasma methyl sterols and cholesterol in the rat: relation to hepatic
cholesterol synthesis. Lipids 1984;3:202 – 5.
[30] Miettinen TA. Diurnal variation of cholesterol precursors squalene and methyl sterols in human plasma lipoproteins. J Lipid Res 1982;23:466 – 73.
[31] Rajaratnam RA, Gylling H, Miettinen TA. Serum squalene in postmenopausal women without and with coronary artery disease. Atherosclerosis 1999;146:61 – 4.
[32] Keys A, Menotti A, Karvonen MJ, et al. The diet and 15-year death in the seven countries study. Am J Epidemiol 1986;124:903 – 15.
[33] Storm HM, Oh SY, Kimler BF, Norton S. Radioprotection of mice by dietary squalene. Lipids 1993;28:555 – 9.