Active immunization of ewes against ovine placental
lactogen increases birth weight of lambs and milk
production with no adverse effect on conception rate
H. Leibovich
a, A. Gertler
a, F.W. Bazer
b, E. Gootwine
c,∗aFaculty of Agriculture, Institute of Biochemistry, Food Science and Nutrition,
The Hebrew University of Jerusalem, Rehovot, Israel
bCenter for Animal Biotechnology and Genomics, Institute of Biosciences and Technology, Texas A&M
University System Health Science Center and Department of Animal Science, Texas A&M University, College Station, TX, USA
cInstitute of Animal Sciences, A.R.O., The Volcani Center, Bet Dagan, Israel
Received 28 March 2000; received in revised form 14 August 2000; accepted 14 August 2000
Abstract
In two experiments, 16 Booroola–Assaf and 35 Assaf ewe-lambs were actively immunized at 5 months of age against recombinant ovine placental lactogen (oPL). At 9 months of age, the ewe-lambs were mated for the first time and then introduced into a frequent mating-system. Anti-oPL antibody titers, reproductive performance, maternal serum levels of oPL during pregnancy, lamb birth weight and milk production of the ewes were followed in the immunized ewes and in their non-immunized control counterparts. All the immunized ewes developed anti-oPL antibodies, which interfered with oPL bioactivity in an in vitro cell proliferation assay. Conception rates did not differ (P >0.05) between immunized and non-immunized ewes. Abundant antibody-bound non-active oPL detected in sera of immunized ewes by western blotting indicated enhanced oPL production by the placenta following immunization. An increase (P < 0.02) in serum oPL bioactivity, but not immunoreactivity, was observed in the immunized ewes in late gestation relative to control ewes. The average litter size was 1.83 and 1.32 lambs born per ewe lambing in the first and second experiments, respectively. Average birth weights of lambs born to the immunized ewes were higher (P < 0.01) than for lambs born to control ewes by 10, 17 and 39% for those born as singles, twins and triplets, respectively. Immunized ewes produced 19 and 33% more milk (P < 0.02) than the control ewes in the first 3.5 months of the first and second lactations, respectively. These findings do not suggest a role for oPL in maternal recognition of pregnancy, but they strongly
∗Corresponding author. Tel.:+972-3-968-3752; fax:+972-8-475-075/3-960-3678. E-mail address: [email protected] (E. Gootwine).
suggest important roles for oPL in fetal growth and mammogenesis. Immunization of ewes against oPL may thus represent a novel practical technique for enhancing birth weights of lambs born to prolific sheep, as well as milk production by both dairy and mutton ewes. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Sheep-placenta; Placental lactogen; Immunization; Lamb production; Milk production
1. Introduction
While the presence of high circulating concentrations of placental lactogen (PL) in preg-nant ewes has long been recognized, the biological role of this hormone has not been clarified. Ovine (o)PL, a 198-amino acid non-glycosylated protein, is a member of the growth hormone/prolactin (GH/PRL) gene family and manifests both somatogenic and lac-togenic activities in heterologous systems (Byatt et al., 1992; Anthony et al., 1995a,b). In contrast, in a homologous (ruminant) system, it displays agonistic activity through lacto-genic receptors only (Herman et al., 1999) or by heterodimerization of somatolacto-genic and lactogenic receptors (Herman et al., 2000). oPL is produced by chorionic binucleated cells of the placenta and is released into both the maternal and fetal circulation, probably under differently regulated secretion patterns (Schoknecht et al., 1991; Kappes et al., 1992). In maternal plasma, oPL is first detected at 40–60 days of gestation, it peaks at days 120–140 when it can reach levels of up to 1mg/ml, and then declines until parturition (Gluckman et al., 1979; Kappes et al., 1992). Fetal oPL plasma concentrations reach maximal levels by day 90 of pregnancy and are relatively low in the range of tens of nanograms per milliliter (Kappes et al., 1992).
Results from studies in which oPL levels were altered by feeding restrictions (Butler et al., 1987; Bauer et al., 1995), by infusion of oPL into the maternal and fetal circulations (Oliver et al., 1995; Currie et al., 1996; Schoknecht et al., 1996), by infusion of antibodies to oPL to neutralize its activity (Waters et al., 1985), by carnuclectomy (Falconer et al., 1985) or by fetectomy (Rueda et al., 1995) have suggested albeit not conclusively, that oPL regulates fetal growth by stimulating repartitioning of maternal nutrients to the fetus, and by stimulating the fetus to use the substrates (Anthony et al., 1995b).
Active immunization against ‘self’ hormone molecules that can inhibit or potentiate en-dogenous hormonal activity has been utilized to investigate physiological roles of hormones (Holder and Carter, 1995; Meloen, 1995; Pell and Aston, 1995). In studies to immunomod-ulate GH activity, infusion of monoclonal antibodies against bovine GH (Aston et al., 1987; Bomford and Aston, 1990), or active immunization against specific peptide regions of oGH (Pell and Aston, 1991) enhanced GH bioactivity and its physiological effects. In swine, vac-cination against a short peptide corresponding to amino acids 54–95 of porcine GH improved growth performance (Wang et al., 1996), and long-term treatment of Snell dwarf mice with GH complexed to anti-GH antibodies stimulated growth rates (Mockridge et al., 1998).
the oPL’s role during pregnancy. The aim of the present study was therefore to investi-gate the physiological role of oPL following active immunization of ewe-lambs against recombinant oPL (roPL) prepared in our laboratory (Sakal et al., 1997).
2. Materials and methods
2.1. Animals and immunization treatments
Experimental protocols were approved by the Volcani Center Animal Care Committee. Two experiments were conducted. The first was carried out at the Central Farm of the Volcani Center at Bet Dagan, where all ewes were kept indoors. Booroola–Assaf crossbred ewe-lambs, born in the spring of 1996, were assigned to immunized and control groups (N =16 and 36, respectively) without knowledge of their genotype at the FecB locus. The second experiment made use of the Katzenelebugen dairy Assaf flock at Moshav Talmai Elazar: 35 and 68 Assaf ewe-lambs, born between January and April 1998, were assigned to immunized and control groups, respectively.
In both experiments, immunization of ewe lambs against roPL was started at 6 months of age. At about 8 months of age, all ewe-lambs were introduced into an accelerated breeding program where mating periods of about 40 days were scheduled every 3–4 months. At the beginning of each mating period, estrus was synchronized in all ewes using progesterone pessaries (Chronogest, Intervet, Boxmeer, The Netherlands) and 500 IU eCG (Synchroject, Vetimax, Bland, Holland). Ewes were mated following the detection of estrus and if they returned to estrus in the subsequent cycle, they were mated again. Pregnancy diagnosis was carried out 20–40 days after the end of each breeding period. Ewes that failed to conceive were resynchronized to estrus and mated again in the next mating period. At lambing, the number of lambs born to each ewe, their sex and birth weights were recorded. Body weight was recorded for control and immunized ewes 3 days after the first lambing in the second experiment. Lambs were raised by their dams (first experiment) or separated from their dams on the day of lambing and moved to an artificial rearing unit (second experiment). Ewes were milked only in the second experiment. Milking was performed twice a day at 08.00 and 16.00 h, from the day of lambing. Milk production was recorded weekly until all ewes completed about 110 days of milking.
Ewes in both experiments were synchronized 70–100 days after lambing and mated again in the succeeding breeding period. Ewes remained in the first experiment until they had had their second lambing. In the second experiment, ewes remained in the experiment until they had completed at least 100 days of milking in the second lactation or up to 12 months after the first lambing, if they did not lamb again.
2.2. Immunization
injections at 3 and 6 weeks after the primary immunization with the same antigen emulsified in incomplete Freund’s adjuvant.
2.3. Antibody detection and measurement
Blood samples were collected from the jugular vein and serum was prepared. In the first experiment, serum was prepared from both control ewes and immunized ewes prior to the immunization procedure (pre-immune samples), 3 weeks after the second booster (first post-immune samples) and on days 60, 80 and 130 of the first pregnancy. In the second experiment, blood samples were collected and serum was prepared at the pre- and first post-immune stages, and at 130 days of gestation in both the first and second pregnancies. The ability of anti-oPL antisera to bind roPL was determined using an ELISA. A stock solution of oPL (2.5mg/ml in 0.1 M Na2CO3buffer; pH 9.6) was prepared and 100ml was added to each well of a 96-well microtiter plate (Nunc-ImmunoTM, NuncTM, Denmark). Plates were incubated for 3 h at 37◦C, then blocked by washing wells three times with PBST (PBS with 0.05% Tween 20). Dilutions of antisera ranging from 1:104to 1:3×106were prepared with PBST and 100ml was added to each well. Following overnight incubation at 4◦C, the samples were decanted, the plates were washed three times as above and 100ml of biotinylated rabbit anti-sheep IgG (1:10,000 dilution in PBST; Zemed, San Francisco, CA) was added. Following incubation at 37◦C for 3 h, the wells were washed with PBST and 100ml of alkaline phosphatase (Zemed) diluted 1:2000 in PBS buffer containing 15% horse serum, 0.5% gelatin and 0.5% BSA was added to each well. After incubation at 37◦C for 30 min, the plates were washed and 100ml alkaline phosphatase substrate (2 mg/ml p-nitrophenyl phosphate; Sigma) in buffer containing, 0.75% glycine and 1 mM MgCl2
adjusted to pH 10.5 using 1N NaOH were added. The color intensity was measured at 405 nm after 20 min of incubation by automated plate reader.
2.4. oPL assays
In the first experiment, serum levels of oPL were measured at 60, 80 and 130 days of the first gestation for all immunized ewes and nine control ewes. In the second experiment, serum levels of oPL were determined at 130 days of both the first and second gestations in 24 and 33 immunized and control ewes, respectively. Serum levels of oPL were detected by both the Nb2-11C lymphoma cell proliferation bioassay (Gertler et al., 1985) and by
radioimmunoassay (RIA). The oPL antiserum used in the RIA was raised in a rabbit against recombinant oPL and used at a final dilution of 1:400,000. The antiserum exhibited low cross-reactivity with oPRL and oGH preparations obtained from NIH. The minimum de-tectable level of oPL was 5 ng/ml serum. The intra- and inter-assay coefficients of variation were<6.4 and<8.8%, respectively.
2.5. Neutralization of oPL activity by anti-oPL auto-antibodies
The neutralizing ability of oPL bioactivity by serum from immunized ewes was expressed in EC50 values. This value represents the titer required to achieve a 50% reduction in
human GH (Strasburger et al., 1989). Both rat lymphoma Nb2-11C cells possessing PRL
receptors (Gertler et al., 1985) and FDC-P1 cells stably transfected with rabbit GH receptor (Rowlinson et al., 1996) were used in the neutralization assays.
2.6. Serum IGF-I measurements
Serum levels of IGF-I were determined at 130 days of pregnancy for immunized and non-immunized ewes by RIA (Breier et al., 1991) and intra- and inter-assay coefficients of variation were 8.7 and 12.4%, respectively. IGF-I concentrations are expressed relative to a reference standard of recombinant human IGF-I (Fujisawa Pharmaceutical Co., Osaka, Japan). Rabbit antiserum to IGF-I (UB2-495) was obtained from the Hormone Distribution Program of NIDDK through the NIH National Hormone and Pituitary Program.
2.7. Determination of free and antibody-bound oPL
Serum aliquots (250ml) from randomly selected immunized and control ewes were ap-plied to a Sephadex G-75 column (25 ml bead volume) equilibrated with PBS (pH 7.0). Then, the column was washed with PBS. The IgG-containing fractions (first 12 ml of elu-ate, as determined in preliminary experiments) and the next 10 ml of eluate that contained IgG-free-fractions were collected and concentrated 10-fold using an ultrafiltration system (Amicon Inc., Beverly, MA). Bioactivity of oPL in those samples was determined using the Nb2test. Western blotting (Towbin et al., 1979) was used to detect oPL in IgG-containing
and IgG-free serum fractions.
2.8. Milk composition analysis
Milk samples for milk constituent analysis were taken during the morning milking from immunized and control ewes once, at the milk recording day of week 13 of the lacta-tion. Concentrations of fat, protein, lactose and total solids in milk were measured us-ing a semi-automated infrared analyzer (Milkoscan 134 A/B, N. Foss Electric, Hillerod, Denmark).
2.9. Statistical analysis
of variance to detect effects of treatment within day of bleeding. Statistical analyses were conducted using the general linear model (GLM) procedure in the SAS computer package (Statistical Analysis System; SAS Institute, 1985). Differences ofP <0.05 were considered significant and all values are expressed as mean and standard errors of the mean.
3. Results
3.1. Anti-oPL antibody titer
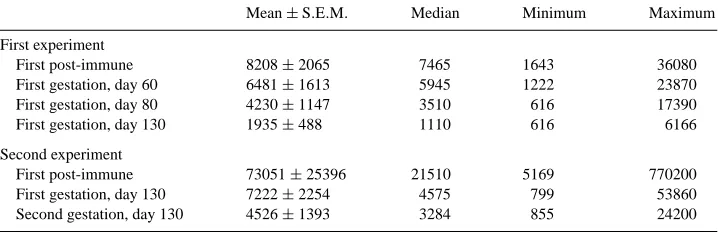
Antibodies against oPL were not detected in serum samples obtained from control ewes or in any of the pre-immune serum samples. In the first experiment, all immunized ewes developed anti-oPL antibodies with titers (dilutions giving 50% of specific antibody binding) of 8208±2065 in the first post-immunization period. For the first pregnancy, anti-oPL antibody titers declined on an average to 79, 51 and 24% of first post-immune titers at 60, 80 and 130 days of gestation, respectively. First post-immune titers were correlated (r=0.87;P <0.05) only with titers measured on day 60 of gestation.
All immunized ewes in the second experiment also developed anti-oPL antibodies with first post-immune titers higher than in the first experiment, at 73,051±25,396 (Table 1). At 130 days of gestation, anti-oPL antibody titers dropped on an average to 10 and 6% of first post-immune titers in the first and second pregnancies, respectively. Whereas no significant association was found between post-immune titers and titers at 130 days of gestation in either pregnancy, a significant correlation (r = 0.49; P < 0.003) was found between titers measured at 130 days of gestation in the first and second pregnancies. No significant association was observed between anti-oPL titers in the immunized ewes and their lambs’ birth weight, or the ewe milk production.
3.2. Serum concentrations of oPL
Distribution of immunoreactive (I) and bioactive (B) oPL in serum on days 60, 80 and 130 of gestation for control and immunized ewes in the first experiment are
pre-Table 1
Anti-ovine placental lactogen (oPL) antibody titers (dilutions giving 50% of specific antibody binding) in ewes immunized against recombinant oPL
Mean±S.E.M. Median Minimum Maximum
First experiment
First post-immune 8208±2065 7465 1643 36080
First gestation, day 60 6481±1613 5945 1222 23870
First gestation, day 80 4230±1147 3510 616 17390
First gestation, day 130 1935±488 1110 616 6166
Second experiment
First post-immune 73051±25396 21510 5169 770200
First gestation, day 130 7222±2254 4575 799 53860
Fig. 1. Immunoreactive (RIA) and bioactive (bioassay) oPL in serum (ng/nl) at 60, 80, and 130 days of gestation in control ewes and in ewes immunized against ovine placental lactogen (oPL).
sented in Fig. 1. Concentrations of oPL increased during gestation and I-oPL serum lev-els were always higher than B-oPL levlev-els in the same samples. At 130 days of gesta-tion, B-oPL serum levels were higher (P < 0.02) in immunized ewes than in control ewes.
Serum oPL levels at 130 days of the first and second pregnancies in the second exper-iment are presented in Table 2. In control ewes, concentrations of I-oPL in serum were higher (P < 0.01) than those of B-oPL, in accordance with results obtained in the first experiment. However, for immunized ewes, the opposite result was obtained: I-oPL con-centrations were lower (P <0.04) than B-oPL concentrations. As in the first experiment, B-oPL levels of immunized ewes were higher (P < 0.05) than B-oPL levels in control ewes. As I-oPL levels were relatively low, they were lower (P < 0.001) than in control ewes.
Table 2
Immunoreactive and bioactive ovine placental lactogen (oPL) serum levels (ng/ml) at 130 days of gestation in control and oPL-immunized Assaf ewes (means±S.E.M.)a,b
Group Immunoreactive oPL serum levels Bioactive oPL serum levels
First gestation
Control 522±36 a (34–1060) 105±12 a (28–266)
Immunized 104±42 b (5–438) 146±15 b (54–375)
Second gestation
Control 465±34 a (94–950) 149±24 a (38–499)
Immunized 53±42 b (0–214) 212±27 b (97–825)
aValues in parentheses are range of values.
bWithin pregnancy and type of oPL assay, values with different letters in the same column are different at
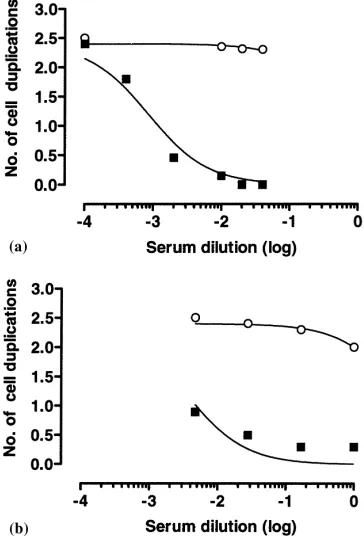
Fig. 2. Inhibition of ovine placental lactogen (oPL)-stimulated proliferation of Nb-2 cells (a) and FDC-P1 cells (b) by serum obtained from ewe no. 4835 immunized against oPL: (s) pre-immune serum; (j) post-immune serum.
3.3. Serum IGF-I concentrations
In the first experiment, concentrations of serum IGF-I on day 130 of gestation did not differ between immunized (179±32 ng/ml) and control (207±36 ng/ml) ewes. Similar results were obtained in the second experiment where on day 130 of gestation, IGF-I levels were 190±12 and 194±13 ng/ml for control and immunized ewes, respectively.
3.4. Neutralization of oPL bioactivity by anti-oPL antisera
While neither serum from control, non-immunized, non-pregnant ewes nor pre-immune serum inhibited cell proliferation following stimulation with oPL, first post-immune serum inhibited proliferation of both Nb2-11C and FDC-P1 cells (Fig. 2). The IC50 inhibition
values for serum from different ewes were not correlated (P >0.05) with antibody titers of the same samples.
3.5. Free and antibody-bound oPL in serum
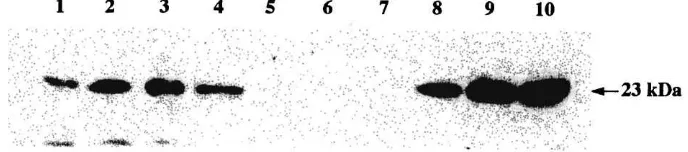
Fig. 3. Western blot analysis of bound ovine placental lactogen (oPL) in fractions containing IgG from control ewes and ewes immunized against oPL. Lanes 1–4: IgG fractions from serum of ewes immunized against oPL; lanes 5–7: IgG fractions from serum of control ewes; lane 8: 2.5 ng recombinant (r)oPL; lane 9: 5.0 ng roPL; lane 10: 10.0 ng roPL.
column (data not shown). No oPL bioactivity was detected in IgG-containing serum frac-tions obtained from control or immunized ewes. However, whereas oPL was not detected by western blotting in IgG-containing fractions of serum from three randomly selected pregnant control ewes, it was detected in IgG-containing serum samples from four ran-domly selected immunized pregnant ewes (Fig. 3). At 130 days of gestation, an esti-mated 50% of the oPL in the circulation of immunized pregnant ewes was bound to antibodies.
3.6. Reproductive performance
Two immunized ewes in the first experiment and four control and two immunized ewes in the second experiment, did not lamb until the age of 26 months. Those ewes were culled. Age at first lambing, which in the first and second experiments was 17.0±1.0 and 14.7±0.4 months, respectively, was not affected by treatment (P > 0.05). The second lambing occurred in the two experiments 7.9±0.4 and 8.4±0.3 months after the first lambing, respectively, and was also not affected by the treatment (P >0.05).
3.7. Lamb birth weights and ewes’ post-partum body weights
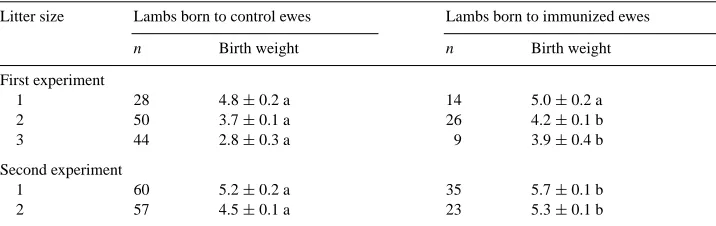
Prolificacy in the first and second experiments, respectively, averaged 1.85 and 1.32 lambs born per ewe per lambing. The relatively high prolificacy in the first experiment was due to the FecB (Booroola) gene segregating in the Booroola–Assaf population. Of the lambs born in the first experiment, 36 and 18% were triplets or more. Treatment, parity and litter size affected (P <0.01) birth weight, whereas the effect of lamb sex was significant (P <0.02) only in the second experiment. Lambs born to immunized ewes were heavier (P < 0.001) than lambs born to control ewes (Table 3) and the greater the number of lambs born in a litter, the greater the advantage in birth weight for lambs born to immunized ewes.
Table 3
Birth weights of lambs born to control ewes and ewes immunized against ovine placental lactogen (means±S.E.M.) in their first and second lambingsa
Litter size Lambs born to control ewes Lambs born to immunized ewes
n Birth weight n Birth weight
First experiment
1 28 4.8±0.2 a 14 5.0±0.2 a
2 50 3.7±0.1 a 26 4.2±0.1 b
3 44 2.8±0.3 a 9 3.9±0.4 b
Second experiment
1 60 5.2±0.2 a 35 5.7±0.1 b
2 57 4.5±0.1 a 23 5.3±0.1 b
aWithin rows, values with different letters are different atP <0.05.
3.8. Milk production
In the first 108 days of the first lactation, milk production in immunized ewes (N =25; 208±12 l), was greater (P <0.01) than that in the control ewes (N =55; 174±8 l). Milk composition was not affected by treatment (data not shown).
Similar results regarding the effect of immunization against oPL on milk production were obtained in the second lactation where during the first 110 days of the lactation, milk production was higher (P < 0.01) in immunized ewes (271±21) than in control ewes (204±15 l).
We studied lactation curve shape by fitting the Pollot model (Pollott and Gootwine, 2000): although milk yield peak occurred for both groups during the first week of lactation, peak yield was significantly higher in the immunized group than in the control group, being in the first lactation 2.1±0.1 and 1.7±0.1 l, respectively. Other curve parameters, including a relative decline in milk production rate throughout the lactation, did not differ between the immunized and control groups.
4. Discussion
Induction of immunity against oPL had no effect on the establishment or maintenance of pregnancy and led to increased in birth weight of lambs and increased milk production of ewes. These results suggest that oPL stimulates, via some unidentified mechanism, fetal growth, and may support mammary gland development before lambing.
4.1. Antibody formation
and vaccination history) factors may have been partially responsible. The highest anti-oPL titers in the serum of immunized ewes were detected at the post-immune stage. Formation and secretion of endogenous oPL during the first pregnancy may serve as a natural booster, elevating anti-oPL titers. Despite this, antibody titers, as measured by ELISA, declined as pregnancy progressed, probably due to increasing amounts of anti-oPL antibodies being bound to endogenous oPL, which interfered with the test.
4.2. Hormone concentration
The significant increase in oPL bioactivity on day 130 of the pregnancy in the immu-nized ewes (Fig. 1; Table 2) resembles the enhancement of in vivo GH activity by anti-GH antibodies (Bomford and Aston, 1990; Wang et al., 1996). In that respect, GH and oPL, which belong to the same gene family, differ from several other hormones whose activity is neutralized by immunization against them (Pell and Aston, 1995). The precise mech-anism by which anti-oPL antibodies enhance oPL activity is not clear. One possibility is that antibodies protect circulating oPL from degradation, prolonging its half-life. Anti-oPL antibodies may also induce conformational changes in oPL, which increase its affinity for its receptor or decrease hormone-receptor internalization rate. Another possibility is that immunoneutralization of oPL activity leads to an increase in its production and secretion by the placenta through an altered feedback mechanism.
While B-oPL was higher in immunized ewes than in control ewes at 130 days of gestation (P <0.02), I-oPL levels were not different between immunized and control ewes in the first experiment (Table 2). This might be due to interference in the RIA by endogenous anti-oPL antibodies. Indeed, the fact that relatively low I-oPL levels (below bioactive levels) were measured in the second experiment (Table 2), where the immune response was much stronger than in the first experiment, supports this suggestion.
4.3. Effect on conception rate
Although recent studies have shown that oPL affects endometrial function (Spencer et al., 1999), results of the present experiments suggest that oPL does not affect recognition or maintenance of pregnancy. Indeed, in humans, there is no direct clinical evidence of a role for PL in maintaining pregnancy, as normal infants have been born to mothers who had no detectable circulating PL (Nielsen et al., 1979; DiRenzo et al., 1982; Rygaard et al., 1998).
4.4. Effect on birth weight of lambs
to the fetus in ewes (Brambell, 1970; McQuoid et al., 1995). Moreover, enhancement of fetal growth is not likely to be mediated by diffusion of oPL from mother to fetus as PL cannot cross the placenta (Kappes et al., 1992; Schoknecht et al., 1992, 1996). It can be suggested that the effect of immunization on fetal growth was mediated via changes in placental growth and function.
Byatt et al. (1991) suggested that the somatogenic effect of PL may be mediated by changes in voluntary feed intake. Indeed, administration of exogenous oPL to lambs (Min et al., 1996) and lactating ewes (Leibovich et al., 2001) increased voluntary food intake. Similarly, the rise in B-oPL levels in immunized ewes in late gestation could stimulate feed intake, which can be a limiting factor for fetal growth in ewes with multiple fetuses. This possibility requires further investigation. Alternatively, increases in maternal oPL levels may affect fetal growth via an indirect effect on fetal hepatic IGFBP-3 gene expression, which in turn affects the somatogenic axis of fetuses, as described for pregnant ewes treated daily with roPL (Currie et al., 1996).
Birth weight is an important factor affecting lamb survival. One of the main limitations of using prolific sheep breeds is that the increase in litter size is associated with the birth of lambs with lower birth weights and higher mortality. Immunization against oPL may be a practical way of increasing lamb survival, especially in prolific breeds.
4.5. Effect on milk production
Immunized ewes produced 19 and 33% more milk than control ewes in the first and second lactations, respectively. This could be related to increased mammogenic activity in immunized ewes due to higher oPL activity. Indeed, we have reported that oPL has mammogenic activity in pseudo-pregnant ewes (Kann et al., 1999), similar to GH. Studies with pregnant ewes (Stelwagen et al., 1993), goats (Knight et al., 1994) and cows (Akers and Cleale, 1990; Stelwagen et al., 1992) have suggested that they produce more milk and have larger mammary glands after parturition following treatment with GH during late gestation.
Milk production is an important economic trait, not only in dairy sheep breeds but also in prolific breeds, where ewes nurse large litters. Immunization of ewe-lambs against oPL appears to be a simple and inexpensive way of improving milk production in sheep. For practical application, immunization should be performed using less harmful, but no less potent adjuvants than Freund’s adjuvant.
Acknowledgements
References
Akers, R.M., Cleale, R.M., 1990. Effect of somatotropin during the dry period on subsequent milk production and induced secretion of somatotropin, prolactin and insulin pre- and post-partum. J. Dairy Sci. 73 (Suppl. 1), 154. Anthony, R.V., Pratt, S.L., Liang, R., Holland, M.D., 1995a. Placental–fetal hormonal interactions: impact on fetal
growth. J. Anim. Sci. 73, 1861–1871.
Anthony, R.V., Liang, R., Kayl, E.P., Pratt, S.L., 1995b. The growth hormone prolactin gene family in ruminant placenta. J. Reprod. Fertil. 49 (Suppl. 1), 83–95.
Aston, R., Holder, A.T., Ivanyi, J., Bomford, R., 1987. Enhancement of bovine growth hormone activity in vivo by monoclonal antibodies. Mol. Immunol. 24, 143–150.
Bauer, M.K., Breier, B.H., Harding, J.E., Veldhuis, J.D., Gluckman, P.D., 1995. The fetal somatotropic axis during long term maternal undernutrition in sheep: evidence for nutritional regulation in utero. Endocrinology 136, 1250–1257.
Bomford, R., Aston, R., 1990. Enhancement of bovine growth hormone activity by antibodies against growth hormone peptides. J. Endocrinol. 125, 31–38.
Brambell, F.W.R., 1970. The transmission of passive immunity from mother to young. In: Neuberger, A., Tatum, E.L. (Eds.), Frontiers of Biology, Vol. 18. North-Holland, Amsterdam, pp. 201–227.
Breier, B.H., Gallaher, B.W., Gluckman, P.D., 1991. Radioimmunoassay for insulin-like growth factor-I: solutions to some potential problems and pitfalls. J. Endocrinol. 128, 347–357.
Butler, W.R., Huyler, S.E., Gradis, A.S., Handwerger, S., 1987. Failure of fasting and changes in plasma metabolites to affect spontaneous fluctuations in plasma concentrations of ovine placental lactogen. J. Endocrinol. 114, 391–397.
Byatt, J.C., Staten, N.R., Schmuke, J.J., Buonomo, F.C., Galosy, S.S., Curran, D.F., Krivi, G.G., Collier, R.J., 1991. Stimulation of body weight gain of mature female rat by bovine GH and bovine placental lactogen. J. Endocrinol. 130, 11–19.
Byatt, J.C., Warren, W.C., Eppard, P.J., Staten, N.R., Krivi, G.G., Collier, R.J., 1992. Ruminant placental lactogens: structure and biology. J. Anim. Sci. 70, 2911–2923.
Currie, M.J., Bassett, N.S., Breier, B.H., Klempt, M., Min, S.H., Mackenzie, D.D.S., McCutcheon, S.N., Gluckman, P.D., 1996. Differential effect of maternal ovine placental lactogen and growth hormone (GH) administration on GH receptor, insulin like growth factor (IGF)-I and IGF binding protein-3 gene expression in the pregnant and fetal sheep. Growth Regulation 6, 123–129.
DiRenzo, G.C., Aneshi, G.G., Volpe, A., 1982. Deficiency of human placental lactogen in an otherwise normal pregnancy. J. Obstet. Gynecol. 2, 153–154.
Falconer, J., Owens, J.A., Allotta, E., Robinson, J.S., 1985. Effect of restriction of placental growth on the concentration of insulin, glucose and placental lactogen in the plasma of sheep. J. Endocrinol. 106, 7–11. Gertler, A., Walker, A., Friesen, H.G., 1985. Enhancement of human growth hormone-stimulated mitogenesis of
Nb2node lymphoma cells by 12-o-tetradecanoyl-phorbol-13-acetate. Endocrinology 116, 1636–1644.
Gluckman, P.D., Kaplan, S.L., Rudolph, A.M., Grumbach, M.M., 1979. Hormone ontogeny in the ovine fetus. II. Ovine chorionic somatomammotropin in mid- and late-gestation in the fetal and maternal circulation. Endocrinology 104, 1828–1834.
Herman, A., Helman, D., Livnah, O., Gertler, A., 1999. Ruminant placental lactogens act as antagonists to homologous growth hormone receptors and as agonists to human or rabbit growth hormone receptors. J. Biol. Chem. 274, 7631–7639.
Herman, A., Bignon, C., Daniel, N., Grosclaude, J., Gertler, A., Djiane, J., 2000. Functional heterodimerization of prolactin and growth hormone receptors by ovine placental lactogen. J. Biol. Chem. 275, 6295–6301. Holder, A.T., Carter, C., 1995. Immunomodulation of the growth hormone-IGF-I axis. Livest. Prod. Sci. 42,
229–237.
Kann, G., Delobelle-Deroide, A., Belair, L., Gertler, A., Djiane, J., 1999. Demonstration of in vivo mammogenic and lactogenic effects of recombinant ovine placental lactogen and mammogenic effect of recombinant ovine GH in ewes during artificial induction of lactation. J. Endocrinol. 160, 365–377.
Knight, C.H., Brown, J.R., Sejrsen, K.A., 1994. A comparison of growth hormone-induced mammogenesis in pregnant and lactating goats. Endocrinol. Metab. 1 (Suppl. B), 52.
Leibovich, H., Gertler, A., Bazer, F., Gootwine, E., 2001. Effects of recombinant ovine placental lactogen and recombinant ovine growth hormone on lamb growth and milk production in ewes. Livest. Prod. Sci., in press. McQuoid, M.R., Hodgkinson, A.J., Hodgkinson, S.C., 1995. Maternal antibodies and immune responsiveness in
growing lambs. Proc. N.Z. Soc. Anim. Prod. 55, 214–217.
Meloen, R.H., 1995. Basic aspects of immunomodulation through active immunization. Livest. Prod. Sci. 42, 135–145.
Min, S.H., Mackenzie, D.D.S., Breier, B.H., McCutcheon, S.N., Gluckman, P.D., 1996. Growth-promoting effects of ovine placental lactogen (oPL) in young lambs: comparison with bovine growth hormone provides evidence for a distinct effect of oPL on food intake. Growth Regulation 6, 144–151.
Mockridge, J.W., Holder, A.T., Beattie, J., 1998. Enhancement of growth hormone (GH) activity by antisera prepared against analogues of an epitope peptide defined by a GH-enhancing monoclonal antibody. Livest. Prod. Sci. 55, 1–11.
Nielsen, P.V., Pedersen, H., Kampmann, E.M., 1979. Absence of human placental lactogen in an otherwise uneventful pregnancy. Am. J. Obstet. Gynecol. 135, 322–326.
Oliver, M.H., Harding, J.E., Breier, B.H., Evans, P.C., Gallaher, B.W., Gluckman, P.D., 1995. The effect of ovine placental lactogen infusion on metabolites, insulin-like growth factors and binding proteins in the fetal sheep. J. Endocrinol. 144, 333–338.
Pell, J.M., Aston, R., 1991. Active immunization with a synthetic peptide region of growth hormone: increased lean tissue growth. J. Endocrinol. 131, R1–R4.
Pell, J.M., Aston, R., 1995. Principles of immunomodulation. Livest. Prod. Sci. 42, 123–133.
Pollott, G.E., Gootwine, E., 2000. Appropriate mathematical models for describing the lactation of dairy sheep. Anim. Sci. 71, 197–207.
Rowlinson, S.W., Waters, M.J., Lewis, U.J., Barnard, R., 1996. Human growth hormone fragments 1–43 and 44–191: in vitro somatogenic activity and receptor binding characteristics in human and nonprimate systems. Endocrinology 137, 90–95.
Rueda, B.R., Dunn, T.G., Anthony, R.V., Moss, G.E., 1995. Influence of fetal death and fetectomy on gestation and initiation of parturition in the ewe. Reprod. Fertil. Dev. 7, 1221–1225.
Rygaard, K., Rovol, A., Esquivel Escobedo, D., Beck, B.L., Barrera Saldana, H.A., 1998. Absence of human placental lactogen and placental growth hormone (hGH-V) during pregnancy: PCR analysis of the deletion. Hum. Genet. 102, 87–92.
Sakal, E., Bignon, C., Grosclaude, J., Kantor, A., Shapira, R., Leibovitch, H., Helman, D., Nespooulous, C., Shamay, A., Rowlinson, S.W., Djiane, J., Gertler, A., 1997. Large-scale preparation and characterization of recombinant ovine placental lactogen. J. Endocrinol. 152, 317–327.
Schoknecht, P.A., Nobrega, S.N., Petterson, J.A., Ehrhardt, R.A., Slepetis, R., Bell, A.W., 1991. Relations between maternal and fetal plasma concentrations of placental lactogen and placental and fetal weights in well-fed ewes. J. Anim. Sci. 69, 1059–1063.
Schoknecht, P.A., Currie, W.B., Bell, A.W., 1992. Kinetics of placental lactogen in mid- and late-gestation ovine fetuses. J. Endocrinol. 133, 95–100.
Schoknecht, P.A., McGuire, M.A., Cohick, W.S., Currie, W.B., Bell, A.W., 1996. Effect of chronic infusion of placental lactogen on ovine fetal growth in late gestation. Domest. Anim. Endocrinol. 13, 519–528. Spencer, T.E., Gray, A., Johnson, G.A., Taylor, K.M., Gertler, A., Gootwine, E., Ott, T.L., Bazer, F.W., 1999.
Effects of recombinant ovine interferon tau, placental lactogen and growth hormone on the ovine uterus. Biol. Reprod. 61, 1409–1418.
Statistical Analysis System Institute, 1985. SAS User’s Guide. SAS Institute, Cary, NC.
Stelwagen, K., Grieve, D.G., McBride, B.W., Rehman, J.D., 1992. Growth and subsequent lactation in primigravid Holstein heifers after prepartum bovine somatotropin treatment. J. Dairy Sci. 75, 463–471.
Stelwagen, K., Grieve, D.G., Walton, J.S., Ball, J.L., McBride, B.W., 1993. Effect of prepartum bovine somatotropin in primigravid ewes on mammogenesis, milk production, and hormone concentrations. J. Dairy Sci. 76, 992– 1001.
Towbin, H., Staehelin, T., Gordon, J., 1979. Eletrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 76, 4350–4354. Wang, B.S., Lumanglas, A.A., Shieh, H.M., Corbett, M.J., Zhang, R.J., Kraft, L.A., 1996. Immunological effect
of a synthetic growth hormone peptide on the growth performance in swine. Mol. Immunol. 33, 609–614. Waters, M.J., Oddy, V.H., McCloghry, C.E., Gluckman, P.D., Duplock, R., Owens, P.C., Brinsmead, M.W., 1985.