viii
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
IDENTIFICATION OF AVIAN INFLUENZA VIRUS S UBTYPE H9 AT CULLING LAYER IN INDONESIA
Siti Hajariyah Fahyuna
ABSTRACT
The aims of this research are to identify Avian Influenza Virus subtype H9 from culling layer in Indonesia and to know the percentage of this virus found based on the positive sample that collected from trachea and cloaca swab. The 350 samples of trachea and cloaca swabs from culling layer were examined by isolating on embryonated SAN chicken egg followed by Hemagglutination and Hemagglutination Inhibition using antisera H9 test. The research was conducted at Professor Nidom Foundation laboratory at Surabaya, inside the Biosafety Cabinet (BSC). The surveillance started in January 2018 and the location of sampling was taken from DKI Jakarta, West Java, Yogyakarta, East Java and South Kalimantan at year 2012 throughout 2018. The 350 samples were collected which is 10 samples each year for each regency. The result from Hemagglutinin Inhibition using antisera H9 showed, 3 positive sample in year 2012 (1.7%), 0 samples in year 2013 throughout 2015 (0%), 16 sample from in year 2016 (4.5%), 0 sample in year 2017 (0%) and 3 samples in year 2018 (1.7%) were positive, indicating the presence of potential Avian Influenza virus subtype H9 in culling layer.
IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
ix
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
ACKNOWLEDGMENT
I would like to acknowledge my gratitude to Allah subhanahu wa ta’ala for blessing, love, opportunity, health, and mercy to complete this undergraduate
thesis. This undergraduate thesis entitled IDENTIFICATION OF AVIAN
INFLUENZA VIRUS SUBTYPE H9 AT CULLING LAYER IN
INDONESIA can be finished. Shalawat and salaam always dedicated to Prophet
Muhammad S.A.W., who lead us to the right path and bring us from the darkness
to the lightness.
With this opportunity, I would like to convey my heartfelt gratitude to all people
who have been always motivating and helping me throughout this research,
especially to:
Prof. Dr. Pudji Srianto, drh., M.Kes. as the Dean of the Faculty of Veterinary
Medicine, Universitas Airlangga, for the given opportunity, so I can carry out the
education in Bachelor program Study in the Faculty of Veterinary Medicine,
Universitas Airlangga.
The supervisor committee, Prof. Dr. Rahaju Ernawati, M.Sc., drh and Dr.
Kadek Rachmawati, drh., M. Kes. as co-supervisor for the time, patience, advice,
precious lesson and guidance, that has been given to me until the completion of this
thesis. The examiner committee, Head of Assessor Prof. Dr. Chairul Anwar Nidom,
drh., Ms and as the research leader for all the support, advice, facility and
accommodation in guiding me to complete this thesis, the examiner secretary Dr.
Eduardus Bimo Aksono H, drh., M. Kes., and examiner member Adi Prijo Raharjo,
x
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
Dr. Kadek Rachmawati, drh., M. Kes., as the academic advisor who has
always been patient and giving me courage and support throughout all these
semesters thru my thesis.
All the Professor Nidom Foundation (PNF) staff, Mbak Ire, Mbak Anis,
Mbak Ana, Mas Khalim and all the other staff that I can't say one by one and
research team for their support and help for my research.
To my beloved parents, Haruna Djawaru and Connie Francis Daimboa for
their endless love, patient, inspiration, pray, material and moral support throughout
my studies. My older brother Fahrun Revak Djawaru, my older sister Mukrima
Fauriska Djawaru and my sister-in-law Dian Sandra Dewi who always giving me
strength, inspiration, advice and fund me to accomplish my ambitions.
My best colleagues, Shendy Canadya Kurniawan, Nina Sagitha Pratiwi,
Reni Ramadhani, and all international class members for the supports and
meaningful days that we spent together and thanks to everyone that cannot be
mentioned one by one for all the help, encourage, and motivation that given to the
author.
My best friend thru thick and thin, Nisha Anggraeny, Rustikanti Ayu
Ningrum and Dian Lastriana for the love, support, and motivation.
My laboratory and research mate, Azrina Khalida Imani Abbas, Kartika
Buana Sari, Indahsari Ahmed and Nur Shabrina for support, motivation, guidance,
and all the hard work we’ve been through together to finish this research.
My research partners who always guide me patiently Ratna, Fariz, Shendy,
IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
xi
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
and Deva for great teamwork and meaningful days that we spent together doing our
research.
Surabaya, July 2018
xii
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
CONTENTS
1.2 Statement of the Problem ... 3
1.3 Theoretical Basis ... 3
1.4 The aims of Research ... 5
1.5 The outcome of Research ... 5
1.6 Hypothesis ... 5
CHAPTER 2 LITERATURE REVIEW ... 6
2.1 Avian Influenza ... 6
2.1.1 Etiology and Morphology Avian Influenza Virus ... 6
2.1.2 Characteristic of Avian Influenza ... 7
2.1.3. Avian Influenza Virus Subtype H9 ... 10
2.1.4. Cycle Infection ... 10
2.1.5. Transmission of Avian Influenza ... 11
2.1.6. Clinical sign of Avian Influenza Virus ... 13
2.1.7. Diagnoses ... 13
2.1.8. Different Diagnoses ... 15
2.1.9. Control and Prevention Avian Influenza ... 15
2.2. Overview of Poultry ... 16
2.2.1 Scientific Classification of Chicken ... 16
2.2.2 Swab Trachea and Cloaca ... 18
2.3 Antigen and Antibody ... 19
2.4 Hemagglutinin Assay Test ... 20
2.5 Hemagglutinin Inhibition Assay Test ... 20
CHAPTER 3 MATERIALS AND METHOD ... 22
3.1 Research Design... 22
3.2 Time and Place of Research ... 22
3.3 Material and Equipment of Research ... 22
3.3.1 Material ... 22
IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
xiii
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
3.4 Research Methods ... 23
3.4.1 Location of Sampling ... 23
3.4.2 Sample Obtaining and Handling ... 23
3.4.3 Sampling Handling in Laboratory ... 24
3.4.4 Inoculation of Embryonated Chicken Eggs ... 24
3.4.5 Harvesting of Embryonated Chicken Eggs ... 25
3.4.6 Preparation of 0.5% Chicken Erythrocyte Suspension ... 26
3.4.7 Hemagglutinin Assay Test ... 27
3.4.8 Retitration of 8 HA Unit Antigen ... 28
3.4.9 Hemagglutination Inhibition (HI) Test using Antisera... 28
3.5 Data Analysis ... 29
3.6 Research Flow Chart ... 30
CHAPTER 4 RESEARCH RESULT ... 32
4.1 Hemagglutination Test Positive Result ... 32
4.2 Hemagglutination Inhibition using Antisera H9 test Positive Result ... 33
4.3 Percentage of Positive Sample According to Year ... 34
4.4 Graphic of Positive H9 ... 34
CHAPTER 5 DISCUSSION ... 36
CHAPTER 6 CONCLUSION ... 41
6.1 Conclusion ... 41
6.2 Suggestion ... 41
REFERENCES ... 42
xiv
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
LIST OF TABLES
Table Pages
IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
xv
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
LIST OF FIGURES
Figures Pages
2.1 Structural of Influenza A virus. ... 7
2.2 Gallus gallus domesticus ... 16
3.1 Research Flow Chart ... 30
xvi
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
LIST OF APPENDICES
Pages
1. The titer of HA and HI using antisera H9 test result. ... 47
2. Hemagglutination Assay test schematic ... 52
3. Hemagglutination Inhibition test using antisera of H9 schematic ... 54
IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
xvii
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
ABBREVATIONS AND SYMBOLS
ºC = Celcius
µ l = Microliter
AIV = Avian Influenza Virus EDS = Egg Drop Syndrome
ELISA = Enzyme Linked Immunosorbent Assay FAO = Food and Agricultural Organization HA = Hemagglutination
HI = Hemagglutination Inhibition HPAI = Highly Pathogenic Avian Influenza IB = Infectious Bronchitis
IBD = Infectious Bursal Disease ILT = Infectious Laryngo Tracheitis IU = International Unit
IUCN = International Union for Conservation of Nature LPAI = Low Pathogenic Avian Influenza
M = Matrix
M1 = Matrix Protein 1 M2 = Matrix Protein 2 ml = Mililiter
mRNA = Messenger Ribonucleic Acid N/NA = Neuraminidase
ND = Newcastle Disease NP = Nucleoprotein
NS1 = Nonstructural Protein 1 NS2 = Nonstructural Protein 2
OIE = World Organization of Animal Health PA = Polymerase Component
PB1 = Polymerase Component 1 PB2 = Polymerase Component 2 PBS = Phosphate Buffer Saline PCR = Polymerase chain reaction pH = Potential of Hydrogen RBC = Red Blood Cell RNA = Ribonucleic Acid Rpm = Rotation per Minute
RT-PCR = Reverse Transcription Polymerase Chain Reaction SAN = Specific Antibody Negative
1
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
CHAPTER 1 INTRODUCTION 1.1 Background
Avian influenza diseases became a special concern as health issues that
spread and threaten some countries such as China, Europe, Thailand, Vietnamese
and also Indonesia. In Indonesia, it remains enzootic for Avian Influenza Virus. The
first outbreaks that happened in Indonesia were H5N1, this influenza was reported
in November 2003, and now the virus has spread to 32 out of the 33 Indonesian
provinces, affecting both intensively farmed birds as well as backyard chickens.
Influenza viruses are classified into 3 types, those are type A, B and C. Influenza
type A is one of the pandemic types, because it can mutate itself, either in the form
of antigenic drift or antigenic shift that form new variants which are more
pathogenic. AI is an influenza disease that infected poultry, such as chicken, duck,
and bird species (Nidom, 2010; Nidom et al., 2012; Neny and Herra, 2014).
Avian Influenza divided into two categories, highly pathogenic avian
influenza (HPAI) viruses, and low pathogenic avian influenza (LPAI) viruses. One
of HPAI virus is H5N1, the infections are constantly monitored worldwide not only
because of the high mortality they produce in poultry (causing great losses of
poultry industry) but also because of the spread to humans and fear of a pandemic
(Jong and Hien, 2006; Jackwood et al.,2012). And LPAI virus reported as H9N2,
even though it is LPAI, it continues to threaten the poultry population worldwide.
H9N2 virus infection in chicken results in mild respiratory sign and egg production
losses. These viruses have been reported to cause flu-like disease in human (Tosh,
2 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
In early 2017, Dirjen Peternakan dan Kesehatan Hewan, Kementrian
Pertanian, I Ketut Diarmita, gave a statement that AIV subtype H9N2 was detected
through a surveillance that conducted by Balai Veteriner Kementrian Pertanian in
South Sulawesi, West Java, Bali, Central Java and Yogyakarta. These cases have
led to a fall in egg supply by the end of 2017 (Dirjen Peternakan, 2017).
H9 first time isolated were reported in the USA in 1966 (Homme &
Easterday, 1970). While in Asian countries, H9N2 is one of subtype AIV that
widespread in domestic poultry (Mosleh, et al., 2009) and have been reported in
various regions including Hong Kong, mainland China, South Africa, the Middle
East, Europe, North America and South Korea (Alexander, 2000; Mo et al., 1997;
Xu et al., 2007). Globally, there are two major, distinct gene pools of H9N2 avian
influenza viruses: The North American and the Eurasian (Gua et al., 2000; Webster
et al., 1992). Eurasian avian influenza subtype H9N2 virus are divided into three
distinct sublineages represented by their prototype strain A/Duck/Hong
Kong/Y280/97 (Y280-like), A/Quail/Hong Kong/G1/97 (G1-like) and
A/Chicken/Korea/38349-p96323/96 (Korean-like) (Li C et al., 2005; Li KS et al.,
2003; Guan et al., 2000).
AI subtype H9N2 cause decreased egg production, cost money for
vaccination in the commercial poultry farms in Iran, also an increased mortality
because H9N2 influenza virus could make chicken more susceptible to secondary
infections, especially Escherichia coli infections with a mortality rate of at least
10%. In addition, the trachea or bronchi are easily embolized by mucus when the
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F 20-60% was reported in the affected broiler farms with clinical signs that were
characterized as swelling of periorbital tissues and sinuses, typical respiratory
discharge and severe respiratory distress (Nili and Asasi, 2003).
A case that reported in human at 1999 was two children were infected with
Influenza A subtype H9 virus in Hongkong, then in 2003, there was also a report of
a child infected with the same virus (Rahardjo and Nidom, 2004).
Until now the case of H9 virus has not been found in the human in Indonesia
but has been found in animals such as laying hens. In some H9 cases in the world,
this virus can be transmitted from animal to human. It is necessary to do this
research to review the transmission of the H9 virus from laying hens to humans
from live poultry market and also to study if H9 virus plays role in the decrease of
egg production. This study was conducted to detect specific antigen to antibodies
of H9 virus in the culling layers in the markets. 1.2 Statement of Problems
Based on the background above, the problem can be formulated: “Is Avian Influenza Subtype H9 can be detected from the sample that collected in Indonesia
from culling layer?”
1.3 Theoretical Basis
AIV has a characteristic the viruses are enveloped, single-stranded,
negative-sense RNA viruses of the family Orthomyxoviridae, and divided into
types A, B, and C. Recently have been detected there are 18 HA (Hemagglutinin)
4 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
birds, except for H17N10 and H18N11, which have recently been identified in bats
(Webster., et al, 1992; Tong., et al, 2012; Tong., et al, 2013).
It causes a variety of infection in avian and mammals. The H9 virus is
widespread in the world and the most prevalent subtype of AI. This subtype was
reported in China over the last decade. Although H9 is characterized as LPAI virus,
occasional infection of human has caused great concerns in poultry that isolated
from domestic birds, however, wildfowl and shorebirds are the natural hosts of AIV
and they facilitate the transmission of avian influenza (Ji K et al., 2013; Olsen et
al., 2006; Peng et al., 2013)
According to Kepala Sub Pengawasan Obat Hewan Direktorat Jendral
Peternakan dan Kesehatan Hewan, Drh. Ni Made Ria Isriyanti, Ph.D., the current
condition of the H9 virus is its spread in many provinces in Indonesia such as Java,
Sumatera, Kalimantan, Sulawesi, and Bali. With H9N2 positive sample amounted
up to 49. The average age of infected chicken is 30–60 weeks. Mortality is generally low, but a symptom like decreasing egg production up to 40-60% of the usual
production, resulting in significant economic losses for farmers is one of the ways
to characterize the H9 virus. (ASOHI, 2017).
The diagnosis of H9 virus in poultry can be done with a test called HA
(Hemagglutination) test and will be followed by Hemagglutinin Inhibition test
using Antisera H9. HA is used to detect a virus that has haemagglutinin. The
presence of hemagglutinin can be seen by its ability to agglutinate erythrocytes of
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F specific antigen H9 in certain organs suspected for AIV (WHO, 2005; Nidom,
2010).
The prevalence of H9N2 virus throughout the world, along with their ability
to infect mammals and human, increases concern about their pandemic potential.
Because of the ongoing concern about the transmission of the H9N2 virus to
mammals and human, continued surveillance of H9N2 virus from live poultry
markets is needed (Fei Fei et al., 2009).
1.4 The Aim of Study
The aims of this study are to identify AIV subtype H9 from culling layer in
Indonesia and to know the percentage of this virus found based on the positive
sample that collected from trachea and cloaca swab.
1.5 Outcome of Study
The outcome of this study is to identify the AIV subtype H9 in poultry
within a certain period of time in several places in Indonesia. Can also assess the
development of the virus.
1.6 Hypothesis
Based on the theoretical basis, researcher can conclude the hypothesis that
Avian Influenza virus subtype H9 can be detected by HA and HI using antiserum
IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
6
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
CHAPTER 2 LITERATURE REVIEW 2.1. AVIAN INFLUENZA
2.1.1. Etiology and Morphology Avian Influenza Virus
Influenza viruses belong to the Orthomyxoviridae family and are divided
into types A, B, and C. Influenza types A and B are responsible for the epidemic
of respiratory illness that is often associated with increased rates of hospitalization
and death. Influenza A is one of the pandemic types because it can mutate
themselves, either in the form of antigenic drift or antigenic shift that form new
variants which are more pathogenic. All influenza viruses are negative-stranded
RNA virus with a segmented genome. Influenza type A and B have 8 genes that
code for 10 proteins, including the surface proteins Haemagglutinin (HA) and
Neuraminidase (NA). In the case of influenza type A, virus further subdivision
can be made into different subtype according to differences in these two surface
proteins. Influenza virus A can be found in chicken, duck, goose, eagles, pigeon,
pigs, and human. Influenza virus type B and C can be found at human (WHO,
2011; Neny and Herra, 2014; Raharjo and Nidom, 2004).
The two glycoprotein of influenza A virus, HA and NA, play essential roles
during virus entry host cells and release from those cells. Influenza A virus
attaches to cells through HA binding to the terminal sialic acid of glycoprotein on
the surface of respiratory epithelial cells. The host range of influenza A virus is
dictated mainly by their affinity for different sialo sides: avian virus preferentially
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F external region (exodomain) of the second transmembrane glycoprotein is NA,
conducting sialolytic enzymatic and releasing the virus progeny that trapped on
the surface of the infected cell. This function prevents the accumulation of virus
and may also facilitate the movement of the virus in the mucous membranes of the
targeted epithelial tissue. Then the virus will stick to the target. (Websters, 1992;
Matrosovich et al., 1997; Rogers et al., 1983; Matrosovich et al., 2004).
2.1.2. Characteristic Avian Influenza
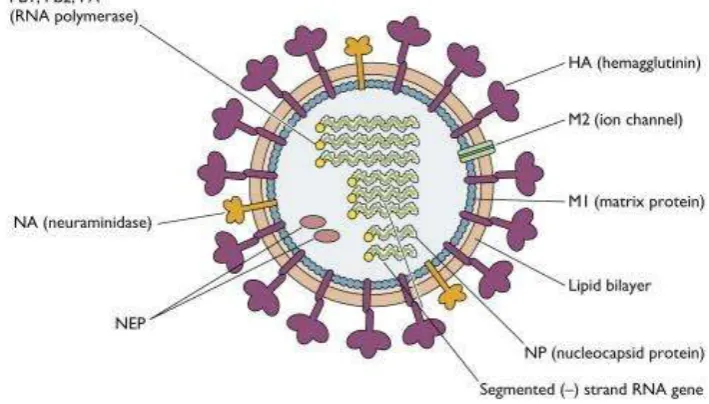
Influenza A has 8 segments (Figure 2.1) that encode for viral genes; Hemagglutinin (HA), Neuraminidase (NA), Matrix 1 (MA1), Matrix 2 (MA2),
Nucleoprotein (NP), non-structural protein 1 (NSP1), NSP2/NEP, polymerase
protein (PA), polymerase basic protein 1 (PB1), PB2-F2.
Figure 2.1 Structural of Influenza A virus (Vincent Racanie llo, 2009)
Transmembrane protein HA, NA, and M2 are the lipid bilayer of Influenza
8 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
AI viruses are classified into two categories, low pathogenic and highly
pathogenic AIV. A virus is defined as HPAI or LPAI by its ability to cause severe
disease in intravenously inoculated young chickens in the laboratory, or by its
possession of certain genetic features associated with HPAI virus. AIV subtype H5
and H7 are the fully virulent virus found in nature, although there are rare examples
of another virus that could technically be considered as HPAI (OIE, 2014).
HPAIV of subtype H5 and H7 have caused severe disease high mortality in
poultry. Historically HPAIV H5N1 infection has resulted in the culling or death of
more than 500 million poultry in more than 62 countries. During 2016 and January
to February 2017, there was no case of H5N1 in humans reported in Indonesia.
HPAIV subtype H5N1 have been found to cause disease in humans since 1997. In
March 2017, reported there have been cases of H5N1 in Indonesia that caused the
death of 12.136 poultries consists of 1018 free-range chicken (Ayam kampung),
4618 ducklings, 2300 quails, 3985 chicken broilers, 15 turkeys and 200 laying
chickens. The World Organization of Animal Health (OIE) recommends the control
of HPAIV at poultry source to decrease the viral load in susceptible avian species,
thereby decreasing the risk of transmission to humans (OIE, 2009; WHO, 2009;
Ditjen PHK, 2017; OIE, 2007).
AIV H9N2 has become widespread among poultry in some areas and also
have been detected in wild birds. This virus characterizes as LPAI. Pigs and dogs
have recently detected of H9N2 virus. A serological test has been conducted in
Bangladesh and China, infection was acquired in macaques in Bangladesh and wild
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F Influenza virus belongs to the segmented genome, making it easy to mutate.
Mutations can occur through two processes antigenic, antigenic drift and antigenic
shift. Changes in the surface of HA and NA proteins are mutations occurring on the
surface of HA (5 epitopes) and NA (4 epitopes), so that when there is a change in
the arrangement of the epitope or even removing the epitope on the surface of HA
and NA, the antibodies in the poultry body cannot recognize the virus, or even be
overcome with an existing vaccine, this process is called “antigenic drift”. Another type of change is the antigenic shift. “Antigenic shift” is a recombinant activity of
two types of influenza A virus that produce new gene segments. This activity leads
to antibodies that have been formed in the body cannot neutralize new viruses. The
results of this recombinant will result in a new subtype that could pose a pandemic
(Dirjen Peternakan, 2014).
AIV can easily die from heat, sunlight, and disinfectant (detergent,
ammonium quarter, formalin 2-5%, etc.). Heat can damage the virus infectivity. In
the temperature 56°C, AIV can live for 3 hours and 30 minutes for 60°C. Fat
solvents such as detergent can damage the double layer of fat in the viral casing.
This virus damage envelope causes the influenza virus to be non-infective again.
Other factors are acidic pH, non-isotonic and dry conditions. Ether compounds or
sodium dodecyl sulfate will disrupt the envelope, thus damaging the proteins of
haemagglutinin and neuraminidase. Viral carriers come from sick chickens, birds
and other animals, feed, chicken manure, fertilizers, transportation equipment, egg
trays, and contaminated equipment. Also, the influenza virus can survive in water
10 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
2.1.3. Avian Influenza Subtype H9
Decreased egg production is one of resulted from H9 across North Africa,
the Middle East, and Asia. Despite being LPAI viruses, these viruses have gained
the ability to cause severe respiratory distress accompanied by high morbidity and
mortality and a marked reduction in egg production. That make some significant
economic losses in the poultry due to moderate to high mortality. The current
circulation Eurasian H9 LPAIV has rapidly spread to become the most prevalent
LPAIV in domestic poultry since their initial isolation in China during 1994 (Fusaro
et al., 2011; Iqbal et al., 2009; Lee and Song. 2013; Zhang et al., 2009).
2.1.4. Cycle Infection
AI subtype H9 infection occurs when the virion spikes with specific
receptors located on the surface of the host cell are attached and the virus enters the
host cell. The virion will enter the cell's cytoplasm and will integrate its genetic
material within the nucleus of its host cell, then the virus can replicate to form new
virions and the virus can re-infect adjacent cells. Avian influenza virus can replicate
in nasopharyngeal cells and in gastrointestinal cells. This virus can also be detected
in the blood, cerebrospinal fluid and feces (Hidaningrum et al., 2016).
The attachment phase is the phase that most determines the viral infection
cycle, whether the virus can enter or not into the host cell to continue the replication.
The entry influenza virus A into the host cell is through the major lipid bilayer,
which is HA. Through the hemagglutinin spike, the influenza A virus binds to
receptors containing sialic acid (SA) present on the surface of the host cell.
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F different from human and avian. There are two major linkages found between sialic
acid and carbohydrates they are bound to in glycoproteins; α (2,3) and α (2,6). In avian influenza viruses can recognize and bind to receptor α (2,3), while influenza
viruses in human can recognize α (2,6)(Hidaningrum et al., 2016; Samji, 2009). Upon HA’s spike binding with host cell's sialic acid residues, receptor
-mediated endocytosis occurs and the virus enters the host cell in an endosome. With
low pH in endosome (5-6), it triggers fusion of the viral and endosomal membrane.
The low pH also opens M2 channel. M2 is a type III transmembrane domain from
a channel that acts as a protein-selective ion channel. Opening the M2 ion channels
acidifies the viral core. This from M1 such that vRPN (PB1, PB2, PA) is free to
enter the host cell. The release of vRPN, influenza virus will transcript and replicate
itself in host cell’s nucleus. After that, all the viruses have to do is form viral
particles and leave the cell. Influenza virus is known as an enveloped virus, so it
uses the host cell's plasma membrane to form the viral particles that leave the cell
with exocytosis and go on to infect neighboring cells (Samji, 2009).
The damage caused by avian influenza comes from one of the following
three processes (1) the direct process of viral replication in cells, tissues and organs,
(2) indirect effects of cell mediators such as cytokines, (3) ischemia (insufficient
blood supply) due to the presence of blood clots (thrombus) in the heart and blood
vessels (Raharjo and Nidom, 2004).
2.1.5. Transmission of Avian Influenza
Wild waterfowl are considered the natural reservoir of all influenza A virus.
12 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
The highly pathogenic avian influenza (HPAI) spreads very rapidly through poultry
flocks, causes disease affecting multiple internal organs, and has a mortality that
can approach 100%, often within 48 hours (WHO, 2007).
Transmission can occur through direct contact of infected poultry and
sensitive birds through the respiratory tract, conjunctiva, mucus, and feces; or
indirectly through dust, feed, drinking water, officers, cage equipment, shoes,
clothing and vehicles that contaminated with AIV and live chicken infected.
Waterfowl such as ducks and geese can act as carriers without showing clinical
symptoms. Direct is presently considered the main route of human infection. The
most cases in human that got infected by avian influenza because there are many
households keep small poultry flocks, and the poultries can roam freely entering
homes or sharing outdoors area where children play. As infected birds shed large
quantities of virus in their faces, the environments easily contaminated by the virus.
It mostly happens in rural areas (WHO, 2007).
Waterfowl usually serves as a source of transmission to a chicken or turkey
farm. Transmission vertically or continentally is not known, because there is no
scientific or empirical evidence. The incubation period is varying from several
hours to 3 days in an individually infected poultry or 14 days in floc.
Migratory birds, humans, and equipment are regarded as risk factors for the
entry of the disease. Bird markets and gathering traders also play an important role
in the spread of the disease. Viral carriers come from sick chickens, birds and other
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F contaminated equipment. Humans spread the virus by moving and selling sick or
dead birds (Dirjen Peternakan, 2014).
2.1.6. Clinical sign of Avian Influenza Virus
Clinical symptoms seen in chickens HPAI sufferers include, comb, eyelids,
feet, and abdomen that is not overgrown feathers look purplish blue (edema and
cyanosis), discharge from the eyes and nose, swelling of the face and head, diarrhea,
coughing, sneezing, and snoring, those are some clinical sign of neurological from
HPAIV. Decreased appetite, decreased egg production and mushy eggshell. The
presence of bleeding in the legs of red spots (petechia) or commonly called a foot
scrap. No signs are pathognomonic but death occurs quickly (Dirjen Peternakan,
2014; OIE, 2014).
According to Dirjen Peternakan dan Kesehatan Hewan, Kementrian
Pertanian, I Ketut Diarmita, H9N2 virus is a type of avian influenza virus that is
low pathogenic Avian Influenza (LPAI), although not deadly, it can cause
decreased immune to the poultry and damage to some organs. Because it can lower
the immune in poultry, it causes the infection along with other infectious diseases
such as Newcastle Disease (ND) or better known as Tetelo, Infectious Bronchitis
(IB) and Egg Drop Syndrome (EDS) it can lead to decreased egg production (Dirjen
Peternakan, 2017).
2.1.7. Diagnoses
LPAIV cannot be diagnosed on bases of the spectrum of clinical signs,
whereas it can be diagnosed by comparison of weight gain of the infected birds.But
14 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
in the infected group most of the birds showed slight depression with low intake of
feed and water between 2 to 7 days PI of H9 virus. Among these birds, 4 birds
suffered from diarrhea while 3 birds revealed depression on 5th day PI. All the birds
were recovered from depression after 7 days PI while diarrhea persisted up to 12th
day PI. No mortality was observed among the birds.
Subclinical infections or mild illnesses in poultry and other poultry are
common in LPAI viruses.Decreased egg production and quality, the respiratory
sign like sneezing, coughing, ocular and nasal discharge and swollen infraorbital
tissue, lethargy, decreased consumption feed and water or somewhat increased
flock mortality rates seen in chickens and turkeys (OIE, 2014).
AIV subtype H9 is one of LPAI, which result from the same decrease in the
feed consumption possible cause of reduction in weight could be effect of viral
infection on pancreatic tissue which results in decreased production of pancreatic
enzymes essential for efficient digestion (Silvano et al., 1997; Shinya et al., 1995).
While on the gross pathology, all visceral organs were found normal with
no abnormal gross changes in the control group while in infected birds only slight
hyperemia and congestion was observed in trachea and lungs in two birds each
which were slaughtered on 5th and 9th day PI. Kidneys were in six out of 14 birds.
The frequency of changes was 43 % in kidneys while only 10% in trachea and lungs
(Subtain et al., 2014).
Isolation virus by inoculation at embryonated chicken egg for detecting a
property of red blood cells precipitation by Hemagglutinin Assay (HA) test, or by
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F positive, then confirm for subtypes by using serum specific for H9 by
Hemagglutination Inhibition test (HI test) to detect the inhibition of red blood cells
precipitation. Reverse Transcription Polymerase Chain Reaction (RT-PCR) or
genetic sequencing as confirmation test to determine the presence of virus (National
Bureau of Agricultural, 2008; Suwarno et al., 2006).
2.1.8. Different Diagnoses
Avian influenza is often confused with Newcastle disease (ND), Infectious
Bronchitis (IB), Fowl Cholera, infectious laryngotracheitis (ILT), duck plague,
acute poising, bacteria cellulitis and Escherichia coli infections. These diseases are
common in HPAI and are mistaken for hemostasis in the wound and comb
accompanied by high mortality. Egg Drop Syndrome is one of LPAI different
diagnoses for AIV subtype H9 (Dirjen Peternakan, 2014; Werner and Harder 2006;
Dirjen Peternakan 2017).
2.1.9. Control and Prevention of Avian Influenza
There are several control and prevention to reduce the virus spreading. H9
is one of LPAI, which mean it won’t harm human as HPAI like H5 or H7. But a
good control of the virus can help the farmers from losing more productivity from
their poultry.
Based on Kepdirjennak No: 17/Kpts/PD.640/F/02.04 there are 9 strategies
to control Avian Influenza biosecurity, selective poultry destruction in infected
areas, vaccinations, traffic controls that include strict regulation of live poultry
16 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
community awareness raising, poultry restocking, stamping out in newly contracted
areas and monitoring (Dirjen Peternakan, 2014).
2.2. Overview of Poultry
Wild bird is the main host of AIV, but occasionally, the virus can spread
from its natural reservoir to poultry. AIVs in the wild bird is generally poorly
adapted to domestic Galliformes (chickens, quail, partridge), but as a condition
permit, the virus can be transmitted and adapt to the new host. Mostly wild bird
does not show clinical sign infection with AIVs. AIVs known can replicate in cells
of both the respiratory and intestinal tracts, but in ducks, they are reported to favor
the intestinal tract. Live bird market (LBM) is a potential source of human infection
with AIV. In LBM we can find many waterfowl species to bought. These waterfowl
species play an important role in AIV transmission and are regardless as a natural
reservoir of AIV (Lou et al., 2017; Wang et al., 2017).
2.2.1. Scientific classification of chicken
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F Classification chicken (IUCN, 2003) is:
Kingdom : Animalia
Phylum : Chordata
Class : Aves
Order : Galliformes
Family : Phasianidae
Genus : Gallus
Species : Gallus gallus
Subspecies : G. gallus domesticus
Laying hens are adult female chickens that are kept to be taken the eggs.
Females over one-year-old known as hens and younger females as pullets although,
in the egg-laying industry, a pullet becomes a hen when she begins to lay eggs at
16 to 20 weeks of age. Laying hens are very efficient to produce eggs and start
laying eggs around ± 5 months with 250 eggs each year of production. (Rasyaf,
2008).
A good layer will have a large, smooth, moist, almost white vent. The two
small bones at the sides of the vent are called the pubic bones. They should be
flexible and wide apart, with at least two finger widths between them (one finger
width = ¾ inch). The abdomen should be deep, soft, and pliable without an
accumulation of body fat. The depth of the abdomen is measured between the tip
of the keel or breastbone and the pubic bones. Laying hens should have a depth of
18 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
Characterize of laying hens (Mississippi State University, 2015):
Comb and Wattles Large, bright red
Head Neat, refined
Eye Bright, prominent
Eye ring Bleached
Beak Bleached
Abdomen Deep, soft, pliable
Pubic bones Flexible, wide apart
Vent Large, moist, bleached
Chicken cull is a chicken that is not actually a broiler type but used as a
meat-producing chicken derived from laying hens put aside as inferior, deform or
productivity down. (Tien R. Muchtadi, et al., 2011). Culling laying hens are laying
hens with low egg production of about 20 to 25% at the age of about 96 weeks
(Gillespie and Flanders, 2010; Eko, et al. 2012).
2.2.2. Swab trachea and cloaca
The trachea of live birds is swabbed by inserting a dry cotton or polyester
swab into the trachea and gently swabbing the wall, and the swab is placed in
transport medium (WHO, 2002).
Cloacal swab was doing of lives birds by inserting a swab deeply into the
vent and vigorously swabbing in the wall. The swab should be deeply stained with
fecal material and is then placed in transport media (WHO, 2002).
Gallinaceous birds typically shed AI viruses in respiratory secretions, so a
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F chickens and turkeys. The virus replicated itself efficiently in tracheas. While
waterfowl typically shed AI viruses in their fecal secretions, so a cloacal swab is
the primary source of virus detection (Li et al., 2005).
2.3. Antigen and Antibody Reaction
Antibodies are immunoglobulins formed by body cells (B cells) as receptors
for antigenic stimulation. All antibody molecules have four basic polypeptide
chains consisting of two heavy chains and two identical light chains linked together
with disulfide bonds. The part consisting of amino acids assigned to bind the
antigen is called site binding antigen. Antibody titer is the antibody content
measured by titration. An antigen is an alien substance that can be recognized and
well-bonded by microorganisms such as viruses, parasites, bacteria, and fungi. Part
of an antigen that can bind to a receptor such as an antibody called an epitope
(Hidaningrum et al., 2016).
Antigen and antibody interactions are divided into two types: interaction of
primary antigen-antibody and interaction of secondary antigen-antibody.
Interaction of primary antigen-antibodies is the binding of molecular level
antibodies that require indicators for example with enzymes or fluorescein dyes and
others. Its testing uses three techniques namely, isotope technique with RIA
(Radioimmunoassay), enzyme labeling technique with ELISA and
Immunofluorescence technique. interaction of secondary antigens is an interaction
20 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
2.4. Hemagglutinin Assay (HA)
The hemagglutination (HA) assay is a tool used to screen cell culture or
amnio-allantoic fluid harvested from embryonated chicken eggs for
hemagglutinating agents, such as type A influenza. The HA assay is not an
identification assay, as other agents also have hemagglutinating properties. Live
and inactivated viruses are detected by the HA test. Amplification by virus isolation
in embrocating chicken eggs or cell culture is typically required before HA activity
can be detected from a clinical sample. The test is, to some extent, quantitative [1
hemagglutinating unit (HAU) is equal to approximately 5-6 logs of virus]. It is
inexpensive and relatively simple to conduct. Several factors (quality of chicken
erythrocytes, laboratory temperature, laboratory equipment, technical expertise of
the user) may contribute to slight differences in the interpretation of the test each
time it is run (Killian, 2008).
2.5. Hemagglutination-inhibition Assay (HI)
The hemagglutination-inhibition (HI) assay is a classical laboratory
procedure for the classification or subtyping of hemagglutinating viruses. For the
AI virus, the HI assay is used to identify the hemagglutinin subtype of an unknown
AI virus isolates or the HA subtype specificity of antibodies to AI virus. Since the
HI assay is quantitative, it is frequently applied to evaluate the antigenic
relationships between different AI virus isolates of the same subtype. The basis of
the HI test is inhibition of hemagglutination with subtype-specific antibodies. The
HI assay is a relatively inexpensive procedure utilizing standard laboratory
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F several hours. However, when working with uncharacterized viruses or antibody
subtypes, the library of reference reagents required for identifying antigenically
distinct AI viruses and/or antibody specificities from multiple lineages of a single
hemagglutinin subtype requires extensive laboratory support for the production and
IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
22
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
CHAPTER 3 METHODS AND MATERIAL 3.1. Research Design
This research using non-experimental laboratory research design that is
descriptive. The swab samples are taken using purposive sampling. The poultry that
was become the sample object was taken without giving any treatment previously.
The sample then inoculated to embryonated chicken eggs, followed with HA test
using allantoic fluid of the embryonated chicken eggs and HI using antisera H9 test
to test the antigen of the allantoic fluid with standard antisera of H9.
3.2. Time and Place of Research
This research conducted at Poultry Disease Laboratory of Professor Nidom
Foundation in Surabaya. The samples swabs are collected from several places in
Indonesia such as DKI Jakarta, West Java, Yogyakarta, East Java and South
Kalimantan from the collection in the year 2012 throughout 2018 by swabbing the
trachea and cloaca of culling layer. The surveillance started in January until June
2018 for samples in the year 2018, and the surveillance was done by a team from
Professor Nidom Foundation for the samples that were collected from 2012-2017.
This research conducted during January – June 2018. 3.3. Material and Equipment of Research 3.3.1. Material
Materials are trachea and cloaca swab from culling layer, M199, SAN
(Specific Antibody Negative) embryonated chicken egg 9-11 days old, Red Blood
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F (PBS), Alcohol 70%, Antibiotics (penicillin 3000 IU/ mL and streptomycin 3000
IU/ mL) and Sterile distilled water.
3.3.2. Equipment
The equipment are gloves, masker, conical tube, cotton swab, ice box, ice
pack, syringe, vortex, tube rack, plastic bag, Microtube tube 1.5 mL, centrifuge,
micropipette (50 L, 100 L and 1000 L), yellow tip, blue tip, micropipette
multichannel 50 L, freezer temperature up to -800C, refrigerator 40C, egg tray,
egg incubator (370C), microplate “V”, scotch tape, tuberculin syringe 1 cc, egg candler, egg hole punch, forceps, pencil, marker, tissue, labelling papers and
autoclave.
3.4. Research Method 3.4.1. Location of Sampling
The location of sampling was taken from traditional markets and farms in
Indonesia. The regencies of traditional markets and farms were from DKI Jakarta,
West Java, Yogyakarta, East Java, and South Kalimantan. Samples were taken from
the farms because there are some reported about high decreased of producing eggs
from the laying hens that probably could cause culling for the hens. And traditional
markets because bad sanitation could cause H9 virus easily transmitted between
poultries.
3.4.2. Sample Obtaining and Handling
In this study, the samples are taken from trachea and cloaca swabs. Trachea
and cloaca swab was taken using a sterile cotton swab from poultry. The trachea of
24 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
swabbing the wall. And for the cloaca of poultry was swabbed by inserting a cotton
swab deeply into the vent and vigorously swabbing the wall. The swab should be
deeply stained with fecal material. After that placed the samples into conical 15
mL containing the medium transport 3 mL (PBS with antibiotics streptomycin 100
IU/mL and penicillin 1000 IU/mL) or conical filled with M199. Each conical filled
with trachea and cloaca swabs were coded before stored in an ice box with the ice
pack inside. The samples that were taken were stored in the refrigerator with a
temperature of 4°C to be tested further (WHO, 2002).
3.4.3. Sample Handling in Laboratory
The swabs sample needs to be vortexed first, in order to mix the swab with
the transport medium, after all the swab samples have been vortexed, the cotton
swab is taken from conical, followed by centrifuging at 3000 rpm for 5 minutes.
The supernatant is accommodated in Microtube and stored in a freezer of -80°C or
keep them at 4°C in the refrigerator for further use to inoculate to embryonated
chicken eggs.
3.4.4. Inoculation Embryonated Chicken Eggs
The virus inoculation to embryonated chicken eggs was performed to grow
the virus inside of the eggs. The 3 supernatant of the poultry swab was pooled into
1 microtube and was added penstrep as much as 50 µL and were inoculated to
‘Specific Antibody Negative' (SAN) embryonated chicken eggs with age 9 – 11
days which has been cleaned. The SAN embryonated chicken eggs were obtained
from Pusvetma, Surabaya, which has been disinfected with alcohol 70%.
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F or from a healthy parent. Examine eggs with an egg candler to sort any infertile
eggs or any bad proportion on the eggs. On the egg candler, give an “X” sign on top eggshell in the area of the air sac. The “X” sign should not near with the blood vessel or near with the embryo. Punch a small hole on the “X” sign. Embryonated
chicken eggs were inoculated with supernatant of swab samples as much as 0.1 ml
using a syringe into the allantoic fluid through a hole that was created on top of the
air sack in 1cm depth parallel to the long axis of the egg then covered with tape.
Eggs were incubated at 37°C for 3-4 days and observed every day with eggs candler
toward the death of the embryo. After 3 days, transferred the eggs to a 4°C
refrigerator and harvested the allantois (WHO, 2002).
3.4.5. Harvesting of Embryonated Chicken Eggs
After 24 hours in the fridge, embryonated chicken eggs allantois fluid is
ready to be harvested. First, the existing tape on the shell surface of Embryonated
chicken Eggs will be released and clean off the eggshell with alcohol 70%. Labeling
the conical 15 mL for each egg with the specimen number. Break and cut shell over
the air sac and push aside the allantois membrane to aside with the forceps. Then
using micropipette and sterile tips to take the allantois fluid carefully and fill the
conical with specimen number. Centrifuge harvested fluids at 3000 rpm for 5
minutes to remove excess blood or tissues. Next centrifuged allantois fluids will be
transferred into 1.5 mL microtube. The results of virus isolation from allantois fluid
are stored at 4°C which is the optimal temperature for the next step to be used for
26 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
3.4.6. Preparation of 0.5% Chicken Erythrocytes Suspension
Red blood cell (RBC) was used in the HA and HI test is 0.5 % of RBC
chicken, chicken blood is used as much as 2 mL, then put into tubes containing
EDTA anticoagulant. The collected blood was transferred to a 15 mL conical tube
and adding 12 mL PBS, then centrifuge it at 3.000 rpm for 5 minutes. The
supernatant is discarded and the rest of the sediment is added with 12 mL PBS, then
centrifuge again at same speed and time. These activities are repeated up to two
times or until the supernatant is clear, so that there is no lysis of erythrocytes.
Making the 0.5 % RBC suspension is by converting the concentration of
RBC 100 % to 0.5 % in 15 mL, formula for 2 microplates, follow by this formula:
N1 x V1 = N2 x V2
100% x V1 = 0,5% x 15 mL
V1 = 0,5% x 15 mL
100%
V1 = 0,075 mL
= 75 µl
Explanation :
N1: Initial erythrocyte concentration
V1: Initial erythrocyte volume
N2: Final erythrocyte concentration
V2: Final erythrocyte volume
So to make a 15 ml of 0.5% Red Blood Cells suspension is by adding 0.075
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F 3.4.7. Hemagglutination Test (HA)
HA test in this research using a microplate "V" bottom. The procedures as
follow: fill the microplate wells with PBS as much as 50 μL starting from columns
1-12 in row A through H. The microplate column 12 will be used as the control
erythrocytes (without antigen). Take one allantois fluid and fill it into well A1, take
another allantois fluid and put it into well A2, it goes on and on from A1 until A11,
with different allantois fluid. Set micropipette multichannel into 50 μl, insert the
eight pipettes (multichannel pipette with 8 channels) into well A1 to A8, make a
dilution in order to mix PBS with allantois fluid, also do this for well A7 to A11.
After dilution, take 50 μl allantois fluid that mixed with PBS from row A to row B
(well A1-A11 to B1-B11), and thoroughly mix it and take as much as 50μl from row B to row C. Do the same thing up to row H, and after mixed until row H, take
50μl from row H (well H1-H11) then discard it. The next step is to fill 0.5% of chicken RBC as much as 50 μl of all wells. Mix it well and cover the microplate
surface with plastic wrap to prevent the contaminant, then incubate at room
temperature for 30 minutes. After 30 minutes, read the agglutination by saw the
titers of the examined samples by tilting the plates to 45°. A positive result is
determined when it showed ≥ 23 (WHO, 2011; OEI, 2012; OIE, 2014).
As expected, column No. 12 will be shown tear shape, because RBC and
PBS won’t bind to each other. Perfect hemagglutination (100%) is clearly visible
hemagglutinate of erythrocytes in the form of a layer and homogenous (diffuse) in
the wells, and clearly fluids on the top of the layer without sedimented erythrocyte
28 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
3.4.8. Retitration of 8 HA Unit Antigen
Antigen standard used in antisera test is 8 HA unit/50 µl it is the same as 25
µ l containing 4 HAU. If the samples under examination have a titer of 24, then the
dilution made for 1 microplate is as follow:
The 0.075 ml antigen 24 mix with 2.25 sterile PBS to provide 3 ml 8HA
unit antigen. To determine the accuracy of 8 HA units antigen that has been made,
retitration antigen testing is necessary to be done.
3.4.9. Hemagglutination Inhibition Test (HI) use Antisera H9
The antisera H9 were tested before using Hemagglutination Inhibition test,
using specific antigen of H9 virus. In this research, the antisera H9 were obtained
from Balai Veteriner with standard titer 25, the titer that shown in the
Hemagglutination Inhibition supposed shows there are inhibition of agglutination
between the antisera and the antigen. The hemagglutination Inhibition test (HI)
using H9 antisera can be used to identify the presence of H9 in the sample that
wants to be tested. The steps in the HI are as follows: provide microplate and filled
with PBS 25 μl in all wells except row A. Row A is filled with 50 μl specific H9
antisera which already known the titer, from A1 taken 25 μl and diluted to hole B1, N1 x V1 = N2 x V2
24 x V1 = 23 x 0.15 Ml
V1 = 23 x 1.5 mL
24
V1 = 0.075 mL
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F serial dilution is carried out until well H1, on H1 is discharged 25 μl. Antisera dilution is needed before used in the HI test. All antigen to be tested is already in
the 4HAU (because in this test was used 25 μl, same as 8HAU/50 μl) retention included 25 μl in holes A1 to H10. Lines 11 and 12 are used as positive and negative
controls. Hole number 11 as a positive control containing RBC 0.5% as much as 50
μl and PBS 50 μl while hole number 12 as negative control contains H9 specific antigen 25 μl, PBS 25 μl and RBC 0,5% 25 μl. The microplate is placed in a
mechanical vibrator until the antisera and antigen are well blended, then incubated
for 30 minutes. After incubation, all holes added 0.5% chicken RBC, then incubated
at room temperature for 30 minutes. The last step is done to see the inhibition of
agglutination or not on the test of HI using specific H9 antisera. Sediment will show
as a red dot on the bottom of the microplate well on a positive result. To ease the
reading, tilting microplate to 45° until it looks like Tear Shape form (Ernawati et
al., 2007).
3.5. Data Analysis
Data were obtained from the positive result of Hemagglutination test and
Hemagglutination inhibition test was present descriptively, which calculate the
percentage found any subtype H9 AIV from trachea swab and cloaca swab at
culling layer in Indonesia. Percentage virus AIV H9 is:
∑ 𝑝𝑜𝑠𝑖𝑡𝑖𝑣𝑒 𝑠𝑎𝑚𝑝𝑙𝑒
∑ 𝑡𝑜𝑡𝑎𝑙 𝑠𝑎𝑚𝑝𝑙𝑒 × 100% = Percentage virus Avian Influenza found
30 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
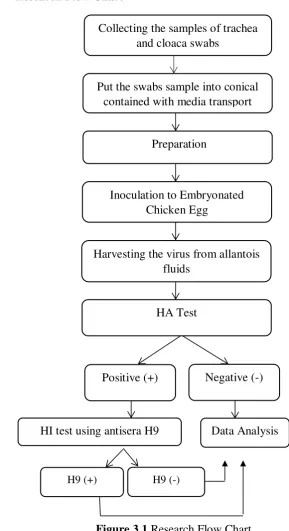
3.6. Research Flow Chart
Figure 3.1 Research Flow Chart Collecting the samples of trachea
and cloaca swabs
Inoculation to Embryonated Chicken Egg
Put the swabs sample into conical contained with media transport
Preparation
Harvesting the virus from allantois fluids
Positive (+)
HA Test
HI test using antisera H9
Negative (-)
H9 (+) H9 (-)
31
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
CHAPTER 4 RESEARCH RESULT
The sample of this research are taken from culling layer at traditional market
and farms in DKI Jakarta, West Java, Yogyakarta, East Java and South Kalimantan,
these samples are the collection from 2012 to 2018. Research has been conducted
from January to June 2018. Examined samples were tracheal and cloacal swabs.
The total amount of sample is 350 where’s 350 samples were obtained from 1050 pooled samples. Which each one pool contains 3 different individuals with the same
species criteria and from the same market or folks. Each code contains 3 samples
that have been pooled into 1 sample and inoculated into 1 embryonated chicken
eggs. The sample has been tested in Poultry Disease Laboratory of Professor Nidom
Foundation in Surabaya inside BSC (Biosafety Cabinet).
The swab samples were centrifuged beforehand. The supernatant was
inoculated to Specific Antibody Negative (SAN) embryonated chicken eggs and
incubated for three days, continued with Hemagglutination (HA) test. Samples that
having titer ≥ 23 HA were continued for with HI test using standard serum of H9 subtype avian influenza virus which is already available. Standardized antigens
must have an HA titer of 4HAU/25µ l (WHO, 2002).
The result of HA test after samples were inoculated to embryonated chicken
eggs showed that the hemagglutination happened to 29 samples out of 350 samples
with HA titer ≥ 23, while for HI test using antisera of H9 subtype Avian Influenza Virus showed result with positive sample as much as 22 positive samples out of 29
32 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
4.1. Hemagglutination Test Positive Result
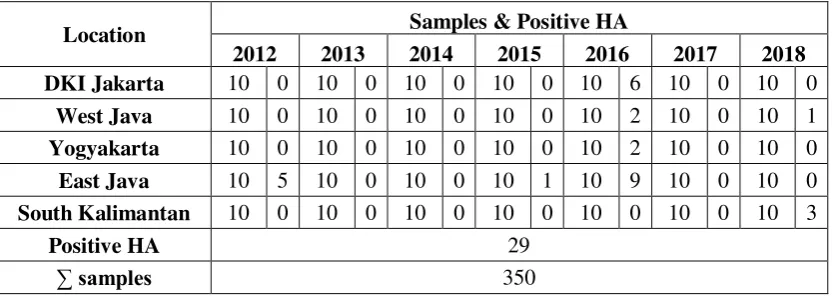
Table 4.1 Data results of positive sample from HA test from culling layer at
traditional market and farm in Indonesia.
Location Samples & Positive HA
2012 2013 2014 2015 2016 2017 2018
Based on table 4.1 there is 29 positive result from HA test out of 350 swab
samples from culling layer in several places in Indonesia. Each year the samples
were taken as much as 10 samples from every place. In DKI Jakarta, with a total of
70 samples from 2012-2018, there is a positive result from HA test, which is 6
samples in 2016. While for West Java, with total samples 70 from 2012-2018, there
is a positive result from HA test, which is 2 samples from Subang in 2016 and 1
sample from Cikidang in 2018. In Yogyakarta, with total samples 70 from
2012-2018, there is a positive result from HA test, which is 2 samples from Yogyakarta
in 2016. Meanwhile East Java, with total samples 70 from 2012-2018, there is a
positive result from HA test, which is 5 samples from Bondowoso and Kediri in
2012, 1 sample in 2015 and 9 samples from Pamekasan, Bangkalan, Sidoarjo,
Lamongan and Sumenep in 2016. Last is South Kalimantan, with total samples 70
from the year 2012-2018, there is a positive result from HA test, which is 3 samples
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F From the result of HA test above showed that some positive samples with
titer ≥23 should be continued with Hemagglutination Inhibition (HI) using antiserum Avian Influenza Virus subtype H9. More detailed results are presented
in Appendix 1.
4.2. Hemagglutination Inhibition using Antisera H9 test Positive Result
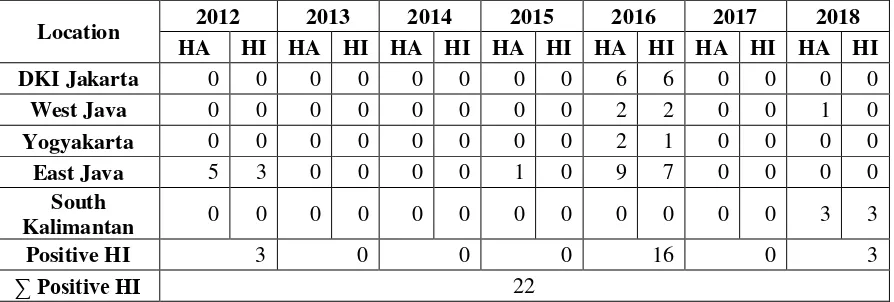
Table 4.2 Data results of positive sample from HI using antisera H9 test from
culling layer at traditional market and farm in Indonesia.
Location 2012 2013 2014 2015 2016 2017 2018
Based on table 4.2 there is 22 positive result from HI using antisera H9 test
out of 350 swab samples from culling layer in several places in Indonesia. Each
year the samples were taken as much as 10 samples from every place.
In DKI Jakarta, with total samples 70 from 2012-2018, there are culling
layer with positive antisera H9, which is 6 samples from RPH in 2016. While for
West Java, with total samples 70 from 2012-2018, there are culling layer with
positive antisera H9, which is 2 samples, 1 sample from Subang in 2016 and
Cikidang in 2018. In Yogyakarta, with total samples 70 from 2012-2018, there are
culling layer with positive antisera H9, which is 1 sample from Yogyakarta in 2016.
34 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
with positive antisera H9, which is 3 samples from Bondowoso and Kediri in 2012,
and 7 samples from Pamekasan, Bangkalan, Sidoarjo, Lamongan and Sumenep in
2016. Last is South Kalimantan, with total samples 70 from 2012-2018, there are
culling layer with positive antibody H9, which is 3 samples from Banjarmasin in
2018.
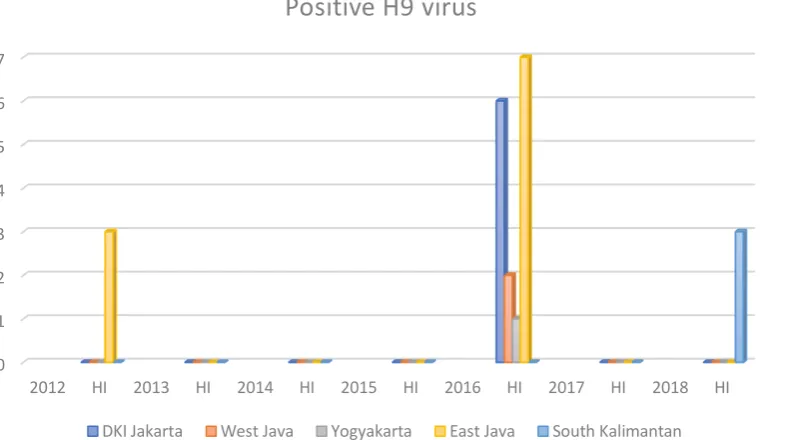
4.3. Percentage of Positive Sample According to Year
The result shows that all regencies are found positive samples tested using HI
antisera H9 Test. In 2012, there is 3 positives sample with antisera H9 from East
Java, which is 1.7% culling layer were infected with AIV subtype H9. In 2013
throughout 2015, there is 0 sample with positive antisera H9, which is 0% culling
layer were infected with AIV subtype H9. In 2016 there are 16 positive samples
with antisera H9 from DKI Jakarta, West Java, Yogyakarta and East Java, which is
4.5% culling layer were infected with AIV subtype H9. In 2018, there are 3 positive
sample with antisera H9 from South Kalimantan, which is 1.7% samples were
infected with AIV subtype H9.
The antisera of avian influenza subtype H9 was obtained from Balai Veteriner
with standardized titer 25. The result above showed the positive samples in the HI
test means those samples have indicated there is hemagglutinin protein of avian
influenza subtype H9. More detailed results are presented in Appendix 1.
4.4 Graphic of Positive H9
The graphic (Fig. 4.4) shows positive sample from HI using antisera H9 test
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F Fig. 4.4 Positive Sample of H9 virus
0 1 2 3 4 5 6 7
2012 HI 2013 HI 2014 HI 2015 HI 2016 HI 2017 HI 2018 HI
Positive H9 virus
IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
36
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
CHAPTER 5 DISCUSSION
This research used method that isolating samples on embryonated SAN
chicken egg using Hemagglutination and Hemagglutination Inhibition test using
Antisera H9. HA test is to detect a virus that has hemagglutinin which will
agglutinate erythrocyte from some species such as poultry, mammals, or human.
Because influenza virus is a virus that has hemagglutinin, so to detect Influenza
virus HA test is needed before any test for screening on the virus and also can be
used to measure the antigen titer for next test is Hemagglutination Inhibition test.
HI test has a function to determine the type of antibody and its titer and also to
identify the virus (Ernawati et al, 2007). While Hemagglutination Inhibition test
using standardized antisera has a function to determine the type of antigen that
match with the specific serum.
Based on the research, the positive result with HA test in table 4.1 shows
there are some samples that have hemagglutinin. The result from HA test with titer
≥23 can be continued to HI test using antisera H9 (WHO, 2002). This research uses
antisera of H9 from poultry that were infected with subtype avian influenza virus
subtype H9 which was obtained from Balai Veteriner, demand to see if the samples
were positive with AIV subtype H9. The positive result of HI test using antisera H9
on table 4.2
In this research, there are 350 samples, where're 29 positive samples in HA
test which is originated from DKI Jakarta, West Java, Yogyakarta, East Java and
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F titer is too low ≤ 22 or the virus inside the antigen could not be detected through HA test.
While in HI test using antisera of H9, there are 22 samples that show
positive result possibilities were infected by Avian Influenza Virus subtype H9
because the hemagglutinin which agglutinates erythrocyte is detected with titer as
much as ≥25. Based on the data above, there are samples that show the positive result with titer ≥23 in HA test but show the negative result in HI test. Means that there is an incompatibility between antigen of the sample with antisera of H9. The
hemagglutination reaction in HA test might occur due to different avian influenza
subtypes or other microorganisms which also have hemagglutinin protein. Another
virus that also could hemagglutinin protein such as paramyxovirus, enterovirus,
arbovirus, and poxvirus (Ernawati et al, 2013).
From the data graphic of positive H9 (Fig. 4.4) shows there is positive
sample H9 virus in 2012 and at 2013 throughout 2015 shows there are no sample
was found be infected with the H9 virus. But mostly positive samples H9 virus were
found at the year 2016 with total 16 positive samples and continued with extremely
decreased of positive sample in 2017 and shows again in 2018 with total 3 positive
samples.
Based on the reported from Dirjen Peternakan dan Kesehatan Hewan,
Kementrian Pertanian, about AIV subtype H9N2 that was detected in early 2017
through a surveillance that conducted by Balai Veteriner Kementrian Pertanian
which led to a fall in egg supply by the end of 2017 (Dirjen Peternakan, 2017), from
38 IR – PERPUSTAKAAN UNIVERSITAS AIRLANGGA
SKRIPSI IDENTIFICATION OF AVIAN … SITI H. F
2017 can be because in mid of 2017, Dirjen Peternakan did establish vaccination
and also asked farmers to more concern about the sanitation in the cages due to
suppressed the infection of H9 virus. The vaccination in 2017 play role the amount
of infection AIV subtype H9 virus in 2018. From the data above in South
Kalimantan still shows some samples from culling layer indicate infected with AIV
subtype H9 with total sample 3. Also, according to the research of Alabio duck in
South Kalimantan in 2018, was found 2 positive samples in HI test using antisera
of H9 out of 53 samples after pooled into 19 samples. These two seropositive
samples are one from farmer group in Hambuku Raya, Kabupaten Hulu Sungai
Utara and the other one is from Landasan Ulin Utara Banjarbaru. The researcher
can conclude the possibilities of positive samples can be because the vaccination
does not reach the South Kalimantan area which led to still there is infected poultry
in South Kalimantan even after the vaccination at the end of 2017.
However, from the result, it showed some regions in this research are
infected with AIV subtype H9N2 even before 2017, wherein year 2017 was the first
time declared its virus infected some poultry in Indonesia. The region that were
indicated infected with AIV subtype H9 such as DKI Jakarta, West Java, East Java
and Yogyakarta. The researcher can conclude, firstly, the Indonesian government
may not have paid attention to AIV H9 subtypes where’s the virus characterized as
LPAI in which LPAI has a low or virtually non-existent death case (OIE, 2014).
Secondly, the limitedness of the equipment required in checking this subtype, such