E
!
ects of carbon dioxide and nitrogen
fertilization on phenolic content in
Poa annua
L.
T. Martijn Bezemer, T. He
"
n Jones
*
, John E. Newington
NERC Centre for Population Biology, Imperial College at Silwood Park, Ascot, Berkshire, SL5 7PY, UK
Received 18 January 1999; received in revised form 20 September 1999
Abstract
Di!erent but partially overlapping hypotheses have been developed to predict the allocation of phenolics in elevated atmospheric CO
2. The carbon}nutrient balance hypothesis predicts
increased allocation to phenolics due to reduced relative availability of nitrogen. The growth}di!erentiation balance hypothesis states that allocation will depend on source and sink strength, while the protein competition model predicts that allocation will remain unchanged. We grew Poa annua at two CO
2 concentrations in soils of three di!erent nutrient levels.
Although plant}tissue nitrogen levels were reduced in high CO
2 and photosynthetic rate
increased, phenolic concentration and biomass allocation remained unchanged. We discuss these data in the context of the three models'predictions of phenolic allocation in conditions of elevated CO
2. ( 2000 Elsevier Science Ltd. All rights reserved.
Keywords:Carbon-nutrient balance; Elevated CO
2; Growth-di!erentiation balance; Nutrients; Phenolics;
Protein competition model
1. Introduction
Rising levels of atmospheric carbon dioxide (CO
2) are likely to have negative
impacts on herbivore performance through CO
2-mediated changes in plant chemistry
(Bezemer and Jones, 1998). Many studies have shown that host}plant quality de-creases in conditions of elevated atmospheric CO
2; foliar nitrogen concentrations
diminish while levels of secondary (carbon-based) compounds tend to increase
*Corresponding author. Tel:#44-20-7594-2483; fax:#44-1344-873173. E-mail address:[email protected] (T. He"n Jones)
(e.g. Lindroth et al., 1993, 1997; Lavola and Julkunen-Tiitto, 1994; Lindroth, 1996; Poorter et al., 1997; Cotrufo et al., 1998; Lavola et al., 1998; Lindroth and Kinney, 1998; Pen8uelas and Estiarte, 1998). Changes in secondary metabolite concentrations may have major implications for plant}insect interactions.
Phenolics are secondary metabolites that are present in all terrestrial higher plants (Harborne, 1997). They can provide protection against herbivores, for example, by reducing the digestibility of leaves (Lambers, 1993). A number of partially over-lapping theories have attempted to explain how concentrations of secondary metab-olites change in conditions of environmental variation (e.g. CO
2elevation). Of these
models, the carbon}nutrient balance hypothesis (CNBH) (Bryant et al., 1983) has received most attention. The CNBH explains changes in secondary metabolism as being the result of imbalances between carbon (C) and nitrogen (N) requirements for growth and the availability of these resources from the external environment. In elevated CO
2 therefore, when more carbon is available but the nutrient availability
does not change, phenolic allocation is predicted to increase because of nutrient limitation. This has been further developed in the growth}di!erentiation hypothesis (GDBH) (Herms and Mattson, 1992), which recognizes that synthesis of secondary metabolites is determined during periods of plant di!erentiation (chemical and morphological changes leading to cell maturation and specialization). The theory states that growth dominates during favourable conditions, and di!erentiation is at a maximum only when conditions are sub-optimal for growth. Extending this to the e!ect of enhanced CO
2 requires an understanding of how the source/
sink balance within the plant is altered. The protein competition model (PCM) of Jones and Hartley (1998, 1999) states that phenolic allocation depends on total protein demand and the activity level of the enzyme phenylalanine ammonia-lyase (PAL) (Jones, 1984) involved in both phenolic and protein synthesis. Cell phenylalanine concentrations are low (Jones and Hartley, 1999) and are a limiting substrate for phenolic synthesis. The PCM postulates that protein and phenolic synthesis compete for phenylalanine; high rates of both protein and phenolic synthesis cannot occur simultaneously in a tissue (Lambers, 1993). In brief, this model predicts that in elevated atmospheric CO
2, phenolic content will generally remain
unchanged as CO
2-induced enhanced plant growth results in increased protein
demand. Even in situations where phenolic content remains constant, the actual concentration of phenolics might decrease in conditions of elevated CO
2 as this
depends on both the degree of leaf-carbon enhancement by photosynthesis and its resultant dilution e!ect.
Investigating changes in secondary metabolism in elevated atmospheric CO
2
and linking these with the existing theoretical framework is complex and intensive, and requires information on plant photosynthesis and growth, as well as tissue nitrogen and phenolic content. In this study we attempt to unravel some of these complexities by concentrating on a common annual grass species. Poa annua L. (Gramineae) plants were grown in soils of three di!erent nutrient levels at two atmospheric CO
2 concentrations; total phenolic
2. Materials and methods
The facilities used have been described by Smith and Jones (1998). In brief, the greenhouse consisted of two separately controlled perspex-walled chambers, each 3.7 m]1.3 m]1.8 m and which could be maintained at di!erent atmospheric CO2 concentrations. Within each chamber natural light was supplemented by two 400 W metal halide bulbs. Chamber temperature was computer-controlled and regulated at 20$13C during day and 12$13C during night. Relative humidity was maintained at 60$10% throughout. Two sensors situated at equal distances along the chamber length continuously relayed information on temperature and humidity to a computer, enabling accurate monitoring and control of the environment. CO
2 levels were
controlled using an infra-red gas analyser (PP Systems, Hitching, UK) that measured the CO
2levels in each chamber every 2 min. CO2 levels were either control (outside
concentration about 350lmol mol~1) or high atmospheric CO
2 (control
#200lmol mol~1).
Seeds of Poa annua from two di!erent sources were sown in 650 ml pots "lled with a loam}silver}sand mixture. The loam}sand mixture was leached with water (low), or fertilized either twice (medium) or six times (high) during the experiment with a slow-release fertilizer (NPK 8.0}4.8}19.1%). At the beginning of the experi-ment NPK levels were 76}63}53 mg l~1, 33}36}49 mg l~1 and 10}15}6 mg l~1
for high, medium and low nutrient soil, respectively. Twelve pots were sown for each seed source/nutrient/CO
2 treatment, each pot contained one plant. The experiment
was set-up with 12 blocks per CO
2 level with each block containing one replicate of
each source/nutrient combination. Weekly, the CO
2 treatments were exchanged
between the chambers and the blocks transferred and randomly re-arranged. Twelve weeks after seedling emergence, leaf photosynthetic rates were measured using a portable infra-red gas analyser (PP Systems, Hitching, UK). Then, all plants were harvested.
At harvest, for six of the 12 replicates of each treatment, leaves were collected and analyzed for leaf nitrogen and total phenolic content. Leaf nitrogen was analysed using a Kjeldahl procedure (Parkinson and Allen, 1975). Total phenolic content was measured following the extraction of 50 mg of dried plant material in 10 ml of boiling 50% (v/v) aqueous methanol for 1 h. The extract was centrifuged and 0.3 ml of supernatant was added to 2.7 ml of deionized water. Saturated sodium carbonate solution (1 ml) and 0.25 ml of Folin-Ciocalteu reagent (Fisons) were added, and the A760 was read 1 h later. Absorbance values were converted to tannic acid equivalents using a standard curve (0}35lg tannic acid)
prepared daily, and results were expressed as % dry weight (see also Waterman and Mole, 1994).
All plants were then harvested and oven-dried at 803C, and above-ground dry-weight determined. Results were analyzed using analysis of variance (ANOVA) with CO
2, nutrient and seed as main e!ects and blocks nested in CO2. Data were "rst
Table 1
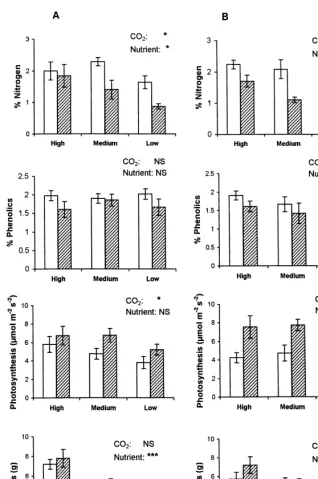
Results from ANOVA test on data from both seed sources.F-value, degrees of freedom and signi"cance are shown. C, N and S refer to CO
2, nutrient and seed, respectively. *p(0.05; ***p(0.001; NS non
signi"cant. Note that as blocks were nested in CO
2, block has been used as error-term for CO2
Source % Nitrogen % Phenolics Photosynthesis Biomass
F-value p F-value p F-value p F-value p
C F
There were no signi"cant di!erences in the results from the two seed sources for % nitrogen concentration, photosynthesis and above-ground biomass (Table 1). Phenolic concentrations did show seed source di!erences. For both overall and separate seed source analyses soil}nutrient and CO
2 concentration had a signi"cant
e!ect on the concentration of nitrogen in leaves ofP. annuawith plants growing in high atmospheric CO
2having lower nitrogen concentrations than plants growing in
the control (Table 1, Fig. 1). When analysed separately phenolic concentrations in the leaves remained unchanged for nutrient and CO
2 treatments for both seed sources
(Fig. 1). Leaf photosynthetic rates were higher in conditions of high atmospheric CO
2,
but when analysed as separate seed sources did not change signi"cantly in di!erent nutrient treatments. Above-ground biomass increased signi"cantly with increased soil nutrient levels, but was not a!ected by atmospheric CO
2 concentration.
4. Discussion
Although leaf photosynthetic rates increased,"xing more carbon in elevated CO
2,
neither nutrients nor CO
2 levels in#uenced the allocation of carbon to phenolic
compounds. This does not support the prediction of the CNBH that allocation of carbon-based secondary compounds, such as phenolics, should be higher in condi-tions of low nutrient and high carbon availability. Although some (e.g. Price et al., 1989), but not all (e.g. BjoKrkman et al., 1991), studies have shown that in conditions of low nutrient or high light availability, increased allocation to carbon-based secondary plant compounds occurs, this study supports the general "nding that phenolic concentration of annual plants is frequently una!ected by CO
2elevation (Fajer et al.,
Fig. 1. E!ect of soil nutrient (high, medium and low), and high (shaded bars) and control (open bars) CO
2
1994, 1995; Lavola and Julkunen-Tiitto, 1994; Lindroth, 1996) have shown an increase in the levels of phenolic compounds, others have not (e.g. Fajer, 1989; Fajer et al., 1989, 1992; Lincoln and Couvet, 1989; Johnson and Lincoln, 1990, 1991).
Both the GDBH and PCM state that total phenolic allocation will depend on the source/sink balance of the plant (Herms and Mattson, 1992; Jones and Hartley, 1998; 1999) the PCM also states that as sink strength increases in elevated CO
2, phenolic
allocation should remain unchanged (Jones and Hartley, 1998). Although our data show no changes in phenolic concentration, thus making the results more consistent with the PCM predictions, we found no changes in biomass production and, hence, sink strength.
Another plausible explanation is that in elevated CO
2, reduced nitrogen levels in
plant tissue are a consequence of the plant requiring less nitrogen-containing RUBISCO to maintain the same photosynthetic e$ciency (see Arp, 1991). Low tissue-nitrogen is not necessarily the result of nutrient}de"ciency but may arise from a reduction in plant requirement. Models that assume that changes in tissue nitrogen are always nutrient stress-related may be far too simplistic.
Our results also show that, at least in terms of biomass allocation, nutrient availability did not change the response ofP. annuato elevated CO
2. Many studies on
other plant species have shown that the response to CO
2 elevation is highest in
conditions of high nutrient availability (e.g. Bazzaz, 1990; Newbery et al., 1995; Bowler and Press, 1996; Cotrufo et al., 1998). However, our results are not entirely unexpected asP. annuais a species with a ruderal primary strategy (Grime, 1974), which shows little or no response in terms of biomass to increased atmospheric CO
2 even in
environments when other growth conditions are non-limiting (Hunt et al., 1991). There are obvious caveats to this study: for example, in considering the appro-priateness of the PCM it should not be assumed that the ratio between plant-nitrogen and protein levels will always remain constant, neither should phenolics be treated as a single entity. However, the data suggest that, at least forPoa annua, the PCM might be a potentially more appropriate working hypothesis for predicting phenolic alloca-tion in condialloca-tions of increased atmospheric CO
2. Future work should be directed to
exploring how applicable this model is across a range of plant species growing in di!ering nutrient conditions.
Acknowledgements
This study was partially funded by the NERC TIGER Initiative (GST/02/646). We thank John Lawton and three anonymous referees for very helpful comments on an earlier draft of the manuscript.
References
Arp, W.J., 1991. E!ects of source}sink relations on photosynthetic acclimation to elevated CO
2. Plant Cell
Bazzaz, F.A., 1990. Response of natural ecosystems to rising CO
2levels. Annu. Rev. Ecol. Syst. 21, 167}196.
Bezemer, T.M., Jones, T.H., 1998. Plant insect}herbivore interactions in elevated atmospheric CO
2:
quantitative analyses and guild e!ects. Oikos 82, 212}222.
BjoKrkman, C., Larsson, S., Gref, R., 1991. E!ects of nitrogen fertilization on pine needle chemistry and saw#y performance. Oecologia 86, 202}209.
Bowler, J.M., Press, M.C., 1996. E!ects of elevated CO
2, nitrogen form and concentration on growth and
photosynthesis of a fast- and slow-growing grass. New Phytol. 132, 398}401.
Bryant, J.P., Chapin III, F.S., Klein, D.R., 1983. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40, 357}368.
Cotrufo, M.F., Ineson, P., Scott, A., 1998. Elevated CO
2reduces the nitrogen concentration of plant tissues.
Global Change Biol. 4, 43}54.
Fajer, E.D., 1989. The e!ects of enriched CO
2atmospheres on plant}insect herbivore interactions: growth
responses of larvae of the specialist butter#y.Junonia coenia(Lepidoptera: Nymphalidae). Oecologia 81, 514}520.
Fajer, E.D., Bowers, M.D., Bazzaz, F.A., 1989. The e!ects of enriched carbon dioxide atmospheres on plant-insect herbivore interactions. Science 243, 1198}1200.
Fajer, E.D., Bowers, M.D., Bazzaz, F.A., 1992. The e!ect of nutrients and enriched CO
2environments on
production of carbon-based allelochemicals inPlantago: a test of the carbon/nutrient balance hypothe-sis. Am. Nat. 140, 707}723.
Grime, J.P., 1974. Vegetation classi"cation by reference to strategies. Nature. 250, 26}31.
Harborne, J.B., 1997. Plant secondary metabolism. In: Crawley, M.J. (Ed.), Plant Ecology. Blackwell Science, Oxford, pp. 132}155.
Herms, D.A., Mattson, W.J., 1992. The dilemma of plants: to grow or defend. Quat. Rev. Biol. 67, 283}335. Hunt, R., Hand, D.W., Hannah, M.A., Neal, A.M., 1991. Response to CO
2enrichment in 27 herbaceous
species. Funct. Ecol. 5, 410}421.
Johnson, R.H., Lincoln, D.E., 1990. Sagebrush and grasshopper responses to atmospheric carbon dioxide concentration. Oecologia 84, 103}110.
Johnson, R.H., Lincoln, D.E., 1991. Sagebrush carbon allocation patterns and grasshopper nutrition: the in#uence of CO
2enrichment and soil mineral limitation. Oecologia 87, 127}134.
Jones, D.H., 1984. Phenylalanine ammonia-lyase: Regulation of its induction, and its role in plant development. Phytochemistry 23, 1349}1359.
Jones, C.G., Hartley, S.E., 1998. Global change and plant phenolic concentrations: towards species level predictions using the protein competition model. In: De Kok, L.J., Stulen, I. (Eds.), Responses of Plant Metabolism to Air Pollution and Global Change. Backhuys Publishers, Leiden, pp. 23}50.
Jones, C.G., Hartley, S.E., 1999. A protein competition model of phenolic allocation. Oikos 86, 27}44.
Julkunen-Tiitto, R., Tahvanainen, J., Silvola, J., 1993. Increased CO
2 and nutrient status changes a!ect
phytomass and the production of plant defensive secondary chemicals inSalix myrsinifolia(Salisb.). Oecologia 95, 495}498.
Lambers, H., 1993. Rising CO
2, secondary plant metabolism, plant}herbivore interactions and litter
decomposition. Vegetatio 104/105, 263}271.
Lavola, A., Julkunen-Tiitto, R., 1994. The e!ect of elevated carbon dioxide and fertilization on primary and secondary metabolites in birch.Betula pendula(Roth). Oecologia 99, 315}321.
Lavola, A., Julkunen-Tiitto, R., Roininen, H., Aphalo, P., 1998. Host-plant preference of an insect herbivore mediated by UV-B and CO
2in relation to plant secondary metabolites. Biochem. Syst. Ecol.
26, 1}12.
Lincoln, D.E., Couvet, D., 1989. The e!ect of carbon supply on allocation to allelochemical and caterpillar consumption of peppermint. Oecologia 78, 112}114.
Lindroth, R.L., 1996. CO
2-mediated changes in tree chemistry and tree-lepidoptera interactions. In: Koch,
G.W., Mooney, H.A. (Eds.), Carbon Dioxide and Terrestrial Ecosystems. Academic Press, London, pp. 105}120.
Lindroth, R.L., Kinney, K.K., 1998. Consequences of enriched atmospheric CO
2and defoliation for foliar
Lindroth, R.L., Kinney, K.K., Platz, C.L., 1993. Responses of deciduous trees to elevated atmospheric CO
2:
productivity, phytochemistry, and insect performance. Ecology 74, 763}777. Lindroth, R.L., Roth, S., Kruger, E.L., Volin, J.C., Koss, P.A., 1997. CO
2-mediated changes in aspen
chemistry: e!ects on gypsy moth performance and susceptibility to virus. Glob. Change Biol. 3, 279}289. Newbery, R.M., Wolfenden, J., Mans"eld, T.A., Harrison, A.F., 1995. Nitrogen, phosphorous and
potassi-um uptake and demand inAgrostis capillaris: the in#uence of elevated CO
2and nutrient supply. New
Phytol. 130, 565}574.
Parkinson, J.A., Allen, S.E., 1975. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Comm. Soil Sci. Pl. Anal. 6, 1}11.
Pen8uelas, J., Estiarte, M., 1998. Can elevated CO
2a!ect secondary metabolism and ecosystem function?
Trends Ecol. Evol. 13, 20}24.
Poorter, H., Van Berkel, Y., Baxter, R., Den Hertog, J., Dijkstra, P., Gi!ord, R.M., Gri$n, K.L., Roumet, C., Roy, J., Wong, S.C., 1997. The e!ect of elevated CO
2 on the chemical composition and construction
costs of leaves of 27 C
3species. Plant Cell Environ. 20, 472}482.
Price, P.W., Waring, G.L., Julkunen-Tiitto, R., Tahvanainen, J., Mooney, H.A., Craig, T.P., 1989. Carbon-nutrient balance hypothesis in within-species phytochemical variation ofSalix lasiolepis. J. Chem. Ecol. 15, 117}1131.
Roth, S.K., Lindroth, R.L., 1994. E!ects of CO
2-mediated changes in paper birch and white pine chemistry
on gypsy moth performance. Oecologia 98, 133}138. Roth, S.K., Lindroth, R.L., 1995. Elevated atmospheric CO
2: e!ects on phytochemistry, insect performance
and insect parasitoid interactions. Global. Change Biol. 1, 173}182. Smith, P.H.D., Jones, T.H., 1998. E!ects of elevated CO
2on the chrysanthemum leaf miner,Chromatomyia
synchenesiae: a greenhouse study. Glob. Change Biol. 4, 287}291.