www.elsevier.comrlocateranireprosci
Reproductive biotechnologies: current status in
porcine reproduction
B.N. Day
)Animal Science Unit, UniÕersity of Missouri, College of Agriculture, 159 Animal Sciences Center, Columbia,
MO 65211, USA
Abstract
During the past decade, considerable attention has been directed toward the development of reproductive technologies for both research purposes and for more controlled swine reproduction. Artificial insemination is an example of a technology that has continued to be expanded from early use in European countries to the USA and Canada where it is now estimated that a majority of the sows bred are artificially inseminated. In addition, several significant technological advancements have been made in the genetic modification of swine and interest has been generated in the possible use of swine as donors of specific tissues and of organs for the improvement of human health. At the same time, the systems for production of swine for human food continue to undergo major changes including, in some countries, the consolidation of swine into large, integrated units. These swine operations are very receptive to the use of technologies to reduce labor costs as well as a basis for increased production efficiency. Therefore, the combined interest in swine reproductive technologies by both the medical field and the swine industry creates an increased effort for the development of new technologies as well as for the implementa-tion of existing ones. One of the more rapid technological advancements this decade has been the
Ž .
progress in in vitro production IVP of swine embryos. Major advancements have been made on the development of procedures for production of large numbers of embryos from oocytes collected
Ž . Ž .
at slaughter houses which are then matured IVM and fertilized IVF in the laboratory. Success in IVP has stimulated increased research in other areas that can be enhanced by the availability of embryos without a requirement for surgical collection from gilts or sows. One example is the combined use of IVF, gender-sorted sperm cells, and embryo transfer to produce offspring of a predicted sex. In a related area, instrumentation for non-surgical embryo transfer has recently been
)Tel.:q1-573-882-7555; fax:q1-573-884-7827.
Ž .
E-mail address: [email protected] B.N. Day .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
developed that results in significant improvement in this technology. Similar achievements have been gained in cryopreservation of embryos by vitrification. These developments will be reviewed with emphasis on the in vitro production of embryos from immature oocytes.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Swine; Reproduction; Embryo culture; In vitro technology
1. Introduction
Considerable interest has developed during the past decade for the use of established as well as emerging reproductive technologies for both research purposes and for more controlled swine production. Several significant technological advancements have been made in the genetic modification of mammals and interest has been generated in the possible use of swine as donors of specific proteins and of organs for the improvement of human health. This interest by the scientific community has resulted in a major increase in the use of swine in biomedical research studies. At the same time, the systems for production of swine for human food continue to undergo major changes including, in some countries, the consolidation of swine units into large, integrated units very receptive to the use of technologies to reduce labor costs as well as a basis for increased production efficiency. Therefore, the combined interest in swine reproductive technologies by both the medical field and the swine industry creates an increased desire for the development of new technologies as well as for the implementation of existing ones.
One research area being given particular emphasis at the present time is studies on oocytes and early pre-attachment embryos. Major strides have been made during the past
Ž .
decade on the development of procedures for the in vitro production IVP of large numbers of embryos from oocytes collected at slaughter houses which are then matured
ŽIVM and fertilized IVF. Ž . in the laboratory. Success in IVP has then stimulated
increased research in other areas that can be enhanced by the availability of embryos without a requirement for surgical collection from gilts or sows. One example is the combined use of IVF, gender-sorted sperm cells, and embryo transfer to produce offspring of a predicted sex.
2. In vitro production of swine embryos
2.1. Historical
For many years, a scientific interest has existed in the capture of genetics present in the ovaries of superior sows in a manner similar to the use of artificial insemination in order to expand genetics of superior boars within the population. Swine are of particular
Ž .
interest in this regard since the ovaries contain large numbers )200,000 of primordial
Ž .
follicles Grupen et al., 1995 . During the early 1980s, media were developed to culture successfully pig embryos for a short period of time prior to transfer to recipient gilts. However, it wasn’t until the early 1990s that one- to two-cell embryos recovered from donor gilts could be cultured through the ‘‘four-cell block’’ to the blastocyst stage of
Ž .
development Petters and Wells, 1993; Beckmann and Day, 1993 . The presence of reduced NaCl concentration or the presence of organic osmolytes in the embryo culture medium was found to provide the capability to culture single-cell embryos through the four-cell stage. These established characteristics of pig oocytes have been later applied in the development of media for the in vitro maturation of oocytes.
Shortly prior to the development of technology to culture in vivo fertilized one- to two-cell embryos to the blastocyst stage, in vitro fertilization of in vivo matured oocytes
Ž .
was achieved Chen et al., 1986 . Therefore, it became possible to produce live offspring from the in vitro fertilization of in vivo matured oocytes. The missing link for the in vitro production of blastocysts from immature oocytes recovered from ovaries was the
Ž .
ability to mature oocytes in vitro IVM . Focus has been directed toward research in this area during the past decade.
2.2. InÕitro maturation of oocytes
In general, oocytes surrounded by a compact cumulus cell mass when collected from control follicles of slaughtered peripuberal gilts have been used for in vitro maturation. Although oocytes collected from sexually-mature sheep and cattle have generally been found to be superior to those from prepuberal animals, this comparison has not been reported for pigs using currently recommended maturation media.
Early studies on IVM clarified the importance to developmental competence of both nuclear and cytoplasmic maturation. Nuclear maturation can be more clearly defined by the development of a metaphase II spindle from the germinal vesicle; whereas, indirect measures must be employed to measure cytoplasmic maturation. In earlier studies, it was noted that a high incidence of nuclear maturation could be attained in media which did
Ž .
not support cytoplasmic maturation see Niwa, 1993; Funahashi and Day, 1996 .
Ž .
However, in a series of studies involving the addition of cysteine Yoshida et al., 1993 ,
Ž . Ž .
follicular fluid Funahashi and Day, 1993a , cysteamine Grupen et al., 1995 and
Ž .
beta-mercaptoethanol Funahashi and Day, 1997; Abeydeera et al., 1998a , this problem
Ž .
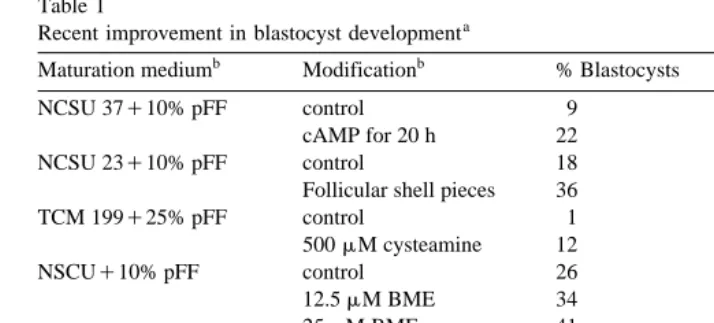
has been minimized Table 1 . Other approaches including reduction of duration of
Ž .
Table 1
Recent improvement in blastocyst developmenta
b b
Maturation medium Modification % Blastocysts Reference
Ž .
NCSU 37q10% pFF control 9 Funahashi et al. 1997b
cAMP for 20 h 22
Ž .
NCSU 23q10% pFF control 18 Abeydeera et al. 1998c
Follicular shell pieces 36
Ž .
TCM 199q25% pFF control 1 Grupen et al. 1995
500mM cysteamine 12
Ž .
NSCUq10% pFF control 26 Abeydeera et al. 1998a
12.5mM BME 34
25mM BME 41
Ž .
NCSU 23q10% pFF control 21 Abeydeera et al. 1998b
1 ngrml EGF 33
10 ngrml EGF 42
Ž .
TCM-199q0.1% PVA control 22 Abeydeera et al. 1999
10 ngrml EGF 37
a Ž .
Day et al., 1999 modified .
b
mWM: modified Whitten’s medium; NCSU: North Carolina State University; pFF: pig follicular fluid; PVA: polyvinylalcohol.
c
BME:b-mercaptoethanol; EGF: epidermal growth factor.
Ž .
and use of reduced media concentrations of NaCL Funahashi et al., 1994 have also been found to enhance pronuclear development.
2.3. Protein free medium
Although high rates of pronuclear formation had been accomplished by the addition
Ž
of porcine follicular fluid, follicular shell pieces, and conditioned media Day and
.
Funahashi, 1996 , a preferred medium for maturation would be one which did not
Ž .
require co-culture with somatic cells or their secretions. Abeydeera et al. 1999 have developed such a medium following a series of studies involving the substitution of
Ž .
polyvinylalcohol PVA for previously used biological materials added to NCSU23, Whittens, Waymouth, and TC199. Results not different from NCSU23 plus porcine follicular fluid were obtained when oocytes were matured in TC199 without follicular fluid but with added PVA.
( )
2.4. Epidermal growth factor EGF
A further improvement in available medium for embryo maturation has been obtained
Ž
by the addition of EGF to either a medium containing follicular fluid Abeydeera et al.,
. Ž .
2.5. Stage of germinalÕesicle at gonadotropin stimulation
Only recently has attention been directed toward the nuclear morphology of oocytes
Ž
collected from ovaries of slaughtered animals Funahashi et al., 1997a,b; Guthrie and
.
Garrett, 1999; Hirao et al., 1995 . The germinal vesicle stage of oocytes selected for in vitro maturation varies considerably when classified according to morphological
charac-Ž .
teristics developed by Motlik and Fulka 1976 . In contrast, nuclear morphology of the GV stage is closely synchronized in gilts at the approximate time of endogenous
Ž
luteinizing hormone release to induce ovulation and oocyte maturation Funahashi et al.,
.
1996 . Improved embryonic development has been obtained by modification of culture condition to synchronize the germinal vesicle stage of oocytes between follicular
Ž .
aspiration and germinal vesicle breakdown GVB . Exposure of oocyte–cumulus
com-Ž . X X Ž .
plexes OCC to dibutyryl cyclic adenosine 3 , 5 -monophosphate dbcAMP , for initial 20 h of maturation culture, synchronized germinal stage and improved embryonic development. Further, simply holding oocytes in culture for 12 h prior to exposure to gonadotropins improved blastocyst formation following IVF. Further research in this area appears to be indicated in order to more clearly establish the most desirable germinal vesicle stage at time of treatment with gonadotropins in order to achieve maximal developmental competence.
( )
2.6. InÕitro fertilization IVF
Along with the development of highly successful methods for the penetration of in vivo or in vitro matured oocytes, there has also occurred a high frequency of polyspermy. Although many of the previous investigations of IVF in pigs have used freshly ejaculated sperm, frozen–thawed sperm techniques have now been developed which provide the major advantage of continued use of the same semen collection over
Ž .
repeated experiments Wang et al., 1991; Abeydeera and Day, 1997 . However, the availability of frozen–thawed sperm has still not led to the development of IVF procedures which yield acceptable rates of monospermy. Reduced polyspermy has been noted following additions to fertilization medium of porcine oviductal cells and
secre-Ž .
tions Nagai and Moor, 1990; Kano et al., 1994; Kim et al., 1996 , porcine follicular
Ž .
fluid Funahashi and Day, 1993a and semi-purified porcine oviduct specific
glyco-Ž .
protein Kouba et al., 1999 . Detailed comparative examination of cortical granule release following IVF of in vivo matured, oviductal and IVM oocytes has not revealed significant differences in degree or time of cortical granule exocytosis following IVF
ŽWang et al., 1997, 1998 , which could be interpreted to be a potential cause of.
polyspermy.
In summary, polyspermy remains the major obstacle to successful production of large numbers of IVP embryos with high level of developmental competence.
2.7. Embryo culture
Several media are available for the successful culture of in vivo embryos to the
Ž .
Ž . Ž .
Whittens medium MWM Beckmann and Day, 1993 , North Carolina State University
Ž . Ž . Ž .
23 NCSU23 medium Petters and Wells, 1993 , Iowa State University ISU medium
ŽYoungs et al., 1993 and Beltsville embryo culture medium 3 BECM-3. Ž . ŽDobrinsky et .
al., 1996 . Recently, a comparison was made of the rate of development to blastocysts of IVMrIVF derived embryos in the above media. A wide variation of 5% to 30% blastocyst development was found with the NCSU23 medium, the most successful and MWM the least. Further preliminary testing of the NCSU23 medium indicated that culture for the initial 72 h in NCSU23 medium without glucose was also beneficial
ŽAbeydeera, unpublished ..
Since well-known morphological differences are known to exist between in vivo
Ž
matured embryos exposed to the oviductal environment and in vitro embryos Wang et
.
al., 1998 , additional research may reveal that reduced development of IVMrIVF embryos may not be due only to inadequate cytoplasmic maturation, but instead this is in combination with polyspermy and inappropriate embryo culture medium, particularly during initial cleavage stages. For example, embryo development to the
morularblasto-Ž
cyst stage of early one-cell hamster embryos collected before pronuclear development 3
.
h post-egg activation was stimulated by an initial 6 h culture in the standard hamster
Ž
culture medium treated to reduce intracellular free calcium levels Lane and Bavister,
.
1998 . Further, it has also been shown that one- to two-cell in vivo embryos recovered from one oviduct, and placed in culture for comparison with embryos recovered from the opposite oviduct 4 days later, are delayed approximately one cell cleavage from the
Ž .
embryos retained in vivo Machaty et al., 1998 . The inner cell mass was particularly delayed in development in the in vitro embryos.
2.8. DeÕelopmental competence
2.8.1. Blastocyst
With the improvements in cytoplasmic maturation, as measured by incidence and timing of male pronuclear development, and the decreased frequency of polyspermy during IVF, major increases have been obtained in the percentage of oocytes subjected
Ž .
to IVM that develop to the blastocyst stage of development Table 1 . At present, IVM and IVF procedures are available which will give repeated results of approximately 35% of subjected immature oocytes that form blastocysts. However, it is now known that fertilized oocytes with more than two male pronuclei form blastocysts at the same
Ž .
frequency as normally fertilized oocytes Han et al., 1999a . There are no reported births of polyploid offspring in pigs and polyploidy may, therefore, be a cause of embryorfetal mortality in recipient gilts receiving morphologically normal IVP embryos. The question remains open as to the fate of extra sperm in polypronuclear oocytes. It has been shown that blastocysts with both normal and abnormal ploidy develop from polypronuclear
Ž .
oocytes Han et al., 1999b .
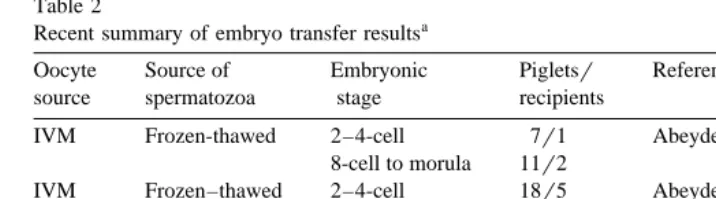
Table 2
Recent summary of embryo transfer resultsa
Oocyte Source of Embryonic Pigletsr Reference
source spermatozoa stage recipients
Ž .
IVM Frozen-thawed 2–4-cell 7r1 Abeydeera et al. 1998b
8-cell to morula 11r2
Ž .
IVM Frozen–thawed 2–4-cell 18r5 Abeydeera et al. 1998d
Ž .
IVM X-sperm 8-cell to morula 24r5 Abeydeera et al. 1998c Y-sperm 8-cell to morula 9r3
Ž .
IVM Frozen–thawed G1-cell 17r3 Kikuchi et al. 1999
Ž .
IVM Frozen–thawed 8-cell to morula 82r12 Abeydeera et al. unpublished data
a Ž .
Day et al., 1999 modified .
2.8.2. Pregnancy rate and litter size
Only during the past 4–5 years has IVP techniques been improved to the extent that expectations for full term development were sufficient to justify more than a very few
Ž .
embryo transfers to recipient gilts Tables 1 and 2 . With improvements in IVMrIVF, gradual increases in number of embryo transfers, pregnancy rate and litter size have occurred to the point whereby consideration can now be given to the use of IVP
Ž
embryos in related studies in biotechnology see review by Abeydeera et al., 1998c; Day
.
et al., 1999 . For example, it has recently been shown that adequate reproduction was obtained in recipient gilts receiving IVP embryos matured in a protein-free medium containing EGF, fertilized with frozen–thawed sperm and cultured to the eight-cell to
Ž .
morula stages of development. Of 25 recipient gilts, 22 88% were diagnosed by ultrasonography to be pregnant at 25–30 days post-estrus. Following slaughter on days
Ž . Ž .
26–45 ns6 or at farrowing ns12 , 18 of 25 gilts were pregnant with an average of 7.2 and 6.8 young, respectively, which represented 23% of the 30 IVMrIVF embryos
Ž .
transferred to each of these 18 recipients Abeydeera, unpublished .
3. Embryo transfer
3.1. Surgical
Surgical techniques for embryo transfer have been available for several decades and are presently in use in several research programs.
3.2. Non-surgical embryo transfer
Ž
An interest in non-surgical embryo transfer has existed for many years Polge and
.
recently, increased research has been given to this technology and success has been achieved with improved designs in transfer instruments and also variations in
synchro-Ž .
nization of donors and recipients see review by Hazeleger and Kemp, 1999 . Further development of present non-surgical transfer techniques would provide major benefits toward the implementation of other swine biotechnologies including transfer of micro-manipulated IVP embryos.
It is interesting to speculate if the endoscopic technology described by Vazquez et al.
Ž1999 for deep uterine AI may provide a much improved method for both non-surgical.
embryo collection and embryo transfer in pigs.
4. Cryopreservation of embryos
4.1. Freezing
Porcine embryos have been successfully frozen, thawed and produced offspring
Ž .
following transfer to recipient gilts Nagashima et al., 1994; Dobrinsky, 1997 . How-ever, the successful birth of piglets from various approaches to cryopreservation has been inconsistent and has involved small numbers of litters.
4.2. Vitrification
Vitrification has become available as an alternative method for cryopreservation of animal embryos. Blastocysts, expanded blastocysts and hatched blastocysts have been shown to have high survival rates in culture following vitrification. More recently, significant improvements have been made in procedures which have resulted in several
Ž
litters of offspring born from embryos transferred following vitrification see Dobrinsky,
.
1999 .
5. Gender preselection
5.1. Sorting of X and Y bearing spermatozoa
It is currently possible to separate X bearing spermatozoa by flow cytometryrcell
Ž .
sorting technology see review by Johnson, 1997 . The spermatozoa DNA are stained with a fluorescence dye which binds equal to the amount of DNA present. In turn, the slightly larger amount of DNA in X bearing sperm provides a basis for sorting the spermatozoa into two populations; i.e., X and Y pools. Offspring have been born as a
Ž
. Ž .
1994 , IVF of in vivo matured oocytes Rath et al., 1997 and IVF of IVM oocytes
ŽAbeydeera et al., 1998c ..
6. Micromanipulation of germ cells
6.1. Cloning and transgenesis
The selection of pigs as a model for biomedical research on human cardiovascular disorders and the increased interest during the past 3 or 4 years on the modification of pig tissues and organs as transplants to humans have resulted in a dramatic increase in the number of laboratories involved in development of transgenic and cloned offspring
Žsee Nottle et al., 1997; Piedrahita et al., 1997 . Cloning and transgenesis technology is.
needed to have a sufficient resource for both desired modification of genotypes for biomedical studies including xenotransplantation and also for an availability of identical
Ž .
piglets. To date, a single cloned pig has been produced Prather et al., 1989 . Although advancements have been made in the processes utilized for nuclear transfer in pigs, such
Ž
as the improved methods for activation of unfertilized oocytes with thimersol Machaty
.
et al., 1997 , full development of additional cloned offspring has not been reported to date. However, chimeric embryos and a live offspring have been produced by transfer to
Ž .
blastocyst of genetically transferred primordial germ cells Piedrahita et al., 1998 .
7. Summary
Past and present research has established reproductive technologies which have become an integral part of the global swine industry. Artificial insemination is an example of a technology that has continued to be expanded from early use in European countries to the USA and Canada where it is now estimated that a majority of the 6–7 million sows bred are artificially inseminated.
Recommended surgical embryo transfer procedures are readily available and numer-ous research laboratories employ the technology.
gained in cryopreservation of embryos by vitrification. Several laboratories await the birth of a cloned ‘‘Miss Piggy’’, hopefully, in the 20th century.
Acknowledgements
The author would like to acknowledge the very beneficial assistance from Dr. Hiroaki Funahashi and Dr. Lalantha Abeydeera in preparation of the manuscript and from long-standing editorial contributions by Betty Nichols.
References
Abeydeera, L.R., Day, B.N., 1997. Fertilization and subsequent development in vitro of pig oocytes inseminated in a modified tris-buffered medium with frozen–thawed ejaculated spermatozoa. Biol. Reprod. 57, 729–734.
Abeydeera, L.R., Johnson, L.A., Welch, G.R., Wang, W.H., Boquest, A.C., Cantley, T.C., Rieke, A., Day, B.N., 1998c. Birth of piglets preselected for gender following in vitro fertilization of in vitro matured pig oocytes by X and Y chromosome bearing spermatozoa sorted by high speed flow cytometry. Theriogenol-ogy 50, 981–988.
Abeydeera, L.R., Wang, W.H., Cantley, T.C., Prather, R.S., Day, B.N., 1998a. Presence ofb-mercaptoethanol can increase the glutathione content of pig oocytes matured in vitro and the rate of blastocyst development after in vitro fertilization. Theriogenology 50, 747–756.
Abeydeera, L.R., Wang, W.H., Cantley, T.C., Rieke, A., Day, B.N., 1998d. Coculture with follicular shell pieces can enhance the developmental competence of pig oocytes after in vitro fertilization: relevance to intracellular glutathione. Biol. Reprod. 58, 213–218.
Abeydeera, L.R., Wang, W.H., Cantley, T.C., Rieke, A., Prather, R.S., Day, B.N., 1998b. Presence of epidermal growth factor during in vitro maturation of pig oocytes and embryo culture can modulate blastocyst development after in vitro fertilization. Mol. Reprod. Dev. 51, 395–401.
Abeydeera, L.R., Wang, W.H., Cantley, T.C., Rieke, A., Prather, R.S., Day, B.N., 1999. Epidermal growth factor can enhance the developmental competence of pig oocytes matured in vitro under protein-free
Ž .
culture conditions. Theriogenology 51, 365, Abstr. .
Beckmann, L.S., Day, B.N., 1993. Effect of medium NaCl concentration and osmolarity ion culture of the early stage porcine embryo and viability of embryos cultured in a selected superior medium. Theriogenol-ogy 39, 611–622.
Chen, W.T.K., Moor, R.M., Polge, C., 1986. In vitro fertilization of pig and sheep oocytes matured in vivo
Ž .
and in vitro. Theriogenology 25, 146, Abstr. .
Day, B.N., Abeydeera, L.R., Prather, R.S., 1999. Recent progress in pig embryo production through in vitro maturation and fertilization techniques. Proc. IV Int. Conf. on Boar Semen Preservation, Beltsville, MD,
Žin press ..
Day, B.N., Funahashi, H., 1996. In vitro maturation and fertilization of pig oocytes. In: Miller, R.H., Pursel,
Ž .
V.G., Norman, H.D. Eds. , Beltsville Symposia in Agricultural Research: II. Biotechnology’s Role in the Genetic Improvement of Farm Animals. Am. Soc. Anim. Sci., Savoy, IL, pp. 125–144.
Dobrinsky, J.F., 1997. Cryopreservation of pig embryos. In: Foxcroft, G.R., Geisert, R.D., Doberska, C.
ŽEds. , Control of Pig Reproduction V. J. Reprod. Fertil. Cambridge Univ. Press, UK, pp. 301–312..
Dobrinsky, J.R., 1999. Cryopreservation of swine embryos: Farrowing super-cooooooool pigs! Proc. IV Int.
Ž .
Conf. on Boar Semen Preservation, Beltsville, MD. in press .
Ž .
Funahashi, H., Cantley, T.C., Day, B.N., 1997a. Preincubation of oocyte–cumulus complexes before exposure to gonadotropins improves the developmental ability of porcine embryos matured and fertilized in vitro. Theriogenology 47, 679–686.
Funahashi, H., Cantley, T.C., Day, B.N., 1997b. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic AMP improves developmental competence following in vitro fertilization. Biol. Reprod. 57, 49–53.
Funahashi, H., Day, B.N., 1993a. Effects of follicular fluid at fertilization in vitro on sperm penetration in pig oocytes. J. Reprod. Fertil. 99, 97–103.
Funahashi, H., Day, B.N., 1993b. Effects of the duration of exposure to hormone supplements on cytoplasmic maturation of pig oocytes in vitro. J. Reprod. Fertil. 98, 179–185.
Funahashi, H., Day, B.N., 1996. Current status of in vitro production of porcine embryos. In: Tumbleson, M.,
Ž .
Schook, L. Eds. , Advances in Swine in Biomedical Research. Plenum, New York, pp. 491–502.
Ž
Funahashi, H., Day, B.N., 1997. Advances in in vitro production of porcine embryos. J. Reprod. Fertil. Suppl.
.
52 , 271–283.
Funahashi, H., Stumpf, T.T., Kim, N.H., Day, B.N., 1994. Effects of sodium chloride concentration in
Ž .
maturation media on histone H1 kinase H1K activity and cytoplasmic maturation of porcine oocytes. J.
Ž .
Reprod. Fertil. 13, 38, Abstr. .
Funahashi, H., Tatemoto, H., Cantley, T.C., Day, B.N., 1996. Nuclear morphology of swine oocytes during
Ž . Ž .
follicular development following stimulation by eCG injection. Biol. Reprod. Suppl. 54 , 156, Abstr. . Grupen, C.G., Nagashima, H., Nottle, M.B., 1995. Cysteamine enhances in vitro development of porcine
oocytes matured and fertilized in vitro. Biol. Reprod. 53, 173–178.
Ž .
Guthrie, H.D., Garrett, W.M., 1999. Changes in germinal vesicle GV development during follicle maturation
Ž . Ž .
in pigs. Biol. Reprod. Suppl. 60 , 186, Abstr. .
Han, Y.M., Abeydeera, L.R., Kim, J.H., Moon, H.B., Cabot, R.A., Day, B.N., Prather, R.S., 1999a. Growth retardation of inner cell mass cells in polyspermic porcine embryos produced in vitro. Biol. Reprod. 60, 1110–1113.
Han, Y.M., Wang, W.H., Abeydeera, L.R., Petersen, A.L., Kim, J.H., Murphy, C., Day, B.N., Prather, R.S., 1999b. Pronuclear location before the first cell division determines ploidy of polyspermic pig embryos. Biol. Reprod. 61, 1340–1346.
Hazeleger, W., Kemp, B., 1999. State of the art in embryo transfer. Theriogenology 51, 81–90.
Hirao, Y., Tsuji, Y., Miyano, T., Okano, A., Miyake, M., Kato, S., Moor, R.M., 1995. Association between p34cdc2 levels and meiotic arrest in pig oocytes during early growth. Zygote 3, 325–332.
Ž .
Johnson, L.A., 1997. Advances in gender preselection in swine. J. Reprod. Fertil. Suppl. 52 , 255–266. Kano, K., Miyano, T., Kato, S., 1994. Effect of oviductal epithelial cells on fertilization of pig oocytes in
vitro. Theriogenology 42, 1061–1068.
Kikuchi, K., Kaziwazaki, N., Noguchi, J., Shimada, A., Takahashi, R., Hirabayashi, M., Shino, M., Ueda, M., Kaneko, H., 1999. Developmental competence, after transfer to recipients, of porcine oocytes matured, fertilized, and cultured in vitro. Biol. Reprod. 60, 336–340.
Kim, N.H., Funahashi, H., Abeydeera, L.R., Moon, S.J., Prather, R.S., Day, B.N., 1996. Effects of oviductal fluid on sperm penetration and cortical granule exocytosis during in vitro fertilization of porcine oocytes. J. Reprod. Fertil. 107, 79–86.
Kouba, A.J., Abeydeera, L.R., Alvarez, I.M., Day, B.N., Buhi, W.C., 1999. Effects of porcine oviduct-specific
Ž . Ž . Ž .
glycoprotein pOSP on fertilization and polyspermy. Biol. Reprod. Suppl. 60 , 228, Abstr. .
Lane, M., Bavister, B.D., 1998. Calcium homeostasis in early hamster preimplantation embryos. Biol. Reprod. 59, 1000–1007.
Machaty, Z., Day, B.N., Prather, R.S., 1998. Development of early porcine embryos in vitro and in vivo. Biol. Reprod. 59, 451–455.
Machaty, Z., Wang, W.H., Day, B.N., Prather, R.S., 1997. Complete activation of porcine oocytes induced by the sulfhydryl reagent, thimerosal. Biol. Reprod. 57, 1123–1127.
Motlik, J., Fulka, J., 1976. Breakdown of the germinal vesicle in pig oocytes in vivo and in vitro. J. Exp. Zool. 198, 155–162.
Nagashima, H., Kashiwazaki, N., Ashman, R., Grupen, C., Seamark, R.F., Nottle, M., 1994. Recent advances in cryopreservation of porcine embryos. Theriogenology 41, 113–118.
Niwa, K., 1993. Effectiveness of in vitro maturation and in vitro fertilization techniques in the pig. J. Reprod.
Ž .
Fertil. Suppl. 48 , 49–59.
Nottle, M.B., Nagashima, H., Verma, P.J., Du, Z.T., Grupen, C.G., Ashman, R.J., MacIlfatrick, S., 1997.
Ž .
Developments in transgenic techniques in pigs. In: Foxcroft, G.R., Geisert, R.D., Doberska, C. Eds. , Control of Pig Reproduction V. J. Reprod. Fertil. Suppl. 52 pp. 237–244.
Ž .
Petters, R.M., Wells, K.D., 1993. Culture of pig embryos. J. Reprod. Fertil. Suppl. 48 , 61–73.
Piedrahita, J.A., Moore, K., Lee, C., Oetama, B., Weaks, R., Ramsoondar, J., Thomson, J., Vasquez, J., 1997. Advances in the generation of transgenic pigs via embryo-derived and primordial germ cell-derived cells.
Ž .
In: Foxcroft, G.R., Geisert, R.D., Doberska, C. Eds. , Control of Pig Reproduction V. J. Reprod. Fertil. Suppl. 52 pp. 237–244.
Piedrahita, J.A., Moore, K., Oetama, B., Lee, C-k., Scales, N., Ramsoondar, J., Bazer, F.W., Ott, T., 1998. Generation of transgenic porcine chimeras using primordial germ cel-derived colonies. Biol. Reprod. 58, 1321–1329.
Polge, C., Day, B.N., 1968. Pregnancy following non-surgical egg transfer in pigs. Vet. Rec. 82, 712. Prather, R.S., Sims, M.M., First, N.L., 1989. Nuclear transplantation in early pig embryos. Biol. Reprod. 41,
414–418.
Rath, D., Johnson, L.A., Dobrinsky, J.R., Welch, G.R., Niemann, H., 1997. Production of piglets preselected for sex following in vitro fertilization with X and Y chromosome-bearing spermatozoa sorted by flow cytometry. Theriogenology 47, 795–800.
Ž .
Rath, D., Johnson, L.A., Welch, G.R., Niemann, H., 1994. Successful gamete intrafollopian transfer GIFT in the porcine. Theriogenology 41, 1173–1179.
Vazquez, J.I., Martinez, E.W., Vazquez, J.M., Lucas X., Gil, M.A., Parrilla, I., Roca, J., 1999. Development of a non-surgical deep intra uterine insemination technique. Proc. IV Int. Conf. on Boar Semen
Ž .
Preservation, Beltsville, MD in press .
Wang, W.H., Abeydeera, L.R., Prather, R.S., Day, B.N., 1998. Morphologic comparison of ovulated and in vitro-matured porcine oocytes, with particular reference to polyspermy after in vitro fertilization. Mol. Reprod. Dev. 49, 308–316.
Wang, W.H., Niwa, K., Okuda, K., 1991. In-vitro penetration of pig oocytes matured in culture by frozen-thawed ejaculated spermatozoa. J. Reprod. Fertil. 93, 491–496.
Wang, W.H., Sun, Q.Y., Hosoe, M., Shioya, Y., Day, B.N., 1997. Quantified analysis of cortical granule distribution and exocytosis of porcine oocytes during meiotic maturation and activation. Biol. Reprod. 56, 1376–1382.
Yoshida, M., Ishigaki, K., Nagai, T., Chikyu, M., Pursel, V.G., 1993. Glutathione concentration during maturation and after fertilization in pig oocytes: relevance to the ability of oocytes to form male pronucleus. Biol. Reprod. 49, 89–94.