Aquaculture 183 2000 195–205
www.elsevier.nlrlocateraqua-online
Antibiotic resistance in bacteria isolated from
Artemia nauplii and efficacy of formaldehyde to
control bacterial load
A.S. Sahul Hameed
), G. Balasubramanian
Aquaculture DiÕision, Department of Zoology, C. Abdul Hakeem College, MelÕisharam - 632 509,
Vellore Dist., Tamil Nadu, India
Accepted 31 July 1999
Abstract
A study was carried out to determine the antibiotic resistance in bacteria isolated from Artemia nauplii and the efficiency of formaldehyde to control the bacteria associated with Artemia nauplii. The total aerobic heterotrophic bacteria of Artemia nauplii was determined on seawater nutrient
agar and TCBS agar, and ranged from 3.8=103to 8.1
=103and 9.4
=102 to 4.3
=103colony
Ž .
forming units CFU per nauplius on seawater nutrient agar and TCBS agar plates, respectively. Among these bacteria, 336 isolates were tested for their resistance to five antibiotics. The minimum inhibiting concentrations of chloramphenicol, erythromycin, nitrofurazone, oxytetracy-cline, tetracyoxytetracy-cline, formaldehyde and sodium hypochlorite for 336 isolates were recorded. The LC50 values of oxytetracycline, formaldehyde and sodium hypochlorite for Artemia nauplii were
determined as 540.5, 293.1 and 5.6 mgrl, respectively, after 24 h of exposure. The efficacy of
formaldehyde was compared with that of antibiotics and formaldehyde was found to be very
effective in controlling the bacteria of Artemia nauplii.q2000 Elsevier Science B.V. All rights
reserved.
Keywords: Artemia; Nauplii; Antibiotic resistant bacteria; Antibiotics; Formaldehyde; Efficacy
1. Introduction
Artemia is widely recognized as the best natural, storable live feed available, and is
used in marine finfish and crustacean hatcheries around the world because of its
Ž .
nutritional and operational advantages Sorgeloos et al., 1986 . Artemia nauplii are also
)Corresponding author.
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
( ) A.S. Sahul Hameed, G. BalasubramanianrAquaculture 183 2000 195–205 196
considered a possible vector for the introduction of pathogens into the rearing systems.
Artemia nauplii carry a large bacterial load that may be transferred from live preys into
the tanks of fish and shellfish larvae. Some bacteria have been reported to be the source of diseases and high mortalities in fish larvae, and live feeds are thought to be
Ž .
responsible Tatani et al., 1985; Muroga et al., 1987; Nicolas et al., 1989 . Therefore,
Artemia nauplii are often treated in order to reduce the bacteria associated with them
prior to feeding them to the larvae. Previous studies often recommend rinsing the nauplii
Ž .
in sterile fresh or seawater Austin and Allen, 1982; Rodriguez et al., 1991 , but some Ž
authors argue that rinsing has little effect on the bacteria Dehasque et al., 1991; .
Verdonck et al., 1991 . In this situation, antibiotics have been tried to disinfect the live Ž
feed before introducing into the rearing systems Hatai et al., 1981; Yamanoi and . Sugiyama, 1987; Tanasomwang and Muroga, 1989, 1992; Gomezgil et al., 1994 . The potential consequences of antibiotic use in the treatments are the development of antibiotic resistant micro-organisms, multiple antibiotic resistance, resistance transfer to pathogenic bacteria, and reduced efficacy of antibiotic treatment for diseases caused by
Ž .
resistant pathogens Frappaolo and Guest, 1986 .
The research on the development of antibiotic resistant bacteria has mainly focused on cattle, poultry and swine. Studies on the development of antibiotic resistance in bacterial fish pathogens has also been reported from all areas of aquaculture, ranging
Ž
from warm water to coldwater, marine to freshwater Rahim et al., 1984; Hjeltnes et al., 1987; Tsoumas et al., 1989; McPhearson et al., 1991; Richards et al., 1991; Spanggaard
.
et al., 1993 . The purpose of the present study is to determine the antibiotic resistance in bacteria isolated from Artemia nauplii, to find out the alternative for the antibiotics used routinely in aquaculture systems, to compare the efficacy of formaldehyde to control the bacteria associated with Artemia nauplii and to determine the tolerance level of
Artemia nauplii to formaldehyde and its optimum exposure time to reduce the bacteria.
2. Materials and methods
2.1. Hatching of Artemia cyst
Ž .
Artemia cysts Artemia franciscana were obtained from Argent Chemical
Laborato-Ž .
ries, USA. The cysts were hatched in filtered artificial seawater 30 ppt salinity at 288C–308C. After 24 h incubation, instar I nauplii were hatched out from the cysts.
2.2. Bacteriological analysis
Groups of 100 freshly hatched nauplii were counted aseptically, placed in a sterile tissue homogenizer with 1 ml of sterile seawater and homogenized. After homogeniza-tion, the samples were serially diluted to 10y5 for the estimation of total aerobic
w
heterotrophic bacterial flora. In the present study, seawater nutrient agar Bacto-peptone ŽDifco , 5.0 g; yeast extract Difco , 2.5 g; ferric phosphate, 0.1 g; Bacto-agar Difco 15. Ž . Ž .
xŽ .
g, and seawater 1000 ml Oppenheimer and Zobell, 1952 and Thiosulphate Citrate Bile
Ž .
A.S. Sahul Hameed, G. BalasubramanianrAquaculture 183 2000 195–205 197 Ž Natural seawater was used for media preparation. It was pumped from the sea Bay of
.
Bengal near Chennai and allowed to sediment to get rid of sand and other particles. In the laboratory, the seawater was filtered through filter paper and used. Salinity varied between 30 and 34 ppt, and for media preparation, the salinity was adjusted to 30 ppt with fresh water. The pour plate technique was followed for estimating the total aerobic heterotrophic bacteria on seawater nutrient agar and the spread plate method was followed for growth of Vibrio spp. on TCBS agar. After incubation for 48–72 h at
Ž .
298C, plates with )30 to 300 colony forming units CFU were counted. Three replicates of each dilution were made. After counting, randomly selected colonies Ž10–20rplate were inoculated onto seawater nutrient agar plates. Purified cultures were. maintained on seawater nutrient agar slants at 48C for further studies.
2.3. Antibiotic resistance determination
Ž .
Mueller Hinton agar Hi-Media, India prepared in seawater was used to determine the antibiotic resistance in bacteria isolated from Artemia nauplii. Antibiotics were mixed with molten agar at approximately 458C or were first diluted in sterile distilled
Ž .
water and then added to the molten agar. Antibiotics and dosage levels mgrml
Ž . Ž .
employed in this investigation were chloramphenicol 30 Parke Davis , erythromycin Ž15. ŽIPCA , nitrofurazone 100. Ž . ŽHi-Media , oxytetracycline 30. Ž . ŽPfizer and tetra-.
Ž . Ž .
cycline hydrochloride 30 Sarabhai Chemicals . The antibiotic plates and control plates without antibiotics were inoculated in duplicate consecutively. The plates were incu-bated at 298C for approximately 24 h, and the drug resistance was determined. An organism was considered resistant to an antibiotic only if it grew as well on the antibiotic plate as on the control plate. After antibiotic resistance, twenty bacterial isolates from each resistance profile were selected and identified according to the
Ž . Ž .
taxonomic schemes of Oliver 1982 and Buchanan and Gibbons 1984 .
Ž .
The Minimum Inhibitory Concentration MIC of antimicrobials against the bacteria isolated from Artemia nauplii were determined by the tube dilution method, in seawater peptone broth composed of 1% peptone and 0.05% beef extract. Varying concentrations Ž0.125, 0.25, 0.5, 1.0, 5.0, 10.0, 20.0, 50.0, 100.0, 150.0, 200.0 and 250 mgrml of. chloramphenicol, erythromycin, nitrofurazone, oxytetracycline, tetracycline, formal-dehyde and sodium hypochlorite were used to determine the MIC of these antimicrobials against bacterial isolates. Two separate dilution series in each antimicrobial agent were employed and average value of MIC was determined.
2.4. Effect of antimicrobials on Artemia nauplii
The toxicity of chloramphenicol, erythromycin, oxytetracycline, tetracycline, nitrofu-razone, formaldehyde and sodium hypochlorite was tested by bathing the Artemia larvae in antimicrobial treated seawater for 24 h. Batches of healthy nauplii at a density of
Ž .
concentra-( ) A.S. Sahul Hameed, G. BalasubramanianrAquaculture 183 2000 195–205 198
Table 1
Ž .
Percentage of bacteria resistant to individual antibiotics. The number of isolates is shown in parenthesis
Antibiotic and dosage Percentage of
Žmgrml. resistant bacteria
Ž . Ž .
Chloramphenicol 30 42.9 144
Ž . Ž .
Erythromycin 15 60.2 202
Ž . Ž .
Nitrofurazone 100 69.2 233
Ž . Ž .
Oxytetracycline 30 71.5 240
Ž . Ž .
Tetracycline 30 21.4 72
tion, including the control, were carried out. The nauplii were fed with rice bran and yeast. The percentage survival of larvae was determined by counting the live larvae after 24 h of treatment and microscopic examination was also made to observe the condition of larvae.
2.5. Antimicrobial efficacy tests
The efficacy of formaldehyde to control the bacteria associated with Artemia nauplii was tested and compared with that of other antimicrobials. Artemia nauplii were collected immediately after hatching and gently washed in sterile seawater. Groups of
Ž .
nauplii 1000rgroup were transferred to the different antimicrobial solutions prepared in seawater. The concentration of antimicrobials used in this test was determined based
Ž .
on MIC and LC50 values Tables 2 and 5 . The treatment was carried out for 8 h and
Ž .
larval samples 100 naupliirsample were collected at 0,2,4,6 and 8 h after exposure to estimate the bacterial flora associated with nauplii. The estimation of bacteria was done as mentioned earlier. Controls consisting of nauplii treated in seawater only were run in parallel during all experiments.
Table 2
Resistance profiles of 336 strains isolated from Artemia nauplii to various antibiotics and minimum inhibiting concentrations of formaldehyde and sodium hypochlorite on bacteria isolated from Artemia nauplii. ND — not done
Antibioticsr No. of Percentage of strains growing at different concentrations of antimicrobials
Ž .
antimicrobials isolates mgrml
0.125 0.25 0.5 1.0 5.0 10.0 20.0 50.0 100.0 150.0 200.0 250.0 Chloramphenicol 336 100 100 100 100 91.7 66.7 50 25 18.4 12.5 0 0 Erythromycin 336 100 100 100 100 100 94.6 59.6 42.3 26.8 16.7 8.3 4.8 Nitrofurazone 336 100 100 100 100 81.5 73.9 69.6 65.3 61.8 59.6 56.8 53.6 Oxytetracycline 336 100 100 100 100 100 95.2 80.4 60.1 47.0 33.3 4.8 0 Tetracycline 336 100 100 95.2 88.1 73.2 68.4 42.9 11.9 8.3 0 0 0
Formaldehyde 336 100 100 94.3 88.2 78.3 66.1 38.7 0 0 0 0 0
Sodium 336 100 100 82.3 72.7 58.9 55.4 47.0 ND ND ND ND ND
A.S. Sahul Hameed, G. BalasubramanianrAquaculture 183 2000 195–205 199 Table 3
Percentage composition of bacteria isolated from Artemia nauplii and no. of isolates of prevalent bacterial genera resistant to various antibiotics. Ch, Chloramphenicol; Er, Erythromycin; Nf, Nitrofurazone; Ot, Oxytetracycline, Tc, Tetracycline
Organisms Composition, % No. of isolates resistant to
Ch Er Nf Ot Tc
Aeromonas 11 4 7 8 8 3
Alcaligenes 16 5 9 10 8 4
Bacillus 3 2 1 3 1 1
Chromobacterium 4 3 2 2 2 1
Cytophaga 3 1 2 2 1 1
FlaÕobacterium 3 2 2 2 2 1
Moraxella 2 1 2 2 1 1
Pseudomonas 10 5 6 8 5 3
Vibrio 36 17 22 28 24 15
Unidentified 12 – – – – –
2.6. Statistical analysis
The statistical significance of the difference in efficacy between formaldehyde treated group and other antimicrobial treated groups was determined with the help of the Student’s t-test. The LC50 values of various antimicrobials for Artemia nauplii were
Ž .
calculated with a computer programme Trevors and Lusty, 1985 based on the method
Ž .
described by Finney 1952 .
3. Results
Total numbers of the aerobic heterotrophic bacterial flora of Artemia nauplii were estimated on seawater nutrient agar and TCBS agar. The total number of bacteria ranged
Table 4
Percentage mortalitya of Artemia nauplii exposed for 24 h by bath treatment against antimicrobials at
Ž .
different concentrations, and 24 h LC50 values mgrl . ND — not done Percentage mortality of Artemia nauplii
Ž .
Antimicrobials Concentrations mgrl used 24 h LC50 values
Žmgrl with 95%.
0 2 4 8 16 50 100 200 400 800
confidence limit Chloramphenicol 0 ND ND ND ND ND 5.7 14.7 21.3 47.4 Not determined
Ž .
Oxytetracycline 0.3 ND ND ND ND ND 0.3 6.7 13.0 84.7 540.5 503.7, 579.9
Tetracycline 0 ND ND ND ND ND 1.7 10.0 19.0 26.3 Not determined
Erythromycin 0 ND ND ND ND ND 17.7 32.7 38.0 46.7 Not determined Nitrofurazone 1.3 ND ND ND ND ND 28.3 36.7 42.0 45.7 Not determined
Ž .
Formaldehyde 0 ND ND ND ND 27.3 31.3 41.3 57.7 ND 293.1 226.0, 380.0
Ž .
Sodium hypochlorite 1 2.3 18 28.7 100 ND ND ND ND ND 5.6 5.4, 5.9 a
()
A.S.
Sahul
Hameed,
G.
Balasubramanian
r
Aquaculture
183
2000
195
–
205
200
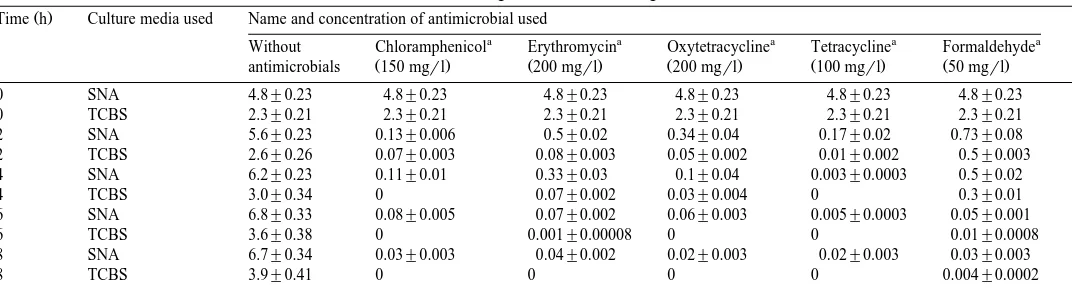
Table 5
Ž .U
Estimated number of bacterial cells thousands of CFU per Artemia nauplius associated with Artemia nauplii in the absence and presence of antimicrobials at different time intervals. Values are mean"S.E. SNA — seawater nutrient agar, TCBS — TCBS agar
Ž .
Time h Culture media used Name and concentration of antimicrobial used
a a a a a
Without Chloramphenicol Erythromycin Oxytetracycline Tetracycline Formaldehyde
Ž . Ž . Ž . Ž . Ž .
antimicrobials 150 mgrl 200 mgrl 200 mgrl 100 mgrl 50 mgrl
0 SNA 4.8"0.23 4.8"0.23 4.8"0.23 4.8"0.23 4.8"0.23 4.8"0.23
0 TCBS 2.3"0.21 2.3"0.21 2.3"0.21 2.3"0.21 2.3"0.21 2.3"0.21
2 SNA 5.6"0.23 0.13"0.006 0.5"0.02 0.34"0.04 0.17"0.02 0.73"0.08 2 TCBS 2.6"0.26 0.07"0.003 0.08"0.003 0.05"0.002 0.01"0.002 0.5"0.003 4 SNA 6.2"0.23 0.11"0.01 0.33"0.03 0.1"0.04 0.003"0.0003 0.5"0.02
4 TCBS 3.0"0.34 0 0.07"0.002 0.03"0.004 0 0.3"0.01
6 SNA 6.8"0.33 0.08"0.005 0.07"0.002 0.06"0.003 0.005"0.0003 0.05"0.001
6 TCBS 3.6"0.38 0 0.001"0.00008 0 0 0.01"0.0008
8 SNA 6.7"0.34 0.03"0.003 0.04"0.002 0.02"0.003 0.02"0.003 0.03"0.003
8 TCBS 3.9"0.41 0 0 0 0 0.004"0.0002
U
Mean of three replicates. a
A.S. Sahul Hameed, G. BalasubramanianrAquaculture 183 2000 195–205 201 from 3.8=103 to 8.1=103 CFU per nauplius on seawater nutrient agar and 9.4=102 to 4.3=103 CFUrnauplius on TCBS agar. A total of 336 isolates was examined for their resistance to five antibiotics. The frequencies of resistances to individual antibiotics are shown in Table 1. More than 60% of the bacterial isolates were resistant to erythromycin, nitrofurazone and oxytetracycline.
The MIC values of seven antimicrobials against the bacterial isolates were deter-mined and the results are presented in Table 2. The growth of all bacterial isolates was inhibited by chloramphenicol at 200 mgrl, oxytetracycline at 250 mgrl, tetracycline 150 mgrl or formaldehyde at 50 mgrl. Erythromycin inhibited the growth of 95.2% of bacterial isolates at 250 mgrl; nitrofurazone at 250 mgrl and sodium hypochlorite at 20 mgrl inhibited the growth of 46.4% and 53% of the bacterial isolates, respectively.
The bacterial isolates were identified to at least generic level and the results are given in Table 3. The percentage of isolates of each genus resistant to each antibiotic is shown in Table 3.
Percentage mortality of Artemia nauplii exposed for 24 h at different concentrations of antimicrobial agents is given in Table 4. High mortality of Artemia nauplii was
Ž .
observed when the larvae were exposed to chloramphenicol 800 mgrl , oxytetracycline Ž800 mgrl , erythromycin 800 mg. Ž rl , nitrofurazone 800 mg. Ž rl , formaldehyde 400. Ž
. Ž .
mgrl or sodium hypochlorite 16 mgrl . Chloramphenicol, oxytetracycline, tetra-cycline, formaldehyde and sodium hypochlorite caused significant mortality in higher concentrations. Nauplii with broken setae and appendage deformities were encountered when the larvae were exposed to higher concentrations of antimicrobials. The LC50 values of various antimicrobials for Artemia nauplii were determined and the results are presented in Table 4. The LC50 values of oxytetracycline, formaldehyde and sodium hypochlorite recorded were 540.5, 293.1 and 5.6 mgrl, respectively, after 24 h of exposure.
The effect of antimicrobial agents on the bacteria associated with the Artemia nauplii was tested and the results are presented in Table 5. The antimicrobials significantly reduced the bacterial load of Artemia on seawater nutrient agar and TCBS agar when compared to the control. In the absence of antimicrobials, the bacterial flora of Artemia nauplii increased to level of 6.7=103and 3.9=103rnauplius on seawater nutrient agar and TCBS agar, respectively, after 8 h of incubation. There is no significant differences between antibiotic and formaldehyde treatments to disinfect the Artemia nauplii.
4. Discussion
Artemia nauplii form an important live feed for a variety of finfishes and shellfishes
( ) A.S. Sahul Hameed, G. BalasubramanianrAquaculture 183 2000 195–205 202
aerobic heterotrophic bacterial flora ranged from 3.8=103 to 8.1=103 CFCrnauplius on seawater nutrient agar and 9.4=102 to 4.3=103 CFCrnauplius on TCBS agar. Earlier studies have also reported the heavy bacterial load associated with Artemia
Ž
nauplii Gilmour et al., 1975; Austin and Allen, 1982; Gatesoupe, 1990; Tanasomwang .
and Muroga, 1990; Verdonck et al., 1991; Straub and Dixon, 1993 . It is therefore necessary to control the bacterial population of Artemia nauplii to minimise the danger of bacterial infection before their use in culture systems. The effects of chemotherapeu-tants, ultraviolet irradiation treatments and freezing have been investigated to minimize
Ž
the danger of bacterial infections associated with feeding live food Hayashi et al., 1975, 1976; Hatai et al., 1981; Tabata et al., 1982; Yamanoi and Sugiyama, 1987; Miyakawa
.
and Muroga, 1988; Yamanoi and Katayama, 1989 . Use of antibiotics, a hypochlorite solution, an iodophor and formaldehyde have all been found to be effective in
suppress-Ž
ing the bacterial flora of Artemia nauplii Gilmour et al., 1975; Coleman et al., 1980; .
Sumitra et al., 1988 .
A wide range of antimicrobial compounds such as chloramphenicol, oxytetracycline, kanamycin, nifurprazine, oxolinic acid, sodium nifurstyrenate and flumequine is now being used in aquaculture. Attention has been drawn to the possible development of antibiotic resistant bacteria and the emergence of resistant bacteria have been repeatedly demonstrated in a variety of environments and media: hospital and barnyard, man and animal. The present data associate the frequency of antibiotic resistant bacteria isolated from Artemia nauplii. In the present study, the common antibiotics used in the aquaculture systems were tested against the bacteria isolated from Artemia nauplii for their resistance and the results showed that more than 60% of bacteria were resistant to erythromycin, nitrofurazone and oxytetracycline, and 43% to chloramphenicol and 21% to tetracycline. The prophylactic use of antibiotics during larval rearing, including disinfecting live feed, results in increasing the frequencies of antibiotic resistant bacteria
Ž
in aquaculture systems. This is true in the present and previous investigations Rahim et al., 1984; Hjeltnes et al., 1987; Richards et al., 1991; Gomezgil et al., 1994; Karunasagar
.
et al., 1994 . It is possible that antibiotic resistance can be transferred to bacterial pathogens of fish and shellfish within the gut of host if the numbers of antibiotic resistant bacteria are ingested along with live feed. This antibiotic resistance may cause economic losses as the outbreak of different diseases may not be treated efficiently. Control of bacterial growth is known to vary with the antibiotic, and no one antibiotic would be expected to inhibit all bacteria under most culture conditions. The best results in controlling bacterial growth in seawater cultures are obtained with addition of two or
Ž
more stable, wide spectrum antibiotics at the same time Cviic, 1953; Walne, 1958; Fitt .
et al., 1987 . But this is not economical to disinfect the live feed in large scale. Moreover some antibiotics, such as tetracycline and penicillin, break down quickly in seawater and are therefore not particularly effective in controlling bacterial growth
Ž .
unless added daily to cultures Fitt et al., 1992 . This is also true in the present investigation. Addition of 100 ppm of tetracycline reduced the bacteria to 5 CFUrnauplius after 6 h of exposure and the bacterial population increased to 20
Ž .
CFUrnauplius after 8 h of exposure Table 5 . This increase might be due to breakdown
Ž .
A.S. Sahul Hameed, G. BalasubramanianrAquaculture 183 2000 195–205 203 breakdown in light to form cytotoxic compounds that affect symbiotic algae and their
Ž .
hosts Rahat et al., 1979 .
In this context, the present study discourages the use of antibiotics for disinfecting the live feed and an attempt was made in the present study to find out some alternatives for the antibiotics to disinfect the live feed. Formaldehyde and sodium hypochlorite were tested for their efficacies to reduce the bacteria associated with Artemia nauplii. The tolerance level of formaldehyde and sodium hypochlorite to Artemia nauplii were also
Ž .
determined. From the foregoing, it is evident that chloramphenicol 150 mgrl ,
erythro-Ž . Ž . Ž .
mycin 200 mgrl , oxytetracycline 200 mgrl or tetracycline 100 mgrl could inhibit the growth of bacteria associated with Artemia nauplii, whereas formaldehyde could eliminate the bacteria at the concentration of 50 mgrl. This concentration of
formal-Ž .
dehyde was found to be less toxic to Artemia nauplii Table 4 . Sodium hypochlorite was omitted as it was not able to inhibit the growth of bacterial flora isolated from
Ž .
Artemia nauplii at the highest concentration 16 mgrl and this concentration caused
100% mortality of larvae. Based upon these observations, the present study strongly recommends the use of formaldehyde to disinfect live feed before introducing into the rearing systems. Hatcheries routinely administer formalin for the control of diseases at
Ž
various concentrations ranging from 250 to 2000 mgrl Piper et al., 1982; Marking et .
al., 1994; Schreier et al., 1996 . Lower concentrations of formalin are effective for
Ž .
prophylactic control of fungus Marking et al., 1994 as observed in the present study for disinfecting Artemia nauplii. Formaldehyde is a good choice as a disinfecting agent
Ž .
based upon studies in penaeid shrimp Hose and Lightner, 1980 , residues should not accumulate in the treated nauplii. In addition, the use of lower treatment concentrations could reduce operational costs and decrease levels of formalin discharged into the environment.
References
Ž .
Austin, B., Allen, D.A., 1982. The microbiology of laboratory hatched brine shrimp Artemia . Aquaculture 26, 369–383.
Buchanan, R.E., Gibbons, N.E., 1984. Bergey’s Manual of Determinative Bacteriology, 8th edn. Williams and Wilkins, Baltimore, MD, 1268 pp.
Cviic, V., 1953. The bactericidal and bacteriostatical action of antibiotics on marine bacteria: I. Penicillin and streptomycin. Acta Adriat. 5, 135–166.
Coleman, D.E., Nakagawa, L.K., Nakamura, R.M., Chang, E., 1980. In: Persoone, G., Sorgeloos Rools, P.,
Ž .
Jaspers, E. Eds. , The Brine Shrimp, Vol. 3. Universa Press Wetteren, Belgium, p. 153.
Dehasque, M., Verdonck, L., Sorgeloos, P., Swings, J., Leger, P., Kristers, K., 1991. Determination of the bacterial contamination in live food production system and in marine fish hatcheries in Souther Europe. In:
Ž .
Lavens, P., Sorgeloos, P., Jaspers, E., Oliver F. Eds. , Larvi-91. Fish and Crustacean Larviculture Symposium, European Aquaculture Society Special Publication No. 15, Ghent, Belgium, pp. 399–401. Finney, D.J., 1952. Statistical Method in Biology Assay. Charles Griffin, London, UK.
Fitt, W.K., Hofmann, D.K., Wolk, M., Rahat, M., 1987. Requirement of exogenous inducers for
metamorpho-Ž .
sis of axenic larvae and buds of Cassiopea andromeda Cnidaria: Scyphozoa . Mar. Biol. 94, 415–422.
Ž
Fitt, W.K., Heslinga, G.A., Watson, T.C., 1992. Used of antibiotics in the mariculture of giant clams F.
.
Tridacnidae . Aquaculture 104, 1–10.
( ) A.S. Sahul Hameed, G. BalasubramanianrAquaculture 183 2000 195–205 204
Gatesoupe, F.J., 1990. The continuous feeding of turbot larvae Scophtalmus maximass and the control of the bacterial environment of the rotifers. Aquaculture 89, 139–148.
Gilmour, A., McCallum, M.F., Allan, M.C., 1975. Antibiotic sensitivity of bacterial isolates from the canned
Ž .
eggs of the California brine shrimp Artemia salina . Aquaculture 6, 221–231.
Gomezgil, B.R.S., Grobois, F.A.A., Jarero, J.R., Vega, M.D.H., 1994. Chemical disinfection of Artemia
Ž .
nauplii. J. World Aquacult. Soc. 25 4 , 574–583.
Hatai, K., Yasumoto, S., Ogawa, S., Yasunaga, N., 1981. Elimination of bacteria associated with baking yeast-fed rotifers by some antibacterial agents. Rep. Nagasaki Prefect. Fish. Stn., pp. 218–222.
Hayashi, K., Kimura, T., Sugahara, I., 1975. Microbiological studies on the artificial seed production of ayu
Ž .
fish Plecoglossus altiÕelis Temminck et Schlegel : III. Bacterial contamination of Brachionus plicatilis
and Moina macrocopa. Bull. Fac. Fish., Mie Univ. 2, 81–91.
Hayashi, K., Kimura, T., Sugahara, I., 1976. Microbiological studies on the artificial seed production of ayu
Ž .
fish Plecoglossus altiÕellis Temminck et Schlegel : IV. On the elimination of bacteria contaminated in
Brachionus plicatilis and Moina macrocopa. Bull. Fac. Fish., Mie Univ. 3, 87–99.
Hjeltnes, B., Anderson, K., Egidius, E., 1987. Multiple antibiotic resistance in Vibrio salmonicida. Bull. Eur. Assoc. Fish Pathol. 7, 85.
Hose, J.E., Lightner, D.V., 1980. Absence of formaldehyde residues in penaeid shrimp exposed to formalin. Aquaculture 21, 197–201.
Karunasagar, I., Pai, R., Malathi, G.R., 1994. Mass mortality of Penaeus monodon larvae due to antibiotic-resistant Vibrio harÕeyi infection. Aquaculture 128, 203–209.
Marking, L.L., Rach, J.J., Schreier, T.M., 1994. Evaluation of antifungal agent for fish culture. Progr. Fish-Cult. 56, 225–231.
McPhearson, R.M., DePaloa, A., Zywno, S.R., Motes, M.L. Jr., Guarino, A.M., 1991. Antibiotic resistance in Gram-negative bacteria from cultured catfish and aquaculture ponds. Aquaculture 99, 203–211. Miyakawa, M., Muroga, K., 1988. Bacterial flora of cultured rotifer Brachionus plicatilis. Suisan Zoshoku 35,
237–243.
Muroga, K., Higashi, M., Keitiku, H., 1987. The isolation of intestinal microflora of farmed red seabream
ŽPagrus major and black seabream Acanthopagrus schlegeli of larval and juvenile stages. Aquaculture. Ž .
65, 79–88.
Nicolas, J.L., Robic, E., Ansquer, D., 1989. Bacterial flora associated with a trophic chain consisting of microalgae, rotifers and turbot larvae: influence of bacteria on larval survival. Aquaculture 83, 237–248. Oliver, J.D., 1982. Instruments and methods, taxonomic scheme for the identification of marine bacteria. Deep
Sea Res. 29, 795–798.
Oppenheimer, C.H., Zobell, C.E., 1952. The growth and viability of sixty three species of marine bacteria as influenced by hydrostatic pressure. J. Mar. Res. 11, 10–18.
Piper, R.G., McElwain, I.B., Orme, L.E., Mc Craren, J.P., Fowler, L.G., Leonard, J.R., 1982. Fish Hatchery Management. US Fish and Wildlife Service, Washington, DC, 517 pp.
Rahat, M., Zeldes, D., Reich, V., 1979. Photoproducts of chloramphenicol in their cytotoxic effects on Hydra
Õiridis and its symbiotic algae. Comp. Biochem. Physiol. 63, 27–30.
Rahim, Z., Sanyal, S.C., Aziz, K.M.S., Huq, M.I., Chowdhury, A.A., 1984. Isolation of entero toxigenic, hemolytic, and antibiotic resistant Aeromonas hydrophila strains from infected fish in Bangladesh. Appl. Environ. Microbiol. 48, 865–867.
Richards, R.H., Inglis, V., Frerichs, G.N., Millar, S.D., 1991. Variation in antibiotic resistance patterns of Aeromonas salmonicida isolated from Atlantic Salmon salar L. in Scotland. Working Papers from the Conference: Problems of chemotherapy from theory to reality. Paris, 12–15 March, 1991.
Rodriguez, J.L., Planas, M., Otero, J.J., 1991. Microflora and antibacterial treatments of rotifers and Artemia.
Ž .
In: Lavens, P., Sorgeloos, P., Jaspers, E., Olleveier, F. Eds. , Larvi-91. Fish and Crustacean Larviculture Symposium. European Aquaculture Society, Special Publication No. 15, Ghent, Belgium, pp. 403–405. Schreier, T.M., Rach, J.J., Howe, G.E., 1996. Efficacy of formalin, hydrogen peroxide and sodium chloride on
fungal infected rainbow trout eggs. Aquaculture 140, 323–331.
Sorgeloos, P., Lavens, P., Legar, P., Tackaert, W., Versichele, D., 1986. Manual for the culture and use of brine shrimp Artemia in Aquaculture. Artemia, Reference Centre, State University of Ghent, Belgium. Spanggaard, B., Jorgensen, F., Gram, L., Huss, H.H., 1993. Antibiotic resistance in bacteria isolated form
A.S. Sahul Hameed, G. BalasubramanianrAquaculture 183 2000 195–205 205
Ž .
Straub, D.V., Dixon, B.A., 1993. Bacteriological flora of the brine shrimp Artemia franciscana from a hypersaline pond in San Francisco Bay, California. Aquaculture 118, 309–313.
Sumitra, V., Ramaiah, N., Chandramohan, D., 1988. Effect of iodophor treatment on the hatching of Artemia
Ž .
cysts. Curr. Sci. 57 5 , 278–280.
Tabata, S., Karata, S., Ruiz, M.S., 1982. Studies on naturally occurring disease during the production of ayu
ŽPlecoglossus altiÕelis in seawater: II. Dynamics of Vibrio anguillarum. Fish Pathol. 23, 77–83..
Tanasomwang, V., Muroga, K., 1989. Effects of sodium nifurstyrenate and tetracycline on the bacterial flora
Ž .
of rotifers Brachionus plicatilis . Fish Pathol. 24, 29–35.
Tanasomwang, V., Muroga, K., 1990. Intestinal microflora of marine fishes at their larval and juvenile stages.
Ž .
In: Hirano, R., Hanyu, I. Eds. , The Second Asian Fisheries Forum. Asian Fisheries Society, Manila, Philippines.
Tanasomwang, V., Muroga, K., 1992. Effect of sodium nifurstyrenate on the reduction of bacterial
contamina-Ž .
tion of rotifers Brachionus plicatilis . Aquaculture 103, 221–228.
Tatani, M., Muroga, K., Sugiyama, T., Hiramoto, Y., 1985. Detection of Vibro anguillarum from reared fry and fingerlings of ayu. Suisan Zoshoku 33, 59–66.
Trevors, J.V., Lusty, C.M., 1985. A basic microcomputer program for calculating LC50 values. Water, Air Soil Pollut. 24, 431–442.
Tsoumas, A., Alderman, D.J., Rodgers, C.J., 1989. Aeromonas salmonicida, development of resistance to 4-quinolone antimicrobials. J. Fish Dis. 12, 493–507.
Verdonck, L., Dehasque, M., Swings, J., Sorgeloos, P., Leger, P., 1991. The microbial environment of rotifer
ŽBrachionus plicatilis. and Artemia production systems. In: Lavens, P., Sorgeloos, P., Jaspers, E.,
Ž .
Ollevier, F. Eds. , Larvi-91, Fish and Crustacean Larviculture Symposium. European Aquaculture Society, Special Publication No. 15, Ghent, Belgium, p. 398.
Walne, P.R., 1958. The importance of bacteria in laboratory experiments on rearing the larvae of Ostrea edulis
Ž .L. . J. Mar. Biol. Assoc. U. K. 37, 414–425.
Yamanoi, H., Katayama, K., 1989. Effects of freezing on bacterial flora of rotifer and brine shrimp nauplii. Nippon Suisan Gakkaishi 55, 2207.