www.elsevier.com / locate / bres
Research report
Neuronal degeneration in the limbic system of weanling rats exposed
to saline, hyperthermia or d-amphetamine
*
John F. Bowyer
Division of Neurotoxicology, National Center for Toxicological Research, Jefferson, Arkansas, AR 72079, USA
Accepted 29 August 2000
Abstract
Neuronal degeneration was detected in the tenia tecta and other regions of the anterior limbic system of male weanling rats 3 days after four doses of 5 mg / kg d-amphetamine (435 mg / kg AMPH) when seizures occurred during AMPH exposure. Neurodegeneration in the parietal cortex, loss of tyrosine hydroxylase immunoreactivity in the caudate-putamen (CPu) and decreases in CPu tissue dopamine levels in weanlings was much less than those previously observed in adults. The neurotoxicity seen in the parietal cortex and CPu of the weanlings was much less than previously seen in adults even though severe hyperthermia and the behavior of retrograde propulsion occurred during AMPH exposure. Neurodegeneration was not detected in any of the previously mentioned brain regions in controls and weanlings made hyperthermic by a warm environment. However, signs of spontaneous neurodegeneration were seen in the posterior piriform cortex (Pir), posteriolateral cortical amygdaloid nucleus (PLCo), and the amygdalopiriform transition area (APir) of control weanlings. The doses of AMPH and the degree of hyperthermia necessary to induce seizures were substantially lower in weanlings compared to those previously observed in adult rats. Further studies will be necessary to determine if the susceptibility of weanlings to AMPH-induced seizures is related to or dependent on the same processes involved in producing degeneration in the posterior limbic system of saline controls. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Neurotoxicity
Keywords: Amphetamine; Limbic System; Seizures; Neurodegeneration; Weanlings
1. Introduction concern that these compounds could affect thalamo-corti-cal pathways [1]. Although AMPH and METH abuse in Because of the extensive abuse of AMPH and metham- children is not nearly as common as in adults, the potential phetamine (METH), the potential of these compounds to for inadvertent poisonings and adverse drug interactions is produce neurotoxicity has been extensively explored using present [10,15]. Thus, studying the neurotoxic potential of laboratory animals [27]. Although AMPH and METH these compounds in juvenile laboratory animals is of clearly produce signs of neurotoxicity in adult laboratory clinical relevance. As well, comparing the neurotoxic animals, the degree to which they produce similar neuro- effects of AMPH in adult versus juvenile animals may toxicity in humans has yet to be resolved [19,32]. Even provide some insight into the mechanisms involved in less clear is what effect AMPH and METH may have on AMPH and METH neurotoxicity.
the developing human brain although there is some Previous data indicates that, relative to adults, weanling rats are more resistant to the dopamine terminal damage in the caudate-putamen (CPu) produced by AMPH and METH [5,6,22]. However, the susceptibility of weanlings Abbreviations: d-amphetamine, AMPH; amygdalopiriform transition to neurodegeneration in the parietal cortex, thalamus and area, APir; caudate / putamen, CPu; Fluoro-Jade, F–J; glial fibrillary
limbic system, which can be produced by AMPH or acidic protein, GFAP; methamphetamine, METH; piriform cortex, Pir;
METH [4,8,9,25] in adults, has not been tested. Degenera-posteriolateral cortical amygdaloid nucleus, PLCo
*Tel.:11-870-543-7194; fax:11-870-543-7745. tion in these later areas involves the loss of neurons and 0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
not just axonal and terminal damage. The doses necessary 2.2. HPLC-quantitation of aromatic monoamine levels in to produce neurodegeneration in the parietal cortex are the striatum
same as those that produce terminal damage in the CPu
[8,9]. The present study evaluated the susceptibility of CPu were dissected from brain on ice immediately after weanling rats (23–24 days of age) to neurodegeneration in sacrifice, and stored at 2708C until analysis using high the forebrain at doses that produce neurotoxicity in adult pressure liquid chromatography (HPLC) separation and rats. Along with aged matched controls given 4 doses of 1 electrochemical detection as previously described [3]. ml / kg saline, weanlings were also given 43saline in an Briefly, each frozen CPu was weighed and diluted with a incubator held at 38–398C to induce a hyperthermic profile measured volume (20%, w / v) of 0.2 M perchloric acid similar to that produced by AMPH exposure. containing 100 ng / ml 3,4-dihydroxybenzylamine (Sigma, The neurodegeneration occurring in the forebrain was St. Louis, MO) as internal standard. After sonication and evaluated 3 days post AMPH or saline exposure using centrifugation the supernatant was removed and 20 ml immunohistology for glial fibrillary acidic protein (GFAP), injected directly onto the HPLC / EC system. Dopamine, histochemical processing with the fluorescent marker 5-HT and their metabolites were separated using a Supel-Fluoro–Jade (F–J) for neurodegeneration and B4 isolectin cosil LC18 3 mm analytical column (7.5 cm34.6 mm, binding to detect activated microglia. Damage to dopamine Supelco, Bellefonte, PA) and an isocratic mobile phase terminals in the CPu was assessed using tyrosine hydroxy- consisted of 92% KH PO -buffer (0.07 M, pH 3.0) and2 4 lase immunohistology and determining tissue dopamine 8% methanol, containing 1 mM Na-1-heptanesulfonic acid
levels in the CPu. and 0.2 mM Na -EDTA per liter. The mobile phase flow2
rate was 1.5 ml / min, and aromatic monoamines and metabolites were detected using a BAS-LC4B amperomet-ric detector and a BAS-LC-17 oxidative flow cell with the
2. Materials and methods electrode operating at an applied voltage of 0.65 V.
2.1. Animals, housing conditions and dosing
2.3. immunocytochemical and Fluoro– Jade B labeling All the procedures involving animals were approved by procedures
the Institutional Animal Care and Use Committee of the
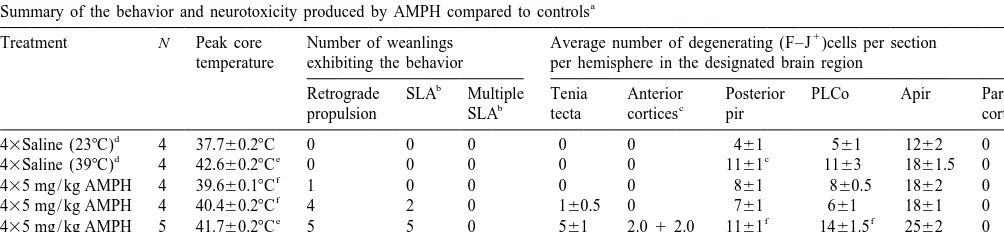
3. Results have episodes of retrograde propulsion, neurodegeneration within the parietal cortex and thalamus is pronounced [4] 3.1. Behavioral effects of amphetamine. yet this was not the case with weanlings where, relative to adults, only sparse neurodegeneration occurred in the The effects of the 435 mg / kg AMPH on peak body parietal cortex (Table 1). In addition, the astrocytic temperature and behaviors that normally precede either hypertrophy (increase in size of GFAP labeled astrocytes terminal damage or neuronal degeneration are shown in seen in coronal sections of CPu), microglial activation and Table 1. The results from weanlings given AMPH are dopamine terminal damage (loss of tyrosine hydroxylase subdivided into 4 groups in Table 1 depending on their immunoreactive fibers) that are normally seen in adults degree of hyperthermia and seizure severity during AMPH were not as pronounced (data not shown). Although the exposure. All of the weanlings dosed with AMPH ex- CPu dopamine levels were significantly affected (P,0.01), hibited intense stereotypic grooming as well as hyperac- they decreased only about 30% (518623 ng / 100 mg tivity. All of the animals that had severe hyperthermia tissue, n56) at 3 days after 435 mg / kg AMPH in rats that (body temperature above 418C) exhibited retrograde prop- were severely hyperthermic (peak temperature was ulsion, and a seizure-like activity called ‘praying’ or 41.760.38C) compared to controls (743627 ng / 100 mg ‘boxing’ (often exhibited by mice given AMPH but not tissue, n56). Serotonin, 5-hydroxyindoleacetic and the adult rats). This initial behavior was often later followed dopamine metabolites, homovanillic acid and dihydrox-by myoclonic events resembling hopping or startle-like yphenylacetic acid, were not significantly affected (data responses. Fully developed myoclonic seizures such as not shown).
those produced in adults by AMPH [4] seldom occurred. In contrast, neurodegeneration within the tenia tecta and Some of these animals had either several episodes of some other areas of the anterior limbic system was seizure-like activity resulting in hind limb splaying, and prominent in those weanlings that had multiple myoclonic were given 15 mg / kg phenobarbital to control seizure episodes (Fig. 1A & B, Table 1). The other limbic areas activity and prevent lethality. Unexpectedly, some of the where neurodegeneration occurred, when AMPH exposure weanlings with a more moderate degree of hyperthermia resulted in pronounced seizure activity, were the orbital (between 40.0 and 40.58C) also exhibited retrograde cortex, agranular insular, entorhinal cortex and, to a lesser propulsion and boxing behavior. Weanlings that were given extent, the anterior Pir. The neurodegeneration in the tenia 43saline at 248C only had transient increases in motor tecta was confined to cells in the granule layer that had the activity after dosing. The animals given 43saline at 398C morphology of granule cells while the neurons in the other became severely hyperthermic (Table 1), and the overall anterior limbic regions were primarily pyramidal in shape. duration of their hyperthermia above 408C was even F–J labeling of neurons (neurodegeneration) within the greater than any of the AMPH treated weanlings. hippocampus was absent among the individual weanlings
even when seizure activity was pronounced.
3.2. Neurodegeneration Weanlings dosed with 43saline in either a 248C or 398C
environment did not have neurodegeneration in the tenia In adult rats, that become severely hyperthermic and tecta or any other area in the more anterior limbic system.
Table 1
a
Summary of the behavior and neurotoxicity produced by AMPH compared to controls
1
Treatment N Peak core Number of weanlings Average number of degenerating (F–J )cells per section temperature exhibiting the behavior per hemisphere in the designated brain region
b
Retrograde SLA Multiple Tenia Anterior Posterior PLCo Apir Parietal
b c
The average number of F–J cells per section per hemisphere for each brain region for each weanling was determined from the mean of 3–5 coronal
1
sections. Data from each brain region of the individual animals were then used to determine the mean6s.e.m. number of F–J cells per section per hemisphere for each group. A one-way analysis of variance (ANOVA) was performed with a post hoc Tukey’s test to determine a significant main effect and significant effects between the groups with respect to the various parameters tested.
b
SLA denotes seizure-like activities while Multiple SLA indicates that multiple episodes of SLA occurred (similar to status epilepticus in adults).
c
Anterior cortices with degeneration included orbital, agranular insular and piriform.
d
Ambient temperature during saline dosing.
e
Significantly greater than 43Saline (238C) and moderately hyperthermic AMPH groups P,0.01.
f
Fig. 1. AMPH-induced and spontaneous neurodegeneration in the tenia tecta, orbital cortex, PLCo and APir. The fluorescent marker F–J was used to label degenerating neurons (appearing bright jade in color against a dark green background) in the tenia tecta (A), orbital cortex (B). PLCo at23.30 mm posterior to the bregma using the coordinates of Paxinos and Watson [21] (C) and the APir at24.30 mm to posterior to the bregma (D) of 435 mg / kg AMPH dosed weanlings. As well, F–J labeled neurons are seen in the APir of control weanlings at lower (E) and higher (F) magnification. The large and lighter staining tubular structures in plate A through F are the vasculature. Isolectin B4 / DAB staining (dark reddish-brown) microglia are seen in the APir of control weanlings at lower (G) and higher magnification (H).
However, unexpectedly, signs of neurodegeneration within a morphology similar to that of granule cells with only a three specific regions of the more posterior limbic system few cells having a morphology similar to that of pyramidal were observed. F–J labeling in two of these areas, the neurons. Hyperthermia significantly potentiated neurode-PLCo (23.3 from the bregma) and Apir (24.3 from the generation in the PLCo of saline dosed rats but did not bregma) is seen in Fig. 1E and F, and F–J labeled cells significantly increase neurodegeneration in the anterior Pir were also seen (not shown in Fig. 1) in the caudal Pir at and APir.
22.8 mm, and greater, posterior to the bregma by the coordinates of Paxinos and Watson [21].
Neurodegenera-tion in these three areas appeared to be potentiated by 4. Discussion
AMPH-induced seizures. There were accompanying B4
isolectin labeled cells in all three regions of the two control We have previously reported [4,25] the similarities in groups which had the overall appearance of microglia the seizure-like behavior patterns seen during developing other than their processes were not as extensive and their amygdaloid kindled seizures [12] and those associated with soma were slightly larger than most acutely activated AMPH and METH induced behavior in adult rats and microglia (Fig. 1G & H). GFAP immunoreactivity also mice. As well, neurodegeneration occurs in the tenia tecta indicated hypertrophy of astrocytes in the three caudal and other areas of the anterior limbic system, excluding the limbic regions of controls (data not shown). However, this hippocampus, in adult rodents when multiple episodes or was observed in all the limbic regions, particularly the status epilepticus occurs during AMPH or METH exposure
1
the tenia tecta and anterior limbic system of weanlings remaining forebrain, there were neither F–J nor B4 after AMPH exposure are not totally unexpected. How- isolectin labeling cells present in the two control groups. ever, the relatively low doses of 435 mg / kg AMPH in The time course of the neurodegenerative events cannot be weanlings compared to 4315 mg / kg AMPH in adults precisely determined. It is possible that this developmen-necessary to produce seizures and neurodegeneration were tally related degeneration could have occurred merely from not expected [4]. In addition, the order of sensitivity of the the saline injection and temperature monitoring. Alter-various brain regions (the parietal cortex being the most natively, the neurodegeneration in these three regions sensitive while the limbic areas being less sensitive) to could be an apoptotic event that is related to some later AMPH-induced neurodegeneration in adults is the opposite stage of limbic development or neuroplastic event timed of weanlings (see Table 1). around weaning. If this were the case the neurodegenera-The minimal damage to the parietal cortex that occurs in tion would have most likely would have occurred within weanlings may be related to or dependent on the lesser 10 days of sacrifice, or after postnatal days 14–18, due to effects seen in the CPu dopamine terminals at this age the normally transient nature of microglial activation after (Results, [6,22]). AMPH-induced damage to the parietal neurotoxic insult [2,18,30]. Potentiation of damage to the cortex and the CPu in adults is believed to be linked to PLCo, APir and posterior Pir appeared to occur from neuronal circuits of the basal ganglia [8,9]. It is not known seizures and hyperthermia but the role the three areas play why the basal ganglia circuits of the weanlings are more in the sensitivity to and the development of AMPH-resistant to neurodegeneration. However, it is possible that induced seizures is unknown. Neonatal animals have been the AMPH-induced increases in extracellular or intracellu- reported to have marked changes in seizure susceptibility lar levels of dopamine in weanlings is not sufficiently and effects of seizures by hypoxia, cortical malformations robust to produce larger dopamine depletions since in and convulsants between the ages 7–24 days [7,14,17]. weanlings CPu tissue levels are only about 70% of adult Therefore, the changes that are observed in the posterior levels. Large increases in either extracellular or intracellu- limbic system might have an overall effect on seizure lar CPu dopamine, supported by continuing synthesis of genesis not solely specific to AMPH-induced seizures. dopamine within the nerve terminals, have been implicated In conclusion, weanling rats are more sensitive to as necessary for long-term dopamine depletions induction of seizure-like activity by AMPH exposure than [11,16,20,23,31]. However, if the dopamine release was adult rats, and significant neurodegeneration in the tenia not robust enough to cause more pronounced dopamine tecta and other areas of the anterior limbic system, depletions, it is not easy to explain why behaviors, such as excluding the hippocampus, is observed when myoclonus retrograde propulsion and head weaving, occurred in the and seizure-like activities occur. It is not known whether weanlings. These behaviors only appear in adults when this greater sensitivity to AMPH-induced seizures is extracellular dopamine levels are sufficient to produce related to, or dependent on, the same processes involved in large dopamine depletions [3]. producing neurodegeneration in the PLCo, APir and caudal Important differences exist between adults and wean- Pir in weanlings. On the other hand, areas like the parietal lings in their behavioral responses to AMPH. When cortex, CPu and thalamus in the basal ganglia are more retrograde propulsion is observed during exposure to either resistant to AMPH-induced neurodegeneration than adult 435 or 4315 mg / kg AMPH, it is invariably accompanied rats.
by severe hyperthermia (.418C body temperature) in adult rats. However, in weanlings, retrograde propulsion was
observed in animals with significantly lower body tempera- Acknowledgements
tures between 40.08C and 40.58C. Also, the seizure-like
behavior was occasionally observed in weanlings with The author wishes to thank Dr. Keri Hopkins and Dr. body temperatures in this lower range. In adult rats, the Larry Schmued for providing the Fluoro–Jade B and convulsive activity is confined to the severely hyperther- methods for F–J labeling in this manuscript as well as their mic. Thus, the two behavioral patterns of retrograde consultation with respect to F–J labeled cells.
propulsion and seizure-like activity are not dependent on such a severe hyperthermia in weanlings as they are in
adults. References
The most unexpected findings of this study were the
evidence of neurodegeneration in the PLCo, APir and [1] O.S. Adrianov, I.A. Shimko, L.M. Gershtein, N.S. Popova, V.F. caudal Pir within the posterior limbic system of control Fokin, Neirofiziologicheskie I biokhimicheski mekhanizmy dozozavisimoi amfetaminovoi moduliatsii neironov sensomotornoi weanlings not exposed to hyperthermia. It is highly
kory u razvivaiushchikhsia zhivotnykh, Uspekhi Fiziologicheskikh unlikely that three relatively specific markers (F–J, B4
Nauk 28 (1997) 78–97.
Ferguson, J. Tor-Agbidye, An evaluation of L-ephedrine neuro- [19] U.D. McCann, D.F. Wong, F. Yokoi, V. Villemange, R.F. Dannals, toxicity with respect to hyperthermia and caudate / putamen mi- G.A. Ricaurte, Reduced striatal dopamine transporter density in crodialysate levels of ephedrine, dopamine, serotonin and glutamate, abstinent methamphetamine and methcathione users: evidence from
11
Toxicol. Sci. 55 (2000) 133–142. positron emission tomography studies with [ C]WIN-35,428, J. [4] J.F. Bowyer, S.l. Peterson, R.L. Rountree, J. Tor-Agbidye, G.J. Neurosci. 18 (1998) 8417–8422.
Wang, Neuronal degeneration in rat forebrain resulting from D- [20] S.J. O’Dell, F.B. Weihmuller, J.F. Marshall, Multiple metham-amphetamine-induced convulsions is dependent on seizure severity phetamine injections induce marked increases in extracellular striatal and age, Brain Res. 809 (1998) 77–90. dopamine which correlate with subsequent neurotoxicity, Brain Res. [5] J.F. Bowyer, W. Slikker Jr., L. Schmued, P. Clausing, Comparison of 564 (1991) 256–260.
the expression of amphetamine (AMPH) neurotoxicity in developing [21] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, versus adult rats, FASEB J. 9 (1995) 3856, Abstract. Vol. 2, Academic Press, San Diego, CA, 1995.
[6] G.D. Cappon, L.L. Moford .an, C.V. Vorhees, Ontogeny of metham- [22] C. Pu, C.V. Vorhees, Developmental dissociation of metham-phetamine-induced neurotoxicity and associated hyperthermic re- phetamine-induced depletion of dopaminergic terminals and as-sponse, Dev. Brain Res. 103 (1997) 155–162. trocyte reaction in rat striatum, Dev. Brain Res. 72 (1993) 325–328. [7] N. Chevassus-au-Louis, S.C. Barban, J.L. Gaiarsa, Y. Ben-Ari, [23] C.J. Schmidt, C.K. Black, V.L. Taylor, G.M. Fadayel, T.M. Humph-Cortical malformation and epilepsy: new insights from animal reys, T.R. Nieduzak, S.M. Sorenson, The 5-HT2 receptor antagonist, models, Epilepsia 40 (1999) 811–821. MDL 28,133A, disrupts the serotonergic-dopaminergic interaction [8] A.J. Eisch, J.F. Marshall, Methamphetamine neurotoxicity: dissocia- mediating the neurochemical effects of
3,4-methylenedioxymetham-tion of striatal dopamine terminal damage from parietal cortical cell phetamine, Eur. J. Pharm. 220 (1992) 151–159.
body injury, Synapse 30 (1998) 433–445. [24] L.S. Schmued, C. Albertson, W. Slikker Jr., Fluoro-Jade: a novel [9] A.J. Eisch, L.C. Schmued, J.F. Marshall, Characterizing cortical fluorochrome for the sensitive and reliable histochemical
localiza-neuron injury with Flouro-Jade labeling after a neurotoxic regimen tion of neuronal degeneration, Brain Res. 751 (1997) 37–46. of methamphetamine, Synapse 30 (1998) 329–333. [25] L.C. Schmued, J.F. Bowyer, Methamphetamine exposure can [10] R.L. Findling, J.W. Dogin, Psychopharmacology of ADHD: children produce neuronal degeneration in mouse hippocampal remnants,
and adolescents, J. Clin. Psychia. 59 (1998) 42–49. Brain Res. 759 (1997) 135–140.
[11] J.W. Gibb, F.J. Kogan, Influence of dopamine synthesis on metham- [26] L.C. Schmued, K.J. Hopkins, Fluoro-Jade B: a high affinity fluores-phetamine-induced changes in striatal and adrenal tyrosine hydroxy- cent marker for the localization of neuronal degeneration, Brain Res. lase, Naunyn-Schmiedeberg’s Arch. Pharmacol. 310 (1979) 185– 874 (2000) 123–130.
187. [27] L.S. Seiden, K.E. Sabol, Neurotoxicity of methamphetamine-related [12] G.V. Goddard, D. McIntyre, C. Leech, A permanent change in brain drugs and cocaine, in: L.W. Chang, R.S. Dyer (Eds.), Handbook of function resulting from daily electrical stimulation, Exp. Neurol. 25 Neurotoxicology, Vol. 2, Marcel Dekker, Inc, New York, Basel,
(1969) 295–330. Hong Kong, 1995, pp. 824–844.
[13] S.M. Hsu, L. Raine, H. Fanger, Use of avidin–biotin–peroxidase [28] L.A. Sternberger, P.H. Hardy Jr., J.J. Cuculis, H.G. Meyer, The complex (ABC) in immunoperoxidase techniques: a comparison unlabeled antibody–enzyme method of immunohistochemistry Prep-between ABC and unlabeled antibody (PAP) procedures, J. Histoch- aration and properties of soluble antigen–antibody complex (horse-em. Cytoch(horse-em. 29 (1981) 577–580. radish peroxidase antihorseradish peroxidase) and its use in the [14] F.E. Jensen, C. Wang, Hypoxia-induced hyperexcitability in vivo identification of spirochetes, J. Histochem. Cytochem. 18 (1970)
and in vitro in the immature hippocampus, Epilepsy Res. 26 (1996) 315–333.
131–140. [29] W.J. Streit, An improved staining method for rat microglial cells [15] P. Kolecki, Inadvertant methamphetamine poisoning in pediatric using the lectin from Griffonia simplicifolia (GSA 1-B ), J. Histoch-4
patients, Ped. Emerg. Care 14 (1998) 385–387. em. Cytochem. 38 (1990) 1683–1686.
[16] M.J. LaVoie, T.G. Hastings, Dopamine quinone formation and [30] W.J. Striet, S.A. Walter, N.A. Pennell, Reactive microgliosis, Prog. protein modification associated with the striatal neurotoxicity of Neurobiol. 57 (1999) 563–581.
methamphetamine: evidence against a role for extracellular dopa- [31] F.B. Weihmuller, S.J. O’Dell, J.F. Marshall, MK-801 protection mine, J. Neurosci. 19 (1999) 1484–1491. against methamphetamine-induced striatal dopamine terminal injury [17] M. Lynch, U. Sayin, J. Bownds, S. Janumpalli, T. Sutula, Long-term is associated with attenuated dopamine overflow, Synapse 11 (1992)
consequences of early postnatal seizures on hippocampal learning 155–163.
and plasticity, Eur. J. Neurosci. 12 (2000) 2252–2264. [32] J.M. Wilson, K.S. Kalasinsky, A.I. Levey, C. Bergeron, G. Reiber, [18] M.J. McCann, J.P. O’Callaghan, P.M. Martin, T. Bertram, W.J. R.M. Anthony, G.A. Schmunk, K. Shannak, J.W. Haycock, S.J. Streit, Differential activation of microglia and astrocytes following Kish, Striatal dopamine nerve terminal markers in human, chronic trimethyl-tin-induced neurodegeneration, Neuroscience 72 (1996) methamphetamine users, Nat. Med. 2 (1996) 699–703.