Plant phenological responses to a long-term experimental
extension of growing season and soil warming in the
tussock tundra of Alaska
R O X A N E H K H O R S A N D R O S A1, S T E V E N F . O B E R B A U E R1, G R E G O R Y S T A R R1 , 2, I N G A P A R K E R L A P U M A1 , 3, E R I C P O P1 , 4, L O R R A I N E A H L Q U I S T1 , 5 and T R A C E Y B A L D W I N1 , 6
1
Department of Biological Sciences, Florida International University, Miami, FL 33199, USA,2Department of Biological Sciences, University of Alabama, Tuscaloosa, AL 35487, USA,3Rutgers University, New Brunswick, NJ 08901, USA,4Bay Area Air Quality Management District, San Francisco, CA 94109, USA,5Parsons Brinckerhoff, San Diego, CA 92101, USA,6NEON, Inc., Boulder, CO 80301, USA
Abstract
Climate warming is strongly altering the timing of season initiation and season length in the Arctic. Phenological activities are among the most sensitive plant responses to climate change and have important effects at all levels within the ecosystem. We tested the effects of two experimental treatments, extended growing season via snow removal and extended growing season combined with soil warming, on plant phenology in tussock tundra in Alaska from 1995 through 2003. We specifically monitored the responses of eight species, representing four growth forms: (i) graminoids (Carex bigellowiiandEriophorum vaginatum); (ii) evergreen shrubs (Ledum palustre,Cassiope tetragona, and Vaccinium vitis-idaea); (iii) deciduous shrubs (Betula nana andSalix pulchra); and (iv) forbs (Polygonum bistorta). Our study answered three questions: (i) Do experimental treatments affect the timing of leaf bud break, flowering, and leaf senescence? (ii) Are responses to treatments species-specific and growth form-specific? and (iii) Which
environ-mental factors best predict timing of phenophases? Treatment significantly affected the timing of all three pheno-phases, although the two experimental treatments did not differ from each other. While phenological events began earlier in the experimental plots relative to the controls, duration of phenophases did not increase. The evergreen shrub,Cassiope tetragona, did not respond to either experimental treatment. While the other species did respond to experimental treatments, the total active period for these species did not increase relative to the control. Air tempera-ture was consistently the best predictor of phenology. Our results imply that some evergreen shrubs (i.e.,C. tetragona) will not capitalize on earlier favorable growing conditions, putting them at a competitive disadvantage relative to phenotypically plastic deciduous shrubs. Our findings also suggest that an early onset of the growing season as a result of decreased snow cover will not necessarily result in greater tundra productivity.
Keywords: Alaska, arctic, climate change, growth form, phenology, season length, snow removal, soil warming, tundra
Received 22 May 2015 and accepted 29 June 2015
Introduction
High-latitude regions are projected to be especially sen-sitive to increases in temperature as a result of climate change (Bonanet al., 1995; Intergovernmental Panel on Climate Change (IPCC), 2014). Although most changes are projected to take place during the cold season (Overpecket al., 1997; Serreze et al., 2000), changes in surface air temperature at the beginning of the growing season have significant effects on timing of snow melt (Clelandet al., 2007; Wipf & Rixen, 2010). Earlier snow melt and reduced snow cover have been reported in North America, particularly for northern Alaska (Frei
et al., 1999; Stone et al., 2002). These changes in snow
properties will drive the growing season as well as affect other important abiotic factors such as thaw depth, soil temperature, water and nutrient availability, and energy fluxes (Billings & Mooney, 1968; Van Wijk et al., 2003). Environmental changes will, in turn, have cascading effects on population dynamics and commu-nity composition of these northern ecosystems (Totland & Alatalo, 2002). Thus, understanding responses of arc-tic plants to changes in snow melt will be criarc-tical to pre-dict shifts in plant community structure and function.
One important consequence of changes in timing of snow melt is the onset and duration of the growing sea-son. Evidence points to a mean of 10–20 days increase
in the growing season over the past three decades (Linderholm, 2006). Tundra plants have the unique characteristic of undergoing extremely rapid leaf and flower expansion (i.e., 1–2 weeks) (Billings & Mooney, Correspondence: Present address: Roxaneh Khorsand Rosa,
School of Biological Sciences, University of Northern Colorado, Greeley, CO 80639, USA, tel. +1 970 351 2923,
1968). Thus, even a small increase of a few days avail-able for plant growth has major implications for plant populations and their pollinators/seed dispersers. However, not all species, nor plant functional types, may show plastic responses to changes in the growing season (Arftet al., 1999; Starr et al., 2000). It is impera-tive, therefore, to consider variation in species and growth form when predicting how the vegetation land-scape will be modified as the climate changes.
In general, arctic regions are experiencing earlier green-up and flowering, as well as longer periods of greening and flowering than in the past (Myneniet al., 1997; Menzel & Fabian, 1999; McCarty, 2001; Jia et al., 2003; Oberbauer et al., 2013; Wu & Liu, 2013). Earlier onset of phenophases is a response to changes in the
abi-otic environment and seasonality (Ernakovich et al.,
2014). For example, the spring flowering index correlates with the incidence of El Nino events and rising sea sur-~
face temperatures (Beaubien & Freeland, 2000), which cause increased surface air temperatures and earlier snow melt (Billings, 1987). Earlier phenology may also be explained by shifts in vegetation cover. Growing evi-dence suggests that rapid climate changes during the twentieth century have caused poleward shifts in species distributions across a wide range of taxonomic groups (Hughes, 2000; Walther et al., 2002; Parmesan & Yohe, 2003). One notable example of a rapid range shift is the increase in abundance of woody shrubs (namelyBetula, Salix, andAlnus) in the Arctic, presumably a response to changes in temperatures, snow melt, and the growing season length (Sturm et al., 2001a; Tape et al., 2006; Higueraet al., 2008; Myers-Smithet al., 2011). Deciduous shrubs in arctic environments have been shown to initi-ate phenological events before other growth forms (Arft
et al., 1999; Molau et al., 2005), potentially advancing
phenology of the entire community.
Given the pervasive changes in arctic plant commu-nities and vegetation distribution, we urgently need field studies that document species-specific and growth
form-specific responses to climate change. Although we
know that plant functional types respond in different ways to environmental manipulation, we still do not
clearly understand which functional types will
acclimate best to altered environmental conditions (Dormann & Woodin, 2002). As part of the ongoing work of the International Tundra Experiment (ITEX) (Webber & Walker, 1991), our study examined the effects of lengthened growing season and soil warming on the vegetative and reproductive phenology of eight species in the tussock tundra of northern Alaska. We simulated conditions predicted for climate change, namely earlier loss of winter snowpack, later develop-ment of snowpack, and greater soil temperature during the growing season. We accomplished these
simula-tions by manually removing snow early in the spring and maintaining plots snow free at the end of the sea-son, as well as warming the soil (Oberbaueret al., 1998; Starret al., 2000). While experimental warming and/or season manipulation is an increasingly common approach used to quantify phenological responses of plants to climate change (Chapinet al., 1995; de Valpine & Harte, 2001; Dunne et al., 2003; Hollisteret al., 2005; Buizer et al., 2012), our research is unique both in its methodology and duration. First, as opposed to using snow fences, which can result in large nutrient inputs
(Fahnestock et al., 2000), we manipulated snow cover
by manually removing snow. Second, our study sepa-rates the effects of earlier snow melt and soil warming. Finally, the duration of our study is one of the longest for active season extension experiments in the low Arctic (Wipf & Rixen, 2010).
Our study aims to answer three primary questions: (i) Do early snow removal and soil warming affect the timing of leaf bud break, flowering, and leaf senes-cence, as well as the proportion of individuals in each phenophase? (ii) Are responses to treatments species
-specific and/or growth form-specific? (iii) Which
envi-ronmental factors best predict timing of leaf bud break, flowering, and senescence? First, we hypothesized that leaf bud break, flowering, and senescence would begin earliest in the soil warming+snow removal plots and
latest in the control plots, with an intermediate effect in the snow removal only plots. Second, we hypothesized that treatment responses would vary among species and growth forms. Deciduous shrubs and graminoids would be the most responsive to treatment effects, demonstrated by earlier greening, flowering, and senes-cence than other growth forms. This hypothesis is based on convincing evidence of an increase in decidu-ous shrubs and graminoids in the Arctic in response to experimental warming (Chapin et al., 1995; Chapin & Shaver, 1996). Finally, we hypothesized that no single environmental variable would be the most important for the duration of the study. Rather, we expected specific environmental variables to have shifting rela-tive importance during different periods of the growing season.
Materials and methods
Experimental design
tus-sock dwarf shrub tundra is defined by the sedge, cotton grass (Eriophorum vaginatum). Intertussock spaces are occupied by deciduous and evergreen shrubs, herbaceous forbs, and peat-forming mosses (Bliss & Matveyeva, 1992).
Our experiment consisted of three treatments: control (C), extended Season (ES), and extended season+warming (ESW). In this study, experimental treatment refers to the two latter treatments. We extended the growing season by care-fully removing snow and preventing new snow accumulation at the beginning of the growing season as well as preventing snow accumulation and light frosts at the end of the growing season (Oberbaueret al., 1998). Here, we define growing sea-son as the period of favorable conditions under which growth can occur. The active period is defined as the period of activity lasting from bud break to leaf senescence or browning. Snow was removed over the days May 1–2, which is 2–5 weeks
before normal snow melt. Once snow was removed, open-ended, polyethylene-covered A-frames were maintained on the ES and ESW plots to keep plots snow free and protect against light frosts until the early-season danger of frosts had passed (usually June 1). The tents were open-ended to mini-mize changes in air temperature. Our intent was to lengthen the season by accelerating the loss of snow, not to provide a season-long warming of air temperature. In the event of an impending snow or windstorms, the ends were closed tem-porarily with transparent fiberglass panels. The tents were placed back on experimental plots during the second week of August and maintained until the end of August, to protect against early winter snow accumulation or light frosts. Because early snow removal did not ensure that the soil would thaw sufficiently for belowground plant metabolism, we included a soil warming treatment. In the warming plots, we installed greenhouse-heating wires uniformly throughout the plots approximately 10 cm below the ground surface in 1994, the year before data collection began. A 1400-watt generator powered the heating wires for two hours per day, releasing 0.4 MJ m2day1of energy to the soil at midday. This energy input is equivalent to 20–30% of the daily soil heat flux for this
ecosystem during summer months (Eugsteret al., 2005), which has been compared to the magnitude of increase in energy pre-dicted with global warming (Maxwell, 1992; Starret al., 2008).
We used a randomized block design, consisting of 18 plots, six replicates per treatment. Each plot was measured 1.5 by 1.5 m, and each plot was separated by 3 m. Our sample size was limited by the intense labor required to (i) manually remove the snow over a short period to maintain a uniform growing season length in all snow removal plots and (ii) main-tain the experimental treatment plots snow free. We accessed plots using an elevated boardwalk so as to minimize effects on the plant community. In each plot, six individual shoots belonging to eight species were tagged and monitored throughout the season. We studied eight species belonging to the four broad functional types of the Alaskan tundra, as out-lined by Molau (1997): sedges (Eriophorumvaginatum L.) and (Carex bigellowii Torr.), evergreen shrubs (Ledum palustre L., Cassiope tetragona(L.) D. Don, andVaccinium Vitis-idaea L.), deciduous shrubs (Betula nana L. and Salix pulchra Cham.), and forbs (Polygonum bistorta L.). We chose these species
because excluding Cassiope tetragona, these seven species occupy greater than 90% of the ground cover of the tussock tundra (McKaneet al., 1997). We includedC. tetragonabecause it is one of the focal species in the ITEX study.
Phenological variables
Weekly or more frequent phenological observations were per-formed for each of the eight species in each plot. During these observations, we recorded the status of three response vari-ables: (i) leaf bud break; (ii) flowering; and (iii) leaf senescence. Leaf bud break was defined as any point where bud scales parted and leaf emergence was visible. We equate leaf bud break to greening and use the terms interchangeably. Direct bud break observations cannot be made for the graminoids, C. bigellowiiandE. vaginatum, and the forb,P. bistorta, because the buds are located beneath the soil surface. ForC. bigellowii andP. bistorta, leaf greening equates to leaf appearance above the surface.Eriophorum vaginatumis a wintergreen species and some leaves remain green over the winter.
We considered both the timing of flowering as well as the intensity of flowering (Larigauderie & Kummerow, 1991). Plants of all species were assessed for flowering status and assigned one of three categories during each phenological observation: (i) floral bud, (ii) anthesis, and (iii) postanthesis. We define anthesis as it relates to functional pollination: The flower is open and reproductive structures (styles and anthers) are intact and not wilted. The total proportion of indi-viduals in bloom was also calculated for each species and plot. For end of season leaf changes, leaves of the current year (new leaves) were assigned one of the three categories: (i) 1–30%
brown, (ii) 31–60% brown, and (iii)>60% brown. Leaf
senes-cence was defined as when the third category occurred. In this study, leaf senescence is synonymous with leaf browning. Leaf senescence in evergreens typically does not occur at the end of the growing season, but rather as new leaves expand. However, leaves of evergreens that are retained undergo browning at the end of the season as plant photo-protective pigments, specifically anthocyanins, accumulate in prepara-tion for winter (Oberbauer & Starr, 2002). We used the timing of this leaf browning to indicate the end of the active period for a given year. We specifically analyzed current-year leaves to be able to compare evergreen and deciduous species.
This study was only concerned with aboveground changes in phenology. Although snow removal and soil warming may have caused significant changes belowground, we restricted our observations to aboveground because these long-term study plots were nondestructive. Leaf data (greening and senescence) were recorded for a total of 9 years (1995 through 2003). Funding constraints shortened the field season in 2003 resulting in no data in August of that year. Flowering data were recorded for 6 years (1995, 1996, 1997, 1998, 2000, and 2003).
Abiotic factors
length or photoperiod was calculated for each day of each study year. In each plot, the active layer was measured on a weekly basis using standard soil probing techniques (Ober-bauer et al., 1991). Two readings were taken at randomly selected intertussock locations in each plot to compute a mean measurement. Soil moisture (volumetric water content) was also measured in each plot using Campbell Scientific 615 Water Content Reflectometers (Campbell Scientific Inc., Logan, UT, USA), following methods of O’Brien & Oberbauer (2001). Steel probes of the soil moisture sensors were inserted at a 45 degree angle relative to the soil surface, integrating soil moisture over the top 20 cm of soil. Sensors were left in the soil throughout the study. Weekly measurements were used to calculate monthly means of depth of thaw and volumetric water content. Soil temperature was not included in our analy-ses. Prior work on these plots shows that although soil tem-peratures were highest in the warming plots, these differences were not statistically significant (Starret al., 2008).
Statistical analyses
One-wayANOVAs were used to compare the duration of each phenophase (greening, flowering, and senescence) among treatments, as well as the total active growing period among treatments. Chi-square tests were conducted to quantify the relationship between treatment and frequency, or proportion of individuals demonstrating leaf bud break, flowering, and leaf senescence. To determine whether the timing of each of the phenological response variables differed among treat-ments, a 2-wayANOVAwas performed for each variable, ana-lyzing main effects (species and treatment) and the interaction between the two terms. We also conducted a 2-way ANOVA testing the effects of growth form, treatment, and the interac-tion on timing of each of the response variables. A one-way ANOVAwas used to determine whether the mean day of year (DOY) of flowering differed significantly among years. Least significant differences, Tukey, and Dunnett C adjustments were applied to pairwise comparisons. We conducted a sim-ple linear regression between DOY and year for each pheno-logical response variable to determine whether the timing of phenophases was progressively earlier as the study pro-gressed. A simple linear regression was also performed to determine the relationship between date of snow melt and bud break in each plot. The relationship between each of the three response variables and air temperature was determined using a Pearson’s correlation. Thawing degree days (Molau & Mølgaard, 1996) were calculated for each plot, species, and year by summing the temperatures between the date each plot was recorded as snow free and the first record of flowering. Flowering frequency, defined as the proportion of population in bloom, was calculated for each DOY postsnow melt. The relationship between flowering frequency and thawing degree days was determined using a Spearman’s rank correlation. Data were tested for normality before proceeding with para-metric tests. Data that were unsuccessfully transformed were analyzed using nonparametric procedures (Zar, 1984).
In addition to evaluating the effect of treatment and species on the timing of response variables, we also asked how abiotic
factors influenced phenology and which factors most strongly predicted changes in phenological outcomes. We conducted a binomial logistic regression for greening and flowering using the Enter Method. An ordinal logistic regression was used for leaf senescence. Species, year, treatment, and snow-free status of plot comprised categorical variables while air temperature, depth of thaw, photoperiod, and soil moisture comprised con-tinuous variables. The CS615 soil moisture sensors were not installed until 1997, so soil moisture data were not available for 1995 and 1996. Consequently, these years were excluded from the bud break and flowering analyses in which all years were analyzed together. Soil moisture was not significant in the leaf senescence model (all years), so 1995 and 1996 were included in the ordinal regression. We defined snow melt for each particular plot as when 50% or more of the plot was snow free (Rumpfet al., 2014). The regression coefficient (b), a measurement of how strongly each predictor variable influ-ences the dependent variable, and the odds ratio (Exp(b)), the likelihood that each phenophase will occur, were calculated for each independent variable. Correlations between indepen-dent variables were verified before proceeding with the regression. Regressions were conducted for all years together to determine general patterns, as well as separately, to explain sources of variation among years and identify the most consis-tent independent variables across years. The goodness-of-fit for each logistic model was assessed by evaluating the2 log likelihood, modelX2, and pseudoR2values before reporting results.
Finally, given results from the logistic regressions, we asked how air temperature of previous years affected bud break and flowering. Simple linear regressions were carried out to deter-mine the relationship between the monthly proportions of individuals in bud break (and anthesis) and mean monthly air temperature 1, 2, and 3 years prior to the study year. Regres-sions were conducted for all species together, as well as each species separately. All analyses were performed in IBM SPSS statistics version 21 (SPSS, Chicago, IL, USA).
Results
Effects of treatment, growth form, and species on phenology
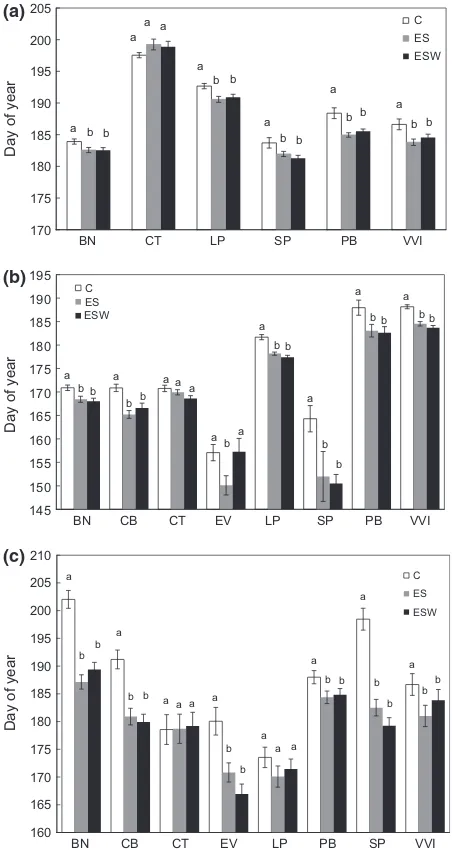
Treatment plots became snow-free a mean of 24 days
before control plots, although we observed large inter-annual variation among treatments, ranging from 1 to 35 days. The earliest snow-free status was recorded in 1998 (DOY 119, April 28), and the latest in 2000 (DOY 160, June 8). We used our plant phenophase data to define total period of activity, that is, the total number of days from onset of leaf bud break to the onset of leaf senescence. Overall, total active period lasted 114 days, independent of treatment. Even when we conducted separate analyses by year, total duration of the active period for all species combined did not differ among the three treatments (F2,24=0.003,P >0.05). However,
the three phenophases and the proportion of the popu-lation in each phenophase.
Bud break. The percent of bud break over the course of
the entire study period was lowest in the control plots and highest in the experimental treatment plots (X2=62.73, df=2, P<0.001). Bud break occurred
2 days later in control plots compared to experimental treatment plots (F2,36102=17.72,P <0.001), although no
difference was found in timing of bud break between
snow removal (ES) and snow removal+warming
(ESW) treatments (Fig. 1a). We also found no signifi-cant difference in duration of bud break among the
experimental treatments and control (F2,24=0.07,
P>0.05). A significant linear relationship was found
between timing of snow melt and timing of bud break (F1,36118=481.31, P<0.001) using the overall data set.
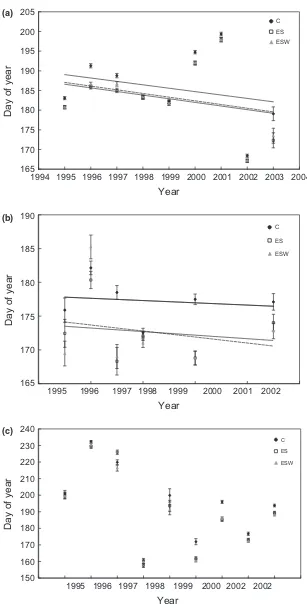
Bud break occurred progressively earlier as the study progressed (Fig. 2a) (F1,36118=152.82, P <0.001). The
timing of bud break was also significantly affected by growth form (F2,36111=397.83,P <0.001) and the
inter-action between growth form and treatment
(F4,36111=2.41,P<0.05). Deciduous shrubs broke their
buds a mean of 7 days earlier than evergreen shrubs and 4 days earlier than the forb. The mean DOY of bud break was up to 3 days earlier in the experimental treatment plots than the control plots for all growth forms.
Flowering. The vast majority of flowering took place
each year between June 1 (DOY 151) and July 9 (DOY
191). However, Eriophorum vaginatum began flowering
earlier than all other species, as early as May 11 (DOY 131). Salix pulchra also tended to flower early, while
Polygonum bistorta and Vaccinium vitis-idaea tended to
flower later than other species. The proportion of indi-viduals in bloom was significantly highest in the treat-ment plots and lowest in the control plots (X2 =49.05,
df=6,P <0.001). Flowering in the experimental
treat-ment plots occurred significantly earlier than in the control plots, by a mean of 5 days (F2,2388 =23.20,
P<0.001), although no significant difference was
found between experimental treatments (Fig. 1b). The duration of flowering did not differ among treatments (F2,21=0.11,P>0.05). The mean day of flowering
dif-fered among years (F5,2586 =45.06, P<0.001).
Flower-ing tended to occur earlier as the study progressed (Fig. 2b). Year was a good predictor of DOY of
flower-ing for both experimental treatments (ES: F1,
751=14.41, P<0.001; ESW: F1,846 =15.59, P<0.001),
but not for the control (F1,788=0.74,P >0.05). Growth
form (F3,2379=386.54, P <0.001) and the interaction
between growth form and treatment (F6,2379=3.56,
P=0.002) significantly affected the timing of flowering.
Graminoids flowered a mean of 9 days earlier than deciduous shrubs, and 20 and 25 days before evergreen shrubs and the forb, respectively. Although flowering began earlier in treatment plots compared to the control plots, it did not last longer (F2,21 =0.11,P >0.05).
Leaf senescence. Treatment significantly affected the tim-ing of leaf senescence (F2,11707=46.45, P <0.001)
170
Fig. 1 Mean day of year of leaf bud break (a), floral anthesis (b),
and leaf senescence (c) for the entire study period. C=control,
ES=extended season, and ESW=extended season+warming;
BN=Betula nana, CB=Carex bigellowii,CT=Cassiope tetragona,
EV=Eriophorum vaginatum, LP=Ledum palustre, PB=
Poly-gonum bistorta, SP=Salix pulchra, VVI=Vaccinium vitis-idaea.
Gra-minoids were not included in the bud break analysis. Different
letters indicate significant differences atP=0.05 within species
165 170 175 180 185 190 195 200 205
1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004
Day of year
Year
C ES ESW
165 170 175 180 185 190
Day of year
Year
C
ES
ESW
1995 1996 1997 1998 1999 2000 2001 2002
150 160 170 180 190 200 210 220 230 240
Day of year
Year
C ES ESW
1995 1996 1997 1998 1999 2000 2002 2002 (a)
(b)
(c)
Fig. 2 Mean day of year of leaf bud break (a), floral anthesis (b), and leaf senescence (c) for each treatment and study year. C=control,
ES=extended season, and ESW=extended season+warming. Error bars represent standard error of mean. Solid trend line
repre-sents control (C), small hashed line reprerepre-sents early snow removal (ES), and large hashed line reprerepre-sents early snow removal+soil
(Fig. 1c). Leaf senescence occurred a mean of 8 days earlier in the experimental treatment plots than in the control plots, but timing did not differ between the treatment plots. A downward trend was detected between year and DOY, that is, senescence occurred earlier in the later years of the project. Negative slopes for the control and treatments suggest a 4-day advance in onset of leaf senescence for the control and 5-day advance for the experimental treatments with each additional study year (Fig. 2c). Year accurately pre-dicted DOY of senescence for the control and experi-mental treatments (C: F1,3229 =219.31, P<0.001; ES:
F1,4156 =366.21, P<0.001; ESW: F1,4340 =404.25,
P<0.001). Growth form (F3,9314 =427.53, P<0.001)
and the interaction between growth form and treatment (F6,9314=10.26, P <0.001) also affected the timing of
leaf senescence. In other words, leaf senescence occurred significantly later in the control plots com-pared to the experimental plots in every single growth form. Each pairwise comparison among the four growth forms was also significantly different, with evergreen shrubs and graminoids browning up to 3 weeks before deciduous shrubs and the forb.
Species-specific responses. Phenological responses to
treatment were species-specific. The highest proportion
of bud break was recorded inPolygonum bistortawhile the highest proportion of flowering occurred in Eriopho-rum vaginatum. Species significantly affected timing of greening (F5,36102=288.14, P<0.001), flowering
(F7,2367=284.91, P<0.001), and leaf senescence
(F7,11707=53.44, P<0.001). The mean day of bud
break, flowering, and leaf senescence differed signifi-cantly among experimental treatments and controls for all species except Cassiope tetragona. In general, C.
te-tragona showed no response to either experimental
treatment. In contrast, Betula nana, Salix pulchra, and
Vaccinium vitis-idaea were highly responsive to both
treatments (Fig. 1a–c). The duration of flowering
dif-fered significantly between Eriophorum vaginatum and
each of the other species (F7,16=13.01, P <0.001).
Duration of flowering did not differ significantly between any other species. Not only did treatment and species affect the timing of each phenological variable, the interaction between species and treatment was also significant (greening: F10,36102=2.42, P=0.007;
anthe-sis: F14,2367=2.46, P =0.002; leaf senescence:
F14,11707=4.65,P<0.001).
Abiotic variables as determinants of phenology
Bud break. When all years were pooled together, each
of the independent variables (year, species, treatment, air temperature, photoperiod, soil moisture, depth of
thaw, and snow-free status of plot) significantly pre-dicted the probability of bud break (Table S1). Within these explanatory variables, the highest probability of bud break (indicated by a high odds ratio) was
associ-ated with Polygonum bistorta, the snow removal and
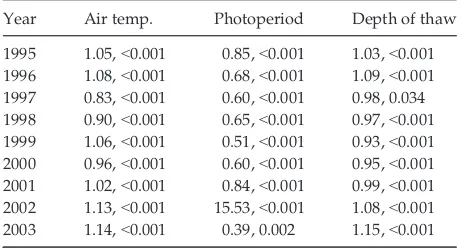
warming treatments, soil moisture and timing of snow melt. However, when the analysis was separated by year, species (specifically Polygonum bistorta) and air temperature were consistently the best predictors across all years (Table 1). Photoperiod, depth of thaw, and treatment were also good predictors, although less so than species and air temperature.
Flowering. When all years were analyzed together, the
following independent variables were associated with a high probability of flowering: year, species, treatment, air temperature, photoperiod, and depth of thaw. Soil moisture and date of snow melt were not significant predictors in the model. The highest probability of anthesis was associated withEriophorum vaginatum, air temperature, and photoperiod (Table S2). On a year-to-year basis,Eriophorum vaginatum, air temperature, and depth of thaw were consistently associated with flower-ing, making them the best predictors of flowering (Table 2). We also found a significant relationship between flowering frequency and thawing degree days
for all species (Spearman’s rank correlation=0.72,
P<0.001).
Leaf senescence. When all years were analyzed together,
year, species, treatment, air temperature, photoperiod, depth of thaw, and snow-free status of plot significantly predicted timing of leaf senescence (Table S3). Of the significant explanatory variables, air temperature, depth of thaw, and year best predicted leaf browning, (indicated by a high odds ratio). Soil moisture was not significant. On a year-to-year basis, air temperature, photoperiod, and depth of thaw most consistently pre-dicted senescence; these three variables were significant in 100% of the models (Table 3). Soil moisture, treat-ment, and snow-free status of plot were significant pre-dictors of leaf senescence for fewer than half of the study years.
Delayed effects of air temperature on phenology
We found a significant relationship between air temper-ature of previous years and the frequency of bud break of all species combined; 1-year time lag (F1,610=137.39,
P<0.001), 2-year time lag (F1,610=175.96, P<0.001),
and 3-year time lag (F1,610 =156.30,P <0.001). Each of
time lag (F1,524=17.31, P <0.001), 2-year time lag
(F1,524=18.44, P<0.001), and 3-year time lag
(F1,526=15.54, P<0.001), were all significant.
How-ever, when analyzed alone, not each species showed a significant response to air temperature carryover effects. This pattern was consistent for 1-, 2-, and 3-year time lags (Table 4).Eriophorum vaginatum,Ledum palus-tre,Polygonum bistorta, andVaccinium vitis-idaea consis-tently responded to air temperature up to 3 years prior to actual flowering, while Betula nana, Carex bigellowii,
Cassiope tetragona, and Salix pulchra showed no
response.
Discussion
Our results clearly show that early snow removal induces earlier leaf bud break, senescence, and floral anthesis relative to the controls. Previous ITEX results also reported earlier leaf burst and flowering in
season-long-warmed plots (Arft et al., 1999). However, our
findings show that although earlier loss of snow cover caused earlier onset of phenophases, the total period of activity did not increase because senescence was also accelerated. These results contrast Arft et al. (1999), which reported delayed senescence in response to warming of air, resulting in longer overall periods of plant activity. Thus, our study suggests that species which do not respond immediately to favorable envi-ronmental conditions will be at a disadvantage relative to phenologically plastic species. Our findings corrobo-rate a growing number of studies showing that plant growth at high latitudes may begin earlier but may not necessarily last longer. In fact, warming temperatures may cause the growing season to shorten (Post et al., 2001; Linderholm, 2006; Shutova et al., 2006). Previous
Table 3 Best predictors of leaf senescence, separated by year. Odds ratio andP-values are indicated for each predictor vari-able: air temperature, photoperiod, and depth of thaw
Year Air temp. Photoperiod Depth of thaw
1995 1.05,<0.001 0.85,<0.001 1.03,<0.001 1996 1.08,<0.001 0.68,<0.001 1.09,<0.001
1997 0.83,<0.001 0.60,<0.001 0.98, 0.034 1998 0.90,<0.001 0.65,<0.001 0.97,<0.001
1999 1.06,<0.001 0.51,<0.001 0.93,<0.001 2000 0.96,<0.001 0.60,<0.001 0.95,<0.001
2001 1.02,<0.001 0.84,<0.001 0.99,<0.001 2002 1.13,<0.001 15.53,<0.001 1.08,<0.001 2003 1.14,<0.001 0.39, 0.002 1.15,<0.001
Table 4 Relationship between 1-year, 2-year, and 3-year time lags in air temperature (°C) and frequency of flowering in each
species; where BN=Betula nana, CB=Carex bigellowii,
CT=Cassiope tetragona, EV=Eriophorum vaginatum, LP=
Le-dum palustre, PB=Polygonum bistorta, SP=Salix pulchra, and
VVI=Vaccinium vitis-idea. Results are from linear regressions
Species 1-year time-lag 2-year time-lag 3-year time-lag
BN F=1.61NS F=1.72NS F=2.46NS
CB F=0.98NS F=1.42NS F=1.84NS CT F=1.93NS F=1.00NS F=0.99NS EV F=16.75** F=4.79* F=9.77**
LP F=8.96** F=5.64* F=4.79* PB F=7.98** F=7.12* F=7.28**
SP F=0.46NS F=0.004NS F=0.00NS VVI F=35.56** F=27.34** F=23.38**
Df values for all categories is 64, 1.P-values <0.05 are indi-cated by*,P-values<0.01 are indicated by**.
Table 1 Best predictors of leaf bud break, separated by year. Odds ratio andP-values are indicated for each predictor variable: Species (PB=Polygonum bistorta), air temperature, photoperiod, depth of thaw, and treatment (ES=extended season, ESW=
extended season+warming)
Year Species–PB Air temp. Photoperiod Depth of thaw ES ESW
1997 28518.13,<0.001 1.72,<0.001 2.89,<0.001 1.05, 0.004 1.43, NS 0.76, NS 1998 154.67,<0.001 1.43,<0.001 0.75, 0.05 1.12,<0.001 1.13, NS 0.66, 0.003
1999 63.98,<0.001 1.06,<0.001 1.06,<0.001 1.06,<0.001 0.79, 0.025 0.98, NS 2000 2.86,<0.001 1.14,<0.001 1.14,<0.001 1.11,<0.001 0.62,<0.001 0.96, NS
2001 4.91,<0.001 1.06,<0.001 1.06,<0.001 1.07,<0.001 2.16,<0.001 0.71,<0.001 2002 39.23,<0.001 1.06,<0.001 1.06,<0.001 1.07,<0.001 0.93, NS 0.98, NS
2003 1.57,<0.001 1.08, 0.004 1.08,<0.001 1.02, NS 0.75, NS 0.99, NS
Table 2 Best predictors of flowering, separated by year. Odds ratio andP-values are indicated for each predictor vari-able: Species (EV=Eriophorum vaginatum), air temperature,
and depth of thaw
Year Species–EV Air temp. Depth of thaw
1995 31.33,<0.001 1.07, 0.035 1.07,<0.001
1996 0,<0.001 0.91,<0.001 1.15,<0.001
1997 292.57,<0.001 0.94, 0.021 1.07,<0.001 1998 118.52,<0.001 1.52,<0.001 1.08,<0.001
work in our plots found that season duration did not increase physiological activity in response to the manipulation (Oberbaueret al., 1998; Starret al., 2000).
Høyeet al.(2013) found an inverse correlation between
duration of flowering and temperature, suggesting that as the summers get warmer in the Arctic, the flowering season will shorten. A shorter flowering season in the Arctic has been linked to decreases in floral resources and visitation rates (Pottset al., 2010).
Species variation is an important factor to consider when evaluating the phenological and physiological responses of tundra communities to climate change (Starret al., 2008; Cooperet al., 2011). Our study shows that species do not respond uniformly to an extended snow-free period and soil warming. In contrast to the
other seven species, Cassiope tetragona exhibited no
response to either treatment. Molau (1997) also found
lit-tle response of C. tetragona to warming treatments.
Therefore, we could classifyC. tetragona, and possibly Polygonum bistorta(Starret al., 2000), as periodic species, or those which have a genetically fixed growth strategy.
Highly responsive species such as Salix pulchra and
Vaccinium vitis-idaeafit the classification of aperiodic, or plastic, species (Sørensen, 1941). Such species can adapt their growth strategy to changing environmental condi-tions and consequently may out-compete species with nonplastic growth strategies (Lechowicz, 1995).
Our data corroborate the idea that present plant growth directly reflects past environmental conditions. Many species in the Arctic produce bud primordia underground, sometimes up to several years prior to flowering, as an evolutionary adaptation to a short growing season (Sørensen, 1941; Billings & Mooney, 1968). Diggle (1997) showed that in the alpine species, Polygonum viviparum, the maximum reproductive out-put of each inflorescence is already determined 1-year prior to actual maturation. Moreover, the development of each leaf and inflorescence, from initiation to func-tional and structural maturity, requires 4 years. Not only may underground buds take longer to develop, but they also may be susceptible to abiotic conditions prior to the season of observation. In other words, the effect of air temperature on plant growth may not man-ifest itself until several years later (Arftet al., 1999). Our results imply that time lags in air temperature do not affect flowering in all species in the same way. The non-plastic response in C. tetragona to air temperature is what we would expect from a periodic species which cannot respond to current, nor past, abiotic conditions. In contrast, we would expect species such as Eriopho-rum vaginatum, which develop preformed buds, to be highly sensitive to past environmental conditions. Although it is implied in the literature that bud forma-tion is susceptible to past abiotic condiforma-tions, few studies
actually address how delayed responses in flowering and greening vary across a range of species.
The timing of phenophases with respect to climate will play a key role in species resiliency and interspeci-fic competition. In tussock tundra, earlier snow melt may affect early greening and flowering species such as
E. vaginatum and S. pulchra more than late greening
and flowering species such asV. vitis-idaeaandB. nana. Earlier flowering species are more dependent on timing of snow melt than late-flowering species (Dunneet al., 2003; Kudoet al., 2008; Wipf, 2010). Abundance of spe-cies with early leaf expansion was higher in early snow melt plots than late snow melt plots in alpine areas of Colorado (Galen & Stanton, 1995). Molau (1993) hypothesized that late-bloomers could be short-term ‘winners’ under climate change. The probability of suc-cessful pollen transfer and seed germination would be higher for these individuals because they could take advantage of the end of the growing season. While ear-lier flowering species have a longer timespan to set fruit and disperse their seeds, they can potentially lose all their reproductive output because of early-season frost, a typical outcome of warming (Inouye, 2008). Although it still remains unclear if plastic growth strategies in response to early snow melt will ultimately be advanta-geous for a species or not, it underlines the possibility that phenotypic acclimation to warming may be more important for specific individuals and populations than general macro-evolutionary adaptations (Totland and Alatalo, 2002). Furthermore, phenotypic adaptation will have direct effects on other biotic interactions. Variation among early-flowering individuals and late-flowering individuals can alter the length of the flowering season, imposing immediate effects on population dynamics and plant–pollinator interactions (Høyeet al., 2013).
In addition to the varying responses of species to treatment, we also found the contribution of each spe-cies to differ in each of the regression models. Although species, in general, significantly predicted bud break and flowering, the likelihood of bud break and flower-ing was an order of magnitude higher inP. bistortaand E. vaginatum, respectively. One interpretation is that such high odds ratios simply reflect the high frequency of these two species and their life histories. Rates of growth (both vegetative and reproductive) and regen-eration tend to be higher in herbaceous than woody plants (Bazzaz, 1979). Thus, we must consider relative abundance of these species when interpreting the results. Alternatively,E. vaginatum andP. bistortamay be more predictable than other species and do well in a more tightly controlled environment. In contrast to bud break and flowering, we did not detect a species-
speci-fic or growth form-specific pattern with respect to leaf
are equally likely to senesce at the end of the season. Oberbaueret al. (2013) found senescence of all growth forms to occur at similar thaw degree day values. It is also important to point out that the most abundant species will not necessarily be more resilient to changes in the growing season. Our results suggest that
E. vaginatum will be at a competitive disadvantage to
deciduous shrubs.
While plant phenology is dependent upon many abi-otic factors, our study suggests that air temperature is the key determinant of the onset of vegetative and reproductive phenology in the tundra. Temperature significantly predicted each of the three phenophases, both when years were pooled and analyzed separately. The strong correlation between thaw degree days and flowering further highlights the critical role air temper-ature plays in phenology. Surface air tempertemper-ature is one of the most useful climate change variables as it accounts for changes in the surface energy budget and atmospheric circulation (Serreze et al., 2000). Air tem-perature is disproportionately important as it dictates, in part, depth of thaw, with indirect effects on other abiotic variables including water and nutrient availabil-ity. There is ample evidence demonstrating that tem-perature determines many phenophases, in general
(Badeck et al., 2004) and particularly in arctic and
alpine species (Dunneet al., 2003; Huelberet al., 2006; Euskirchen et al., 2014). Timing of bud break inBetula
nanaandSalix pulchrawas found to be a function of air
temperature (Popet al., 2000). Air temperature was the strongest predictor of the commencement of photosyn-thesis in an evergreen boreal forest (Tanjaet al., 2003).
With the exception of air temperature, environmental variables such as snow melt, thaw, and photoperiod exert different degrees of importance at different peri-ods in the growing season. Date of snow melt was the most relevant for bud break, which suggests that plants are limited by snow cover early in the season, when the majority of growth occurs. These results corroborate the strong, positive linear relationship we found between timing of snow melt and bud break. Other studies have also found bud break of evergreen and deciduous species to be dependent on timing of snow
melt (Shaver & Kummerow, 1992; Shevtsova et al.,
1997). Earlier snow melt since the 1950s is well docu-mented in the Arctic and is predicted to be one of the primary consequences of climate change (Foster, 1989; Aurela et al., 2004; Post et al., 2009). Thus, we predict earlier bud break in the tundra as the timing of snow melt advances.
Soil thaw also explained leaf bud break in our mod-els, consistent with other studies. Soil thaw accurately
predicted bud break ofB. nana 3 years in a row (Van
Wijket al., 2003). In our plots, photoperiod significantly
predicted vegetative phenology for the majority of study years, but not for flowering. These results make intuitive sense; an increase in hours of light is a key trigger for plants to begin vegetative processes, while a decrease in hours of light is a key trigger for plants to prepare for winter (Habjørg, 1972). Photoperiod is at a maximal constant during peak flowering (June/July), however. Photoperiod may play an important role in the timing of early- or late-bloomers, but has a negligi-ble effect for the majority of flowering individuals. Our results agree with other studies suggesting that snow cover and depth of thaw may be especially important in the early season (when greening occurs), while pho-toperiod and genetic constraints become increasingly important in the late-season (associated with senes-cence) (Shaver & Kummerow, 1992; Oberbauer et al., 1998; Van Wijket al., 2003; Estiarte & Pe~nuelas, 2015).
While early snow melt allows plants to get a ‘jump-start’ on growth, it also exposes them to early-season low temperatures, damaging frost, and drier conditions (Wookey & Robinson, 1997; Sturm et al., 2005). Soil warming likely aggravated drying effects in our study, probably explaining why we did not observe a differ-ence in phenology between the two experimental treat-ments. Drier tundra organic soils are highly insulative, reducing thermal transfer from the soil surface to dee-per layers (Hinkelet al., 2001); as a result, the warmed plots had slightly shallower depth of thaw than the extended season plots. Although a lengthened snow-free period offers plants the opportunity for a longer period of potential growth, it can also lead to water stress. A lack of water obstructs effective nitrogen assimilation (Kramer & Boyer, 1995) and prevents plants from taking advantage of a longer growing sea-son. Other studies have attributed delayed reproduc-tive phenology (Dorjiet al., 2013) and declines in flower production (de Valpine and Harte, 2001) to soil drying caused by soil warming.
Growth form affects snow accumulation and has a consequential ripple effect through the landscape (Sturmet al., 2001b, 2005). Furthermore, different plant functional types with different life histories will respond heterogeneously to climate change, potentially introducing asynchronies into plant–plant and plant–
1996). Evergreen shrubs tend to be slow growing and rely heavily on internal cycling of nitrogen, while deciduous shrubs are characterized by rapid growth and can access soil-available nitrogen (Aerts, 1995).
Starr et al. (2008) demonstrated that photosynthetic
rates (directly affecting and affected by phenology) in our plots were highest for deciduous shrubs and lowest for evergreen shrubs. Also, deciduous shrubs must ‘take a chance’ and break buds before other plant func-tional types (Billings & Mooney, 1968), monopolizing resources and increasing recruitment probability. In our study, deciduous shrubs senesced later than ever-greens and graminoids, suggesting that deciduous shrubs are best suited to take advantage of an extended growing season. These results support growing evi-dence of deciduous shrub expansion in the tundra (Sturmet al., 2001b; Stowet al., 2004; Tapeet al., 2006; Walker et al., 2006). We are not the first to predict a
decline in evergreen shrubs including C. tetragona
(Grime, 1979; Billings & Peterson, 1992; Molau, 1997; Buizer et al., 2012) and a consequential reduction in species diversity (Walkeret al., 2006).
As expected, temporal variation was significant among years. Anomalous weather events explain, to an extent, seasonal phenology. In alpine communities, 15–
40% of among-year variation in phytomass was
attribu-ted to interannual climate variation (Walker et al.,
1994). The dynamic interaction between abiotic factors and interannual variation also may explain nonlinear phenological responses to climate (Ileret al., 2013). In our study, 1999 was marked by unusually early snow melt, warm spring conditions, and an extended dry period. Other seemingly random events such as the
complete absence of flowering in S. pulchra in 1996
make generalizing temporal patterns difficult.
Although our results suggest a unidirectional, down-ward trend in onset of phenology, we interpret these results with caution. Further work is crucial so we can describe with more certainty the relationship between timing of phenophases and year. Nevertheless, our results add to a growing body of evidence demonstrat-ing earlier onset of phenology at northern latitudes (Zhou et al., 2001; Badeck et al., 2004; Postet al., 2009; Bocket al., 2014).
Finally, our work highlights the possibility that advanced snow melt and phenology will not necessar-ily be accompanied by a net increase in productivity. While greening began significantly earlier in the treat-ment plots, the total duration of activity did not increase, suggesting that an earlier start to the growing season, in and of itself, is not enough to increase pro-ductivity. Our phenological observations are consistent with finding of La Pumaet al.(2007), who reported no increase in net ecosystem productivity at Toolik despite
increased season length. Perhaps plants need addi-tional nutrients to be able to take advantage of a longer growing season. Nataliet al. (2012) point to N limita-tion as the primary explanalimita-tion why ecosystem-level net primary productivity did not increase despite experimental lengthening of the growing season. Fertil-ization effects have been shown to have a greater influ-ence on productivity in the Arctic than warming effects (Chapin et al., 1995; Dormann & Woodin, 2002; Van
Wijk et al., 2004; but see Larigauderie & Kummerow,
1991).Eriophorum vaginatumappears to be more limited by nutrients than photosynthesis in the Alaskan tussock tundra (Tissue & Oechel, 1987). Growth inLedum and
Eriophorumwas highly constrained by nutrients in the
late-season (Chapin & Shaver, 1996). Earlier phenology may cause a feedback mechanism by which nutrient limitation is further exacerbated, thereby limiting pro-ductivity (Kremerset al., 2015). Our results provide evi-dence that an earlier onset of the growing season alone will not result in increased growth. These findings con-trast with satellite data that indicate an increase in tun-dra plant growth (Myneni et al., 1997; Tucker et al., 2001), implying the role of other factors beyond changes in timing of snow melt. Late-season nutrient supply and plants’ ability to access nutrients may be key factors determining ecosystem productivity. Limi-tations in growth will, in turn, cause limiLimi-tations in carbon sequestration, making the tundra potentially a larger source than sink of carbon (Starr & Oberbauer, 2003).
Acknowledgements
This work is based, in part, on funding from National Science Foundation grants OPP-9321626, 9615845, 9907185, and 0856710. Logistical support by the staff of the Institute of Arctic Biology Toolik Field Station is greatly appreciated. Kevin R. T. Whelan, Carlo Calandriello, Esperanza Rodriguez, Brook Shamblin, Car-rie Beeler, Michael Rasser, and Tara Madsen provided much appreciated field assistance. The work benefited greatly from statistical advice by Dr. Jianbin Zhu.
References
Aerts R (1995) The advantages of being evergreen.Trends in Ecology and Evolution,10, 402–407.
Arft AM, Walker MD, Gurevitch Jet al.(1999) Responses of tundra plants to experi-mental warming: meta-analysis of the International Tundra Experiment.Ecological Monographs,69, 491–511.
Aurela M, Laurila T, Tuovinen JP (2004) The timing of snow melt controls the annual CO2balance in a subarctic fen.Geophysical Research Letters,31, L16119.
Badeck FW, Bondeau A, Bottcher K, Doktor D, Lucht W, Schaber J, Sitch S (2004) Re-€ sponses of spring phenology to climate change.New Phytologist,162, 295–309. Bazzaz FA (1979) The physiological ecology of plant succession.Annual Review of
Ecology and Systematics,10, 351–371.
Beaubien EJ, Freeland HJ (2000) Spring phenology trends in Alberta, Canada: links to ocean temperature.International Journal of Biometeorology,44, 53–59.
Billings WD, Mooney HA (1968) The ecology of arctic and alpine plants.Biological Reviews,43, 481–529.
Billings WD, Peterson KM (1992) Some possible effects of climatic warming on arctic tundra ecosystems of the Alaskan North Slope. In:Global Warming and Biological Diversity(eds Peters RL, Lovejoy TE), pp. 233–243. Yale University Press, New Haven.
Bliss LC, Matveyeva NV (1992) Circumpolar vegetation. In:Arctic Ecosystems in a Changing Climate: An Ecophysiological Perspective(eds Chapin FS III, Jeffries RL, Reynolds JF, Shaver GR, Svoboda J), pp. 59–86. Academic Press, San Diego. Bock A, Sparks TH, Estrella N (2014) Changes in first flowering dates and flowering
duration of 232 plant species on the island of Guernsey.Global Change Biology,20, 3508–3519.
Bonan GB, Chapin FS III, Thompson SL (1995) Boreal forest and tundra ecosystems as components of the climate system.Climate Change,29, 145–167.
Buizer B, Weijers S, van Bodegom PMet al.(2012) Range shifts and global warming: ecological responses ofEmpetrum nigrumL. to experimental warming at its north-ern (high Arctic) and southnorth-ern (Atlantic) geographical range margin. Environmen-tal Research Letters,7, 025501.
Chapin FS III, Shaver GR (1996) Physiological and growth responses of arctic plants to a field experiment simulating climatic change.Ecology,77, 822–840.
Chapin FS III, Shaver GR, Giblin AE, Nadelhoffer KJ (1995) Responses of arctic tun-dra to experimental and observed changes in climate.Ecology,76, 694–711. Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD (2007) Shifting plant
phenology in response to global change.Trends in Ecology and Evolution,22, 357– 365.
Cooper EJ, Dullinger S, Semenchuka P (2011) Late snowmelt delays plant develop-ment and results in lower reproductive success in the High Arctic.Plant Science,
180, 157–167.
Diggle PK (1997) Extreme preformation in alpinePolygonum viviparum: an architec-tural and developmental analysis.American Journal of Botany,84, 154–169. Dorji T, Totland Ø, Moe SR, Hopping KA, Pan J, Klein JA (2013) Plant functional
traits mediate reproductive phenology and success in response to experimental warming and snow addition in Tibet.Global Change Biology,19, 459–472. Dormann CF, Woodin SJ (2002) Climate change in the Arctic: using plant functional
types in a meta-analysis of field experiments.Functional Ecology,16, 4–17. Dunne JA, Harte J, Taylor KJ (2003) Subalpine meadow flowering phenology
responses to climate change: integrating experimental and gradient methods. Eco-logical Monographs,73, 69–86.
Ernakovich JG, Hopping KA, Berdanier AB, Simpson RT, Kachergis EJ, Steltzer H, Wallenstein MD (2014) Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change.Global Change Biology,20, 3256–3269. Estiarte M, Pe~nuelas J (2015) Alteration of the phenology of leaf senescence and fall in
winter deciduous species by climate change: effects on nutrient proficiency.Global Change Biology,21, 1005–1017.
Eugster W, McFadden JP, Chapin FS III (2005) Differences in surface roughness, energy, and CO2fluxes in two moist tundra vegetation types, Kuparuk watershed, Alaska, U.S.A.Arctic, Antarctic, and Alpine Research,37, 61–67.
Euskirchen ES, McGuire AD, Chapin FS III, Yi S, Thompson CC (2014) Changes in vegetation in northern Alaska under scenarios of climate change, 2003–2100: impli-cations for climate feedbacks.Ecological Applications,19, 1022–1043.
Fahnestock JT, Povirk KL, Welker JM (2000) Ecological significance of litter redistri-bution by wind and snow in Arctic landscapes.Ecography,23, 623–631. Foster JL (1989) The significance of the date of snow disappearance on the arctic
tun-dra as a possible indicator of climate change.Arctic and Alpine Research,21, 60–70. Frei A, Robinson DA, Hughes MG (1999) North American snow extent: 1900–1994.
In-ternational Journal of Climatology,19, 1517–1534.
Galen C, Stanton ML (1995) Responses of snowbed plant species to changes in grow-ing-season length.Ecology,76, 1546–1557.
Grime JP (1979)Plant Strategies and Vegetation Processes. Wiley and Sons, New York. Habjørg A (1972) Effects of photoperiod and temperature on growth and
develop-ment of three latitudinal and three altitudinal populations ofBetula pubescensEhrh. Norges Landbrukshøgsk Meld,51, 1–27.
Higuera PE, Brubaker LB, Anderson PM, Brown TA, Kennedy AT, Sheng HUF (2008) Frequent fires in ancient shrub tundra: implications of paleorecords for arctic envi-ronmental change.PLoS ONE,3, e0001744.
Hinkel KM, Paetzold F, Nelson FE, Bockheim JG (2001) Patterns of soil temperature and moisture in the active layer and upper permafrost at Barrow, Alaska: 1993– 1999.Global and Planetary Change,29, 293–309.
Hollister RD, Webber PJ, Tweedie CE (2005) The response of Alaskan arctic tundra to experimental warming: differences between short- and long-term responses.Global Change Biology,11, 525–536.
Høye T, Post E, Schmidt NM, Trøjelsgaard K, Forchhammer MC (2013) Shorter flow-ering seasons and declining abundance of flower visitors in a warmer Arctic. Na-ture Climate Change,3, 759–763.
Huelber K, Gottfried M, Pauli H, Reiter K, Winkler M, Grabherr G (2006) Phenologi-cal responses of snowbed species to snow removal dates in the central Alps: impli-cations for climate warming.Arctic, Antarctic, and Alpine Research,38, 99–103. Hughes L (2000) Biological consequences of global warming: is the signal already
apparent?Trends in Ecology and Evolution,15, 56–61.
Iler AM, Høye TT, Inouye DW, Schmidt NM (2013) Nonlinear flowering responses to climate: are species approaching their limits of phenological change?Philosophical Transactions of the Royal Society B,368, 20120489.
Inouye DW (2008) Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers.Ecology,89, 353–362.
Intergovernmental Panel on Climate Change (IPCC) (2014)Climate Change 2013: The Physical Science Basis: Working group I contribution to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cam-bridge, UK.
Jia GS, Epstein HE, Walker DA (2003) Greening of Arctic Alaska, 1981–2001. Geophysi-cal Research Letters,30, 2067.
Kramer PJ, Boyer JS (1995)Water Relations of Plants and Soils. Academic Press, San Diego, CA.
Kremers KS, Hollister RD, Oberbauer SF (2015) Diminished response of arctic plants to warming over time.PLoS ONE,10, e0116586.
Kudo G, Ida TY, Tani T (2008) Linkages between phenology, pollination, photosyn-thesis, and reproduction in deciduous forest understory plants.Ecology,89, 321– 331.
La Puma IP, Philippi TE, Oberbauer SF (2007) Relating NDVI to ecosystem CO2 exchange patterns in response to season length and soil warming manipulations in arctic Alaska.Remote Sensing of Environment,109, 225–236.
Larigauderie A, Kummerow J (1991) The sensitivity of phenological events to changes in nutrient availability for several plant growth forms in the Arctic.Holarctic Ecol-ogy,14, 38–44.
Lechowicz MJ (1995) Seasonality of flowering and fruiting in temperate forest trees. Canadian Journal of Botany,73, 175–182.
Linderholm HW (2006) Growing season changes in the last century.Agricultural and Forest Meteorology,137, 1–14.
Maxwell B (1992) Arctic climate: potential for change under global warming. In: Arc-tic Ecosystems in a Changing Climate: An Ecophysiological Perspective(eds Chapin FS III, Jeffries RL, Reynolds JF, Shaver GR, Svoboda J), pp. 11–31. Academic Press, San Diego, CA.
McCarty JP (2001) Ecological consequences of recent climate change.Conservation Biology,15, 320–331.
McKane RB, Rastetter EB, Shaver GR, Nadelhoffer KJ, Giblin AE, Laundre JA, Chapin FS III (1997) Climatic effects on tundra carbon storage inferred from experimental data and a model.Ecology,78, 1170–1187.
Menzel A, Fabian P (1999) Growing season extended in Europe.Nature,397, 659. Molau U (1993) Relationships between flowering phenology and life-history
strate-gies in tundra plants.Arctic and Alpine Research,25, 391–402.
Molau U (1997) Responses to natural climatic variation and experimental warming in two tundra plant species with contrasting life forms:Cassiope tetragonaand Ranun-culus nivalis.Global Change Biology,3(Suppl. 1), 97–107.
Molau U, Mølgaard P (1996)ITEX Manual. Danish Polar Center, Copenhagen, Denmark. Molau U, Nordenh€all U, Eriksen B (2005) Onset of flowering and climate variability in an alpine landscape: a 10-year study from Swedish Lapland.American Journal of Botany,92, 422–431.
Myers-Smith IH, Forbes BC, Wilmking Met al.(2011) Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities.Environmental Research Let-ters,6, 6045509.
Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR (1997) Increased plant growth in the northern high latitudes from 1981 to 1991.Nature,386, 698–702. Natali SM, Schuur EAG, Rubin R (2012) Increased plant productivity in Alaskan
tun-dra as a result of experimental warming of soil and permafrost.Journal of Ecology,
100, 488–498.
Oberbauer SF, Starr G (2002) The role of anthocyanins for photosynthesis of Alaskan arctic evergreens during snowmelt.Advances in Botanical Research,37, 129–145. Oberbauer SF, Tenhunen JD, Reynolds JF (1991) Environmental effects on CO2efflux
from water track and tussock tundra in arctic Alaska, USA.Arctic, Antarctic, and Alpine Research,23, 162–169.