L
Journal of Experimental Marine Biology and Ecology 242 (1999) 59–74
www.elsevier.nl / locate / jembe
Effects of two organochlorine compounds on hatching and
viability of calanoid copepod eggs
a ,* a a a b ,1
J.A. Lindley , P. Donkin , S.V. Evans , C.L. George , K.F. Uil
a
Centre for Coastal and Marine Sciences, Plymouth Marine Laboratory, Prospect Place, The Hoe, Plymouth PL1 3DH, UK
b
Wageningen Agricultural University, Landbouwuniversiteit, Wageningen, The Netherlands Received 21 May 1998; received in revised form 10 June 1999; accepted 28 June 1999
Abstract
Two organochlorine compounds, pentachlorophenol (PCP), a respiratory uncoupler, and 1,2-dichlorobenzene (DCB), a non-polar narcotic, were selected for experiments on their toxicity to eggs of estuarine and neritic planktonic calanoid copepods. Experiments on freshly laid eggs in aqueous solutions of the toxicants showed that no viable nauplii of Eurytemora affinis or Acartia
bifilosa hatched from eggs incubated in initially saturated solutions and a reduced percentage of
eggs hatched after temporary exposure (|16 h) to saturated solutions. The percentage of eggs of
E. affinis that hatched was reduced below control values in 5 and 10% saturated solutions with EC50 (concentration reducing the response by 50%) estimated at 2.1% saturated solution of PCP and 3.2% saturated DCB (initial concentrations). Viability of nauplii was reduced at lower concentrations with estimated LC50 (concentration lethal to 50% of population) values of 0.7% saturated PCP and 1.7% saturated DCB. Equivalent values for Acartia bifilosa were higher, with no reduction below control values in percentage hatch at 10% saturation and LC50 values for nauplii of 0.8% PCP and 14.2% DCB. The percentage of eggs of A. clausi that hatched when incubated in sealed vials to eliminate loss of DCB through evaporation was not reduced in 10% saturated solutions but the estimated LC50was 2.0% saturation. In sediment exposed to a saturated solution of DCB very high mortality occurred within 1 day. 1999 Elsevier Science B.V. All rights reserved.
Keywords: Calanoid eggs; Dichlorobenzene; Hatching; Nauplii; Pentachlorophenol; Viability
1. Introduction
Diaptomoid calanoid copepods, usually the dominant planktonic calanoids of neritic
*Corresponding author.
1
Present address: Beltstraat 98a, 7512 AB Enschede, The Netherlands.
and estuarine waters (Lindley, 1997), produce eggs that may accumulate in the sediments. These eggs may remain dormant for periods far exceeding their normal temperature dependent development times. The eggs include diapause eggs and delayed hatching eggs, the development of which is delayed by endogenous processes as well as subitaneous eggs (Marcus, 1996). The subitaneous eggs normally develop at temperature dependent rates but development of all types of egg may be delayed by unfavourable environmental conditions for hatching, including the effects of burial in sediment. Anthropogenic contaminants and organic matter, the decay of which can cause anoxia particularly where eutrophication occurs, also accumulate in coastal and estuarine sediments. Long et al. (1996), in experiments on sediments from 22 US estuaries, found that sediments from 10.9% of the area and pore waters from 43% of the area were toxic. Reductions in viability of calanoid copepod eggs have been attributed to anoxia (Uye et al., 1984), to hydrogen sulphide (Marcus et al., 1997) and to toxicants, for example rotenone (Naess, 1991b). Zooplankton eggs can remain dormant in sediments, usually for periods of days to months, but recent work has indicated that at least a proportion of eggs can remain viable over decades (Marcus, 1996). There is potential for impact on eggs in polluted sediment and hence on recruitment to the zooplankton through direct effects of accumulated toxicants and decay of organic matter or indirectly through decreased bioturbation increasing anoxia and sulphide accumulation. Interspecific differences in susceptibility of the eggs or the hatching nauplii could result also in changes in plankton community structure and species diversity. This investigation was undertaken to supplement existing information on effects of contaminants on estuarine and neritic zooplankton eggs and to complement field studies on viability of eggs from estuaries with a range of histories of industrial and urban pollution (Lindley et al., 1998) Two compounds were selected for experiments, pentachlorophenol (PCP) and 1,2-dichlorobenzene (DCB). PCP is an extensively used biocide, which is on the UK Red List of priority pollutants, in addition to EEC and US EPA priority lists. It has been detected in significant quantities in freshwater sediments in the USA (Hoke et al., 1993), in the Scheldt estuary, Netherlands (van Zoest and van Eck, 1991) and in river and coastal monitoring programmes in the UK (National Rivers Authority, 1995a,b,c). It is very hydrophobic in its unionised form (log n-octanol / water partition coefficient, log Kow55.12), so can readily be absorbed by sediments, and bioaccumulated, including into mitochondria where it uncouples respiration (Mahler and Cordes, 1966). This mode of action provides a link with rotenone, another respiratory uncoupler, one of the few other organic compounds known to reduce the viability of zooplankton eggs (Naess, 1991b). The dichlorobenzenes are toxic by non-polar narcosis (van Wezel et al., 1996) and are more likely to partition into water. The 1,2-isomer was chosen for study since it is a liquid at the experimental temperatures, eliminating the possibility of crystal formation during experiments at high concentrations. Its log Kow is 3.38. 1,2-DCB occurs in sewage sludge (Rogers et al., 1989a,b; Wang and Jones, 1994) and is a widespread contaminant of freshwater (Hoke et al., 1993) and estuarine environments (Rogers et al., 1989a; van Zoest and van Eck, 1991).
J.A. Lindley et al. / J. Exp. Mar. Biol. Ecol. 242 (1999) 59 –74 61
mid-estuarine species, and Acartia spp., mainly A. bifilosa which overlaps with E. affinis but is more abundant at higher salinities (e.g., Collins and Williams, 1981). These species dominated our plankton samples from the Exe and Tamar estuaries. E. affinis carries its eggs in a sac. Diapausing eggs are shed after a few days but eggs may reach the sediment also following predation on the egg carrying females, which are more conspicuous to predators than those without egg sacs (Webb and Weaver, 1988). Conway et al. (1994) and Flinkman et al. (1994) found that the eggs of E. affinis remain viable after passage through fish guts. Acartia spp. spawn freely and the eggs sink.
2. Methods
2.1. Source of eggs
Plankton samples were taken with a hand net of 0.5 m opening and 100 mm mesh size. Samples were taken by streaming the net in the tidal current of the near surface waters mainly from a pontoon jetty on the Exe estuary, Devon, England. This was near to the West Muds site described by George and Lindley (1997). Samples were taken here in April and October 1994 and May 1996. These samples were supplemented during June and July 1996 by sampling from the shore in the Tamar estuary, again streamed in
21
the tidal current, or towed at about 1 m s for about 10 min by research vessels in Plymouth Sound. The dates of sampling are given in Tables 1–3.
Small quantities of the plankton samples were poured or transferred using a wide mouth pipette into Petri dishes. When Acartia spp. were numerous they were concen-trated in the original sample by exploiting their positive phototaxis (e.g., Stearns and Forward, 1984). Later on the same day or on the following day adult female calanoid copepods were transferred, using Pasteur pipettes, to 0.1mm filtered sea water (FSW) in Petri dishes. They were incubated in the FSW overnight (|16 h). The eggs of Acartia spp. and Eurytemora affinis with egg sacs extracted were removed with pipettes. The sea water was collected off the Eddystone Rocks (508109N, 48169W) in the English Channel about 15 km from the nearest land. It has a salinity of |35‰ and the concentrations of heavy metals and anthropogenic organic pollutants are much lower in these offshore waters than in most estuaries (Marine Pollution Monitoring Management Group, 1998). All handling of the copepods and eggs in this and subsequently described procedures was performed at room temperature (approx. 208C).
Table 1
Effect of (initially) saturated DCB solution on hatching of Eurytemora affinis eggs; females collected 18 April 1994. FSW, filtered sea water
Treatment Eggs % Hatch % Viable
Control (FSW throughout) 132 (5) .9961 .9961
DCB solution throughout 209 (5) ,161 ,161
Table 2
21
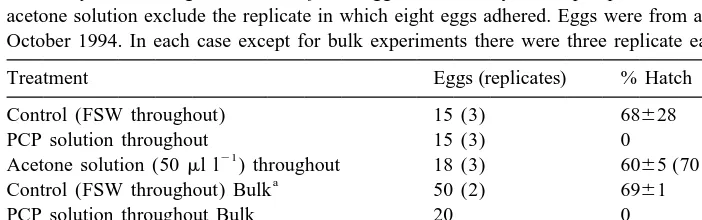
Effect of saturated PCP solution in FSW with acetone carrier and of acetone carrier (50ml l solution) in FSW only on hatching of Acartia bifilosa eggs and viability of nauplii produced. Values in parentheses for acetone solution exclude the replicate in which eight eggs adhered. Eggs were from adult females sampled 11 October 1994. In each case except for bulk experiments there were three replicate each with about five eggs
Treatment Eggs (replicates) % Hatch % Viable
Control (FSW throughout) 15 (3) 68628 68628
PCP solution throughout 15 (3) 0 0
21
Acetone solution (50ml l ) throughout 18 (3) 6065 (7062) 3961 (4060)
a
Control (FSW throughout) Bulk 50 (2) 6961 28.06,1
PCP solution throughout Bulk 20 0 0
21
Acetone (50ml l ) throughout Bulk 20 90.0 65.0
FSW overnight, washed, incubated in FSW 13 (3) 86612 6164
PCP overnight, washed, incubated in FSW 15 (3) 0 0
Acetone overnight, washed, incubated in FSW 15 (3) 7462 6762
a
Two experiments, one with 20 eggs one with 30 eggs.
2.2. Egg incubation experiments with saturated solutions
Eggs produced by the females were used in experiments carried out in Petri dishes that were fitted with glass cover dishes but not sealed. Eggs were treated in the following
21 ways: (1) incubated in a saturated solution of DCB or PCB, the latter with 50 ml l acetone carrier solvent; (2) incubated in FSW; (3) incubated in a saturated solution of the contaminant overnight, washed in FSW then incubated in FSW; and (4) incubated in FSW overnight, washed in FSW then incubated in FSW. When PCP solutions were used in treatments 1 and 3, two further treatments were used to test for the effects of the acetone carrier: (5) incubated in FSW with carrier; and (6) incubated in FSW with carrier overnight, washed in FSW then incubated in FSW. The incubations were in Petri dishes in constant light at 158C except for the initial overnight stages of treatments 3 and 4 where the samples were kept at 58C in the dark to minimize the chance of hatching during this stage. The Petri dishes were examined on the day following the start of the incubation and then at 1–3 day intervals afterwards to find the numbers of nauplii that hatched, and whether they were still alive when examined. These data were used to calculate percentage hatch and percentage viability, the means and standard deviations of which were calculated from arcsine-transformed data.
The concentrations of the chemicals in the dishes were monitored by ultra-violet (UV) spectrophotometry (at 322 nm for PCP and at 269 and 277 nm for DCB). The saturated concentration, based on comparison with UV absorbance calibration curves prepared
21
using standard solutions of PCP, was 14 mg l . This is the same figure as quoted by Howard (1991) for the aqueous solubility of PCP. The solubility of 1,2-dichlorobenzene
21
in pure water at 258C is 91 mg 1 (Miller et al., 1984). Since the solubility of organic chemicals in sea water is usually less than in freshwater, this calculated mass value could be an overestimate by approximately 25%.
2.3. Egg incubations in low concentration solutions
J.
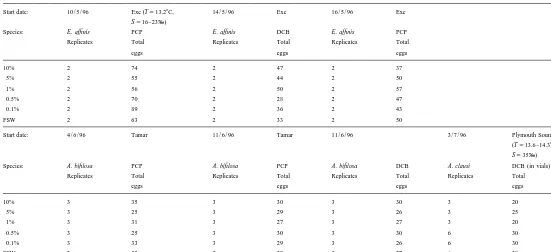
Experiments with calanoid eggs in a range of (initial) concentrations of PCP and DCB. Dates, sampling locations with (temperature, T, and salinity, S, where available) species (Eurytemora affinis, Acartia bifilosa or Acartia clausi ), numbers of replicates and numbers of eggs
Start date: 10 / 5 / 96 Exe (T513.28C, 14 / 5 / 96 Exe 16 / 5 / 96 Exe S516–23‰)
Species: E. affinis PCP E. affinis DCB E. affinis PCP Replicates Total Replicates Total Replicates Total Species: A. bifilosa PCP A. bifilosa PCP A. bifilosa DCB A. clausi DCB (in vials)
Replicates Total Replicates Total Replicates Total Replicates Total
PCP and DCB and to determine whether there were interspecific variations in the effects. The saturated solutions were diluted to 10, 5, 1, 0.5 and 0.1% with FSW controls. By expressing toxicological data for different compounds in terms of percentage saturation, comparisons between the potencies of the compounds can be made in a mechanistically meaningful way. Since eggs cannot take up particulate material, partitioning from aqueous solution is the primary route of uptake of the toxicants. Saturated solutions thus represent the highest level of exposure achievable. Furthermore, Ferguson (1939) proposed that narcosis (the primary toxicological response caused by many organic pollutants) occurs at a constant thermodynamic activity of the chemical. Effectively, this means a constant ratio to the aqueous solubility of the chemical. In these experiments the eggs were exposed continuously to the toxicant with no rinsing stage. In order to address the problem of the loss of DCB through evaporation, experiments were conducted in which the eggs in their experimental medium were sealed in vials in addition to experiments in Petri dishes.
Values for EC50 (concentrations reducing the selected response by 50%) for hatching and LC50 (concentration causing 50% mortality) for larval viability were calculated by probit analysis. As substantially less than 100% of eggs of Acartia spp. hatched in all controls (in FSW), the values for this genus were calculated from 50% of the control values rather than 50% of the initial numbers of eggs.
2.4. Experiments with eggs in sediment samples
Sediment samples for the DCB experiment were taken on 7 June 1994 at West Muds on the Exe estuary (George and Lindley, 1997) using a Perspex tube of 19 mm diameter. The experiments were started on the same day. The top 3 cm of sediment, which was not blackened by reduction reactions, was used in the experiments described here. The sediment from each core was extruded from the tube and used in an incubation in a 100 ml beaker (covered by a watch glass) either under a saturated solution of the DCB or under FSW at 5–78C in the dark. At intervals of 1, 3, 6, 9, 17 and 23 days, five replicate samples (beakers containing the sediment and supernatant) exposed to the toxicant and five FSW controls were removed. The sediment from each beaker was washed through a 50mm mesh gauze for 3 min and incubated under FSW. The numbers of viable nauplii
21 hatching were determined as for the similar experiments with a solution of 4mg l PCP described by Lindley et al. (1998).
Mortality due to the DCB was calculated by M5100T /C where M is mortality, T the mean numbers hatched from sediments treated with solution of the toxicant and C mean numbers hatched from FSW controls.
3. Results
3.1. Egg incubation experiments with saturated solutions
Eurytemora affinis dominated a plankton sample taken on 18 April 1994 (temperature
J.A. Lindley et al. / J. Exp. Mar. Biol. Ecol. 242 (1999) 59 –74 65
were used in each replicate experiment with DCB and FSW controls. Five replicates were used for each of the treatments 1 and 2. Three replicates were used for each of treatments 3 and 4. The results are given in Table 1. Only one egg failed to hatch in the FSW controls and all the nauplii were alive when examined, whereas three hatched in the DCB solution, none of which survived. Temporary immersion in DCB reduced the percentage hatch but all the nauplii that hatched were alive. The percentage hatch varied between the three replicates for the temporary immersion, the results were 0% of 37 eggs, 12.5% of 40 eggs and 100% of 50 eggs. Typically, 90% of the DCB was lost through evaporation from solution in the Petri dishes within the first 24 h, and none was detectable after 48 h. All hatching took place within 4 days of the start of incubation. Adult female Acartia bifilosa were extracted from a plankton sample taken on 11 October 1994 (temperature 13.28C, salinity 26–29‰) in which that species was dominant. The eggs that these produced overnight (i.e. in approximately 16 h) were used in three replicates of each of the treatments described above but with PCP with acetone carrier as the toxicant. Three replicates of each of two additional treatments were carried
21
out, continuous incubation in 50ml l acetone solution in FSW and initial incubation in acetone solution followed by transfer to FSW after |24 h. In each replicate five eggs were used except for one acetone replicate where eight eggs adhered to each other and could not be separated without damage. Remaining eggs were used in bulk experiments with 20 or 30 eggs per Petri dish. The results are listed in Table 2 with the values for the acetone replicates calculated for including and excluding that in which the eggs were clumped. Both continued immersion and overnight immersion in initially saturated PCP solution completely suppressed hatching. PCP concentrations fell to approximately 80%
21
of the saturated concentration, approximately 11 mg l , within the first 24 h of an experiment. The acetone carrier had no consistent effect. All hatching took place within 4 days of the start of incubation. A single Eurytemora affinis egg sac containing seven
21
eggs was incubated in 50ml l acetone solution. All the eggs hatched and the nauplii were viable.
3.2. Egg incubations in low concentration solutions
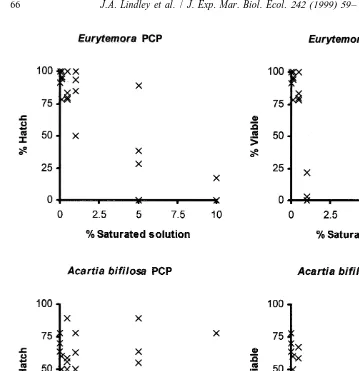
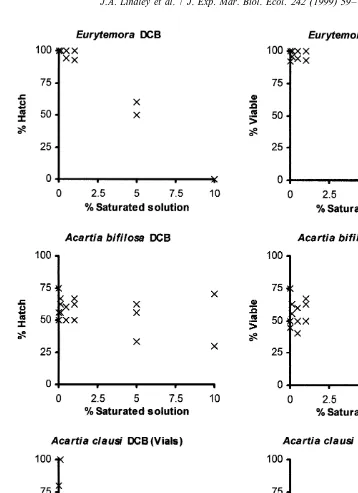
The experiments on a range of concentrations of toxicants and to determine interspecific variability were carried out on eggs from females collected between May and July 1996. The species, numbers of replicate experiments and the numbers of eggs are listed in Table 3 and the percentages of eggs which hatched and the percentages which yielded viable (alive when first examined) nauplii are illustrated in Figs. 1 and 2.
Eurytemora females were extracted from samples taken from the Exe in May
(temperature 11–138C, salinity 12–25‰). No Eurytemora affinis eggs hatched success-fully at a dilution of 10% of a saturated solution of DCB and only three hatched of a total of 111 eggs in PCP. Overall, 40% hatched in 5% PCP and 42% in 5% DCB. Solutions of 0.5% PCP or less and 1% DCB or less had little effect on hatching. The calculated EC50 values for hatching and LC50 values for viability are summarised in
21 21
Table 4. The EC50 values were equivalent to 291mg l or 1.1 mmol l PCP and 3.5
21 21
Fig. 1. Percentage hatch and percentage viable nauplii from eggs of Eurytemora affinis and Acartia bifilosa in a range of dilutions of initially saturated solutions of PCP.
21 21
calculated LC50 were equivalent to a maximum of 96mg l or 0.37mmol l PCP and
21 21
a maximum of 15.9 mg l or 74.1 mmol l DCB initial concentration. The hatching success of A. bifilosa did not differ significantly between 10% of a saturated solution or lower concentrations of both toxicants and filtered sea water but all dilutions of PCP reduced viability in comparison with filtered sea water and none survived in the 10%
21 21
solution. These data gave a calculated LC50 equivalent to 980mg l or 3.7mmol l . The effects on viability were not significant at 1% and below. At DCB concentrations of 10% dilution viability was greatly reduced in experiments in Petri dishes and no Acartia
clausi nauplii were viable in experiments in vials where loss of DCB through
J.A. Lindley et al. / J. Exp. Mar. Biol. Ecol. 242 (1999) 59 –74 67
Table 4
Eurytemora affinis, Acartia bifilosa and A. clausi. Calculated EC50and LC50levels (initial concentrations) for PCP and DCB solutions expressed as % saturation. Nt, no downward trend in the range 0–10%
Species Compound Vessel EC50 LC50
Eurytemora affinis PCP Petri dishes 2.1 0.7
DCB Petri dishes 3.2 1.7
Acartia bifilosa PCP Petri dishes nt 0.8
a
DCB Petri dishes nt 14.2
A. clausi DCB Vials nt 2.0
a
Extrapolated.
these experiments the viability of control nauplii of A. clausi was less than 50%. The
21 21
LC50 was equivalent to a maximum concentration of 2.1 mg l or 16.6mmol l . Some of the dead nauplii which hatched from eggs in the higher concentrations of PCP and DCB were malformed as Tester and Costlow (1981) found with nauplii hatched from eggs from Acartia tonsa that had been exposed to Diflubenzon (DFB, marketed as Dimlin). No deformed nauplii were alive when examined.
3.3. Experiments with eggs in sediment samples
The results of the incubations following treatments with DCB and the respective controls are shown in Fig. 3. DCB was rapidly lost, presumably mainly due to evaporation, and was not detectable in the samples after 1 day. No hatched nauplii were recovered from samples which had been incubated in the DCB solution at low temperature and in darkness for 1 day. Some nauplii were found in samples that had been incubated in initially saturated DCB solution for longer periods. This created the apparent anomaly of estimated mortality declining from 100% to lower levels at later
J.A. Lindley et al. / J. Exp. Mar. Biol. Ecol. 242 (1999) 59 –74 69
dates, but it should be noted that the value for each treatment duration interval was calculated from a separate set of treated and control samples. The differences between the treated and control samples in numbers of nauplii recovered were significant (t.6.00, P,0.001 except after 1 day, t54.51, P,0.01 and after 17 days t53.13,
P,0.05) on each occasion. The value of t for the results for samples treated for 6 days, when the number of nauplii recovered from the DCB-treated samples was greatest, was 7.75, the second highest value. All hatching occurred within 14 days of the start of incubation at 158C and within 28 days from collection of the samples.
4. Discussion
Exposure of the eggs of Eurytemora affinis and Acartia spp. to PCP and DCB reduced hatching success and viability of the hatched nauplii. The results of experiments to determine the effects at different concentrations indicated that the viability of nauplii was more sensitive than the developmental processes prior to hatching. The experiments including washing of the eggs and incubation in FSW after exposure to the PCB and DCB solutions indicated that the embryos can be damaged or accumulate and retain sufficient toxicant to have deleterious effects. Therefore, contamination of the eggs in sediment can inhibit recruitment even if the eggs are resuspended in relatively clean water.
The hatching of nauplii from sediment samples that had been washed and incubated after being maintained in initially saturated DCB solution for periods.1 day when none hatched in those washed after only 1 day may be due to volatility of DCB, stimulation of the eggs by more prolonged exposure to low temperature or a combination of the two factors. Although DCB was no longer detectable in solution after 1 day, some may have remained in the sediment or adsorbed onto the egg surface after the compound had ceased to be detectable in solution and subsequently damaged any nauplii that did attempt to hatch. As the DCB is not strongly hydrophobic such residues would have steadily dispersed into the water and evaporated, reducing in significance with increasing duration of treatment. It should be noted that numbers of nauplii hatching from the control samples reached a maximum in those removed from the cold (5–78C) dark conditions after 6 days. Naess (1991a) demonstrated that refrigeration can stimulate hatching in subsequent incubations of sediment, so this increase in hatching success may be a result of stimulation of the eggs by the length of time during which they were kept at the low storage temperature.
There are no accounts of the effects of DCB on zooplankton eggs prior to the work described here and the only previously described work on PCB in Lindley et al. (1998) is placed in the context of a wider range of experiments. There has been work on Cladocera and the copepodite stages of copepods. Hauch et al. (1980), in experiments on copepodites of the neritic calanoid Pseudodiaptomus coronatus, calculated the LC50 for
21 96 h exposure to sodium pentachlorophenate (the sodium salt of PCP) as 68 mg l . According to Le Blanc (1980), the LC50 for 48 h exposure to DCB of the freshwater
21
21
here. Calamari et al. (1983) gave an even lower value (0.78 mg l ) for 24 h exposure of the same species. The calculated EC50 and LC50 values for Acartia spp. were influenced by the comparatively low hatching success of the (FSW) controls. Due to the small numbers of eggs in most of the experiments with Acartia spp. and the low viability of the controls the results should be treated with caution.
The low viability was not necessarily a consequence of the experimental methods as Burkart and Kleppel (1998) recorded ,50% successful hatch from Acartia clausi eggs using a method for incubating eggs under field conditions. The use of full salinity sea water for estuarine species could potentially have had an effect on the percentage hatch and viability in these experiments. However, it should be noted that the hatching success of E. affinis, normally found in brackish waters, was higher than the values for A. clausi, an open sea species. Transferring the adult females from ambient conditions to the experimental temperatures and full salinity and temperature changes between different stages of experiments with eggs could potentially effect hatching and viability, but in each experiment the controls were treated in the same way as the exposed animals / eggs except for the absence of the toxicant. In all cases the actual manipulation of material took place at normal room temperature, and the small volumes in Petri dishes and beakers would have started to adjust to that level before transfer to the new medium. In the case of eggs in intertidal sediment samples, exposure to ambient atmospheric temperatures and twice daily inundation by sea water will expose them naturally to a wide range of rapidly changing temperatures. Other studies with resting eggs in sediment (e.g., Naess, 1991a,b; Viitasalo, 1992) have involved transfer from refrigerator to incubator involving increases of .108C.
The use of specimens from two areas may have been the source of differences in viability. The Tamar drains areas including igneous and metamorphosed rocks, which are a rich natural source of heavy metals and past exploitation of these has probably increased the load above natural background levels. In contrast, the area drained be the Exe is mostly non-metamorphosed sedimentary rocks. Also, Plymouth on the Tamar is a much larger source of industrial and urban contamination than exists in the Exe catchment. However, in all experiments the control specimens and specimens treated with the toxicants were from the same source.
Higher concentrations of DCB than of PCP were needed to have an effect on viability, but in terms of percentage saturation the results from the experiments with DCB in sealed vials, where the DCB content was maintained, the results for the two compounds were comparable. It is interesting to note that hatching from sediment samples was
21 reduced significantly after 2 days exposure to Rotenone concentrations of 0.5 mg l and
21
very few hatched after similar treatment at 5 mg l (Naess, 1991a,b). The LC50 values for PCP were lower, but it should be noted that after treatment with rotenone, the eggs were transferred to FSW, whereas in the present experiments with sediments the eggs were maintained in the original solutions.
J.A. Lindley et al. / J. Exp. Mar. Biol. Ecol. 242 (1999) 59 –74 71
and Labidocera aestiva hatched at lower oxygen concentrations than did newly laid eggs and that anoxia had less effect on fully developed eggs than on newly laid eggs of the former species. Initial exposure of eggs to the solutions may have occurred at differing stages of egg envelope development. Those that were still at an early stage may have been more vulnerable than those with completely formed envelopes, although as eggs were laid during a 16 h period prior to the start of the experiments, only a small proportion would be expected to have incompletely developed. Santella and Ianora (1990) described subitaneous eggs of Pontella mediterranea with a simple thin layer surrounding the plasma membrane as well as both subitaneous eggs and diapausing eggs with a complex four layered enveloping structure. This type of variation may explain the variable effect of temporary exposure. The more complex envelopes were presumably more resistant than the thin layer. There was no evidence for diapausing eggs amongst those obtained from adult females in the laboratory and used in the experiments described here.
Differences between Eurytemora affinis and Acartia spp. in the levels at which the DCB and PCB solutions reduced hatching or naupliar viability below that of the FSW controls indicated that Acartia spp. were more resistant. The 10% saturated solutions and lower concentrations did not reduce the percentage hatch of Acartia spp. and at least some nauplii were viable in the 5% saturated solutions, whereas hatching of E. affinis was very significantly reduced in 5% solutions of both compounds, no nauplii were viable at that level and a 1% solution of PCP significantly reduced percentage viability. Differences between species in vulnerability to contaminants have clear implications for effects of pollution on diversity and community structure. The cumulative effect with time of a lower concentration of PCP indicates that dormant eggs in sediments may be vulnerable to lower concentrations of toxicants than the subitaneous eggs developing rapidly in optimum conditions. Anderson et al. (1984) proposed that toxicity is a function of duration of exposure as well as of concentration. Marcus and Lutz (1998) demonstrated decreasing hatching success of subitaneous eggs of Centropages hamatus with increasing time of exposure to anoxia.
The concentrations of PCP and DCB shown to have an effect here are well above levels normally encountered in estuaries and coastal waters. For example, PCP level at
21
sites in the Mersey estuary in 1993 were 0.07–0.09 mg l , well below the UK
21
Environmental Quality Standard (EQS) of 2.0 mg l (National Rivers Authority, 1995c). The EQS is half the concentration shown here to have a cumulative effect on eggs in the sediment. However, it would be unusual for DCB or PCP to be the only contaminants in polluted areas, for example in addition to the PCP in the Mersey in
21
1993, there were also concentrations of up to 4.1 mg l of carbon tetrachloride and
21
.0.5 mg l of several other organochlorine compounds at several sites (National Rivers Authority, 1995c). In very polluted locations it is likely that saturated solutions of some organic contaminants can exist in the poorly flushed interstitial waters of fine sediments.
contaminants on oogenesis within the female or on the post-hatching developmental stages rather than on the egg itself. Similarly, egg viability was reduced due to exposure of the females of Acartia tonsa (Tester and Costlow, 1981) and Eurytemora affinis (Wright et al., 1996) to Diflubenzon. Thus the reproductive success of calanoids is vulnerable to contamination through all stages of the life cycle but effects on eggs in the sediment is likely to be particularly important due to accumulation of contaminants in sediments and long exposure times due to dormancy during periods when the pelagic population is absent or rare.
Acknowledgements
This work was funded under contract to the UK Department of the Environment as a contribution to its coordinated programme of research on the Irish Sea. It is part of the PML Strategic Research Programmes on coastal biodiversity and stress effects and health in the oceans. K.F. Uil worked on the project from March to July 1996. He was primarily responsible for the experiments to determine the effects of a range of concentrations of PCP and DCB which were carried out as a final year undergraduate project for the University of Wageningen.
References
Anderson, J.W., Keisser, J.M., McQuerry, D.L., Riley, R.G., Fleishmann, M.L., 1984. Toxicity testing with constant or decreasing concentrations of chemical dispersed oil. In: Allen, T.E. (Ed.), Oil Spill Chemical Dispersants: Research, Experience and Recommendations, ASTM Special Publication 840, American Society for Testing and Materials, Philadelphia, pp. 14–22.
Blades-Eckelbarger, P.I., Marcus, N.H., 1992. The origin of cortical vesicles and their role in egg envelope formation in the spiny eggs of a calanoid copepod, Centropages velificatus. Biol. Bull. 182, 41–53. Burkart, C.A., Kleppel, G.S., 1998. A new incubation system for the measurement of copepod egg production
and egg hatching success in the field. J. Exp. Mar. Biol. Ecol. 221, 89–97.
Calamari, D., Galassi, S., Setti, F., Veghi, M., 1983. Toxicity of selected chlorobenzenes to aquatic organisms. Chemosphere 12, 253–262.
Collins, N.R., Williams, R., 1981. Zooplankton of the Bristol Channel and Severn Estuary.The distribution of four copepods in relation to salinity. Mar. Biol. 64, 273–283.
Conway, D.V.P., McFadzen, I.R.B., Tranter, P.R.G., 1994. Digestion of copepod eggs by larval turbot Scophthalmus maximus and egg viability following gut passage. Mar. Ecol. Prog. Ser. 106, 303–309. Davies, J.M., Baird, I.E., Massie, L.C., Hay, S.J., Ward, A.P., 1980. Some effects of oil derived hydrocarbons
on a pelagic food web from observations in an enclosed ecosystem and a consideration of their implications ´
for monitoring. Rapp. P-v. Reun. Cons. Int. Explor. Mer 179, 201–211.
Ferguson, J., 1939. Use of chemical potentials as indices of toxicity. Proc. R. Soc. London (B) 127, 387–404. Flinkman, J., Vuorinnen, I., Christiansen, M., 1994. Calanoid copepod eggs survive passage through fish
digestive tracts. ICES J. Mar. Sci. 51, 127–129.
George, C.L., Lindley, J.A., 1997. Hatching of nauplii of calanoid copepods from intertidal estuarine sediments. J. Mar. Biol. Assoc. UK 77, 899–902.
Hauch, R.G., Norris, D.R., Pierce, R.H., 1980. Acute and chronic toxicity of sodium pentachlorophenate to the copepod Pseudodiaptomus coronatus. Bull. Environ. Contam. Toxicol. 25, 562–568.
J.A. Lindley et al. / J. Exp. Mar. Biol. Ecol. 242 (1999) 59 –74 73 Hoke, R.A., Geisy, J.P., Zabik, M., Unger, M., 1993. Toxicity of sediments and sediment pore waters from the Grand Calumet River–Indiana Harbour, Indiana area of concern. Ecotoxicol. Environ. Saf. 26, 86–112. Howard, P.H. (Ed.), 1991. Pesticides, Handbook of Environmental Fate and Exposure Data for Organic
Chemicals, Vol. III, Lewis, Michigan.
Le Blanc, G.A., 1980. Acute toxicity of priority pollutants to water flea (Daphnia magna). Bull. Environ. Contam. Toxicol. 25, 684–691.
Lindley, J.A., 1997. Eggs and their incubation as factors in the ecology of planktonic Crustacea. J. Crustac. Biol. 17, 569–576.
Lindley, J.A., George, C.L., Evans, S.V., Donkin, P., 1998. Viability of calanoid copepod eggs from intertidal sediments: a comparison of 3 estuaries. Mar. Ecol. Prog. Ser. 162, 183–190.
Long, E.R., Robertson, A., Wolfe, D.A., Hameedi, J., Sloane, G.M., 1996. Estimates of the spatial extent of sediment toxicity in major U.S. estuaries. Environ. Sci. Technol. 30, 3585–3592.
Lutz, R.V., Marcus, N.H., Chanton, J.P., 1994. Hatching and viability of eggs at two stages of embryological development: anoxic / hypoxic effect. Mar. Biol. 119, 199–204.
Mahler, H.R., Cordes, E.H., 1966. Biological Chemistry, Harper and Row, New York.
Marcus, N.H., 1996. Ecological and evolutionary significance of resting eggs in marine copepods: past, present and future studies. Hydrobiologia 320, 141–152.
Marcus, N.H., Lutz, R.V., 1998. Longevity of subitaneous and diapause eggs of Centropages hamatus (Copepoda: Calanoida) from the northern Gulf of Mexico. Mar. Biol. 131, 249–257.
Marcus, N.H., Lutz, R.V., Chanton, J.P., 1997. Impact of anoxia and sulphide on the viability of eggs of three planktonic copepods. Mar. Ecol. Prog. Ser. 146, 291–295.
Marine Pollution Monitoring Management Group, 1998. National Monitoring Programme. Survey of the quality of UK coastal waters. Marine Pollution Monitoring Management Group, Aberdeen.
Miller, M.M., Ghodbane, S., Wasik, S.P., Tewari, Y.B., Martire, D.E., 1984. Aqueous solubilities, octanol / water partition coefficients, and entropies of melting of chlorinated benzenes and biphenyls. J. Chem. Eng. Data 29, 184–190.
Naess, T., 1991a. Marine calanoid resting eggs in Norway: abundance and distribution of two copepod species in the sediment of an enclosed marine basin. Mar. Biol. 110, 261–266.
Naess, T., 1991b. Tolerance of marine calanoid resting eggs: effects of freezing, dessication and Rotenone exposure—a field and laboratory study. Mar. Biol. 111, 455–459.
National Rivers Authority (NRA), 1995a. Contaminants entering the sea. A report on contaminant loads entering the seas around England and Wales for the years 1990–1993. Water Quality Series No. 24. HMSO, London.
National Rivers Authority (NRA), 1995b. Pesticides in the aquatic environment. Water Quality Series No. 26. HMSO, London.
National Rivers Authority (NRA), 1995c. The Mersey Estuary; a report on environmental quality. HMSO, London.
Rogers, H.R., Crathorne, B., Leatherland, T.M., 1989a. Occurence of chlorobenzene isomers in the water column of a UK estuary. Mar. Pollut. Bull. 20, 276–281.
Rogers, H.R., Campbell, J.A., Crathorne, B., Dobbs, A.J., 1989b. The occurence of chlorobenzenes and permethrins in twelve U.K. sewage sludges. Water Res. 23, 913–921.
Santella, L., Ianora, A., 1990. Subitaneous and diapause eggs in Mediterranean populations of Pontella mediterranea (Copepoda: Calanoida): a morphological study. Mar. Biol. 105, 83–90.
Stearns, D.E., Forward, Jr. R.B., 1984. Photosensitivity of the calanoid copepod Acartia tonsa. Mar. Biol. 82, 85–89.
Tester, P.A., Costlow, Jr. J.D., 1981. Effect of insect growth regulator Dimlin (TH6040) on fecundity and egg viability of the marine copepod Acartia tonsa. Mar. Ecol. Prog. Ser. 5, 297–302.
Uye, S.-I., Yoshiya, M., Ueda, K., Kasahara, S., 1984. The effect of organic sea-bottom pollution on survivability of resting eggs of neritic calanoids. Crustaceana 7 (Suppl.), 390–403.
Van Wezel, A.P., De Vries, D.A.M., Kostense, S., Sijm, D.T.H.M., Opperhuizen, A., 1996. Intraspecific variarions in lethal body burdens of narcotic compounds. Aquat. Toxicol. 33, 325–342.
Viitasalo, M., 1992. Calanoid resting eggs in the Baltic Sea: implications for the population dynamics of Acartia bifilosa (Copepoda). Mar. Biol. 114, 387–405.
Wang, M.-J., Jones, K.C., 1994. The chlorobenzene content of contemporary U.K. sewage sludges. Chemosphere 28, 1201–1210.
Webb, D.G., Weaver, A.J., 1988. Predation and the evolution of free spawning in marine calanoid copepods. Oikos 51, 189–192.