www.elsevier.com / locate / bres
Research report
Acute thermal hyperalgesia elicited by low-dose morphine in normal
mice is blocked by ultra-low-dose naltrexone, unmasking potent opioid
analgesia
*
Stanley M. Crain , Ke-Fei Shen
Department of Neuroscience, Albert Einstein College of Medicine, Yeshiva University, 1300 Morris Park Avenue, Bronx, NY 10461, USA Accepted 19 September 2000
Abstract
Our previous electrophysiologic studies on nociceptive types of dorsal root ganglion (DRG) neurons in culture demonstrated that extremely low fM–nM concentrations of morphine and many other bimodally-acting mu, delta and kappa opioid agonists can elicit direct excitatory opioid receptor-mediated effects, whereas higher (mM) opioid concentrations evoked inhibitory effects. Cotreatment with pM naloxone or naltrexone (NTX) plus fM–nM morphine blocked the excitatory effects and unmasked potent inhibitory effects of these low opioid concentrations. In the present study, hot-water-immersion tail-flick antinociception assays at 528C on mice showed that extremely low doses of morphine (ca. 0.1mg / kg) can, in fact, elicit acute hyperalgesic effects, manifested by rapid onset of decreases in tail-flick latency for periods.3 h after drug administration. Cotreatment with ultra-low-dose NTX (ca. 1–100 pg / kg) blocks this opioid-induced hyperalgesia and unmasks potent opioid analgesia. The consonance of our in vitro and in vivo evidence indicates that doses of morphine far below those currently required for clinical treatment of pain may become effective when opioid hyperalgesic effects are blocked by coadministration of appropriately low doses of opioid antagonists. This low-dose-morphine cotreatment procedure should markedly attenuate morphine tolerance, dependence and other aversive side-effects. 2001 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Pain modulation: pharmacology
Keywords: Acute low-dose morphine hyperalgesia; Bimodally-acting opioid agonists; Tail-flick assay (528C); Ultra-low-dose naltrexone; Subanalgesic etorphine; Mouse strains 129 / SvEv / Tac vs. SW
1. Introduction extremely low doses (fM–nM) of morphine (or other mu, delta and kappa opioid agonists) [10,11]. Cotreatment with Our previous studies demonstrated that cotreatment of pM NLX or NTX plus fM–nM morphine unmasked potent mice with ultra-low doses of naltrexone (NTX) markedly inhibitory APD-shortening effects which required mM enhances the magnitude and duration of antinociceptive concentrations of morphine when applied alone. Further-effects of morphine and many other opioid agonists more, recent evidence indicates that opioid receptors can [10,34]. This cotreatment paradigm was guided by pharma- be interconverted rapidly between an inhibitory (Gi / Go-cologic studies on nociceptive types of mouse dorsal root coupled) and an excitatory (Gs-coupled) mode following ganglion (DRG) neurons in culture [7–12]. These in vitro physiological alterations in the concentration of a specific studies showed that low (pM) concentrations of naloxone cyclic AMP-dependent glycolipid, GM1 ganglioside, in the (NLX) or NTX can selectively antagonize high-efficacy neuronal cell membrane [11–13,36]. These studies of the excitatory opioid receptor-mediated effects [e.g., prolonga- bimodal excitatory / inhibitory actions of opioid agonists on tion of the action-potential duration (APD)] elicited by DRG neurons in vitro suggested that treatment with morphine in vivo may result not simply in analgesia, but also in anti-analgesic or hyperalgesic effects which would
*Corresponding author. Tel.: 11-718-430-2481; fax: 1
1-718-430-tend to mask the expected analgesia.
3381.
E-mail address: [email protected] (S.M. Crain). Hot-water-immersion tail-flick antinociception assays in
mice carried out at 558C revealed that morphine does, into which the animal voluntarily enters with no applica-indeed, elicit anti-analgesic excitatory effects as evidenced tion of any force. During the tail-flick assay only the by the marked increase in the analgesic potency of cylinder is handled without direct contact with the animal. moderate doses of morphine (0.1–3 mg / kg) following One third of the tail from the tip is immersed into a water cotreatment with ultra-low-dose NTX [10,34]. Progressive bath maintained at 528C (60.18C) with an electronic increases in the magnitude and duration of analgesia were thermoregulator (Yellow Springs). The latency to a rapid observed in these assays as the dose of morphine alone tail-flick was recorded; mice with control latencies .8 s was increased from 0.1 mg / kg to 10 mg / kg (s.c.) [10,34]. were excluded from these tests and a 10 s cut-off was used However, tail-flick latencies of mice treated with much to minimize tissue damage. Six sequential control tests lower doses of morphine (ca.1 mg / kg) did not differ were made, each with a 10 min interval. The latencies of significantly from control mice. Because the average the last four tests were averaged to provide a predrug control tail-flick latencies were only about 2 s, we realized value. Time–effect curves were plotted using tail-flick that possible hyperalgesic effects of low doses of morphine latencies as the ordinate (see also [13]).
would be difficult to quantitate with this type of assay. To determine if low doses of morphine elicit hyperalgesic
2.2. Animal test groups effects in mice analogous to the excitatory effects evoked
in DRG neurons by ,nM opioid concentrations, we
Comparative tests were generally carried out on the decreased the temperature of the hot-water bath from 558C
same day with two or more groups of eight mice receiving to 528C.
a specific cotreatment plus an appropriate control group of Tail-flick assays at 528C showed that 1000-fold lower
eight mice treated with morphine alone. All animal test doses of morphine can, indeed, elicit acute hyperalgesic
groups were used for only one assay, except in chronic effects, manifested by rapid onset of opioid-induced
de-drug treatment tests. creases in tail-flick latency for periods .3 h after drug
administration. Cotreatment with ultra-low-dose NTX blocks this opioid-induced hyperalgesia and unmasks
2.3. Statistical analyses potent opioid analgesic effects. These in vivo results are
remarkably consonant with the results of our in vitro
Differences between treatment groups were examined studies of the acute excitatory effects of low-dose
for statistical significance by means of ANOVA with morphine6ultra-low-dose NTX on DRG neurons [10]. The
Neuman–Keuls’ tests or by means of Student’s t-test [35]. present evidence indicates that doses of morphine far
below those currently required for clinical treatment of
pain may become effective when opioid hyperalgesic 2.4. Materials effects are blocked by co-administration of appropriately
low doses of NTX or other opioid antagonists. This low- The following drugs were used: NTX, morphine dose-morphine plus NTX cotreatment procedure should (Sigma); etorphine (gift from Dr E.J. Simon).
markedly attenuate morphine tolerance, dependence and other aversive side-effects.
3. Results 2. Materials and methods
3.1. Low doses of morphine elicit acute thermal 2.1. Antinociception assays in mice hyperalgesic effects in normal mice
dosing. These delayed ‘hyperalgesic’ effects were not detected in our previous assays at 558C [34].
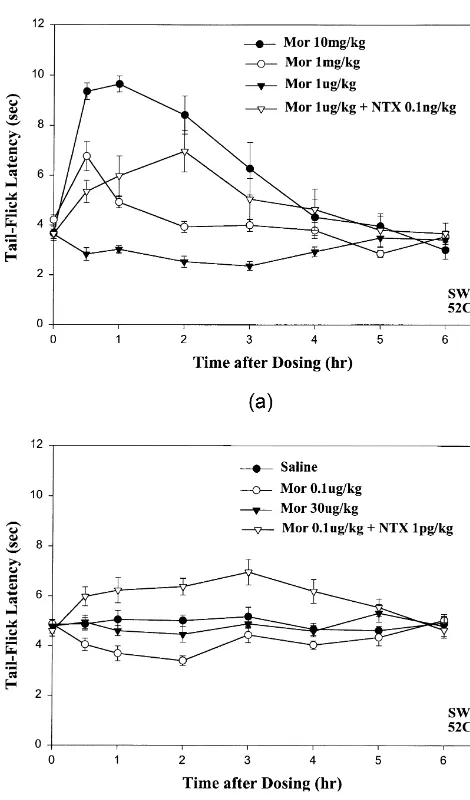
Administration of 1000 to 10,000-fold lower doses of morphine, e.g., 0.1 and 1 mg / kg (s.c.), resulted in rapid onset of decreases in tail-flick latencies (ca. 1–2 s) which lasted for.3 h after drug injection (Fig. 1A: .; Fig. 1B: s). Preliminary assays indicate that doses of morphine as
low as 1–10 ng / kg can elicit significant decreases in tail-flick latencies, but more systematic studies are required to characterize the dose–response properties of the high-efficacy excitatory opioid receptor system mediating these hyperalgesic effects. Interestingly, whereas 100 mg / kg morphine elicited analgesia (slightly weaker than 1 mg / kg morphine: Fig. 1A; time–effect curve not shown for simplicity) and 1mg / kg morphine resulted in hyperalgesia, injection of an intermediate dose of morphine, 30 mg / kg showed no significant difference from saline (Fig. 1B: .). The latter result suggests that about 30 mg / kg morphine elicits equipotent inhibitory / analgesic and excitatory / hy-peralgesic effects that effectively neutralize opioid modula-tion of nocicepmodula-tion. In other groups of mice, however, the dose of morphine that elicited equipotent analgesic / hy-peralgesic effects appeared to be closer to 5–10 mg / kg (see Section 4.1). In some groups of mice, especially where the mean baseline tail-flick latencies were about 5–6 s (e.g., Fig. 2A), the decreases in tail-flick latencies elicited by 1mg / kg morphine were larger in magnitude (ca. 2–3 s) and the hyperalgesic effects lasted .6 h. By contrast, in previous assays at 558C, no significant alterations in tail-flick latency were detected during tests carried out for 4 h after injection of 1 mg / kg morphine [34].
Control assays were carried out in order to evaluate whether the observed low-dose morphine-induced hy-peralgesia might be inadvertently exaggerated by the repeated hot-water exposures used in our routine assay
Fig. 1. Hyperalgesia elicited by low-dose morphine is blocked by procedure. Groups of mice were injected with 1 mg / kg
cotreatment with ultra-low-dose naltrexone (NTX) unmasking potent morphine and the initial tail-flick test was delayed until 1, opioid analgesia. (A) Time-effect curves show results of hot-water 2 or 3 h after drug administration. Characteristic decreases (528C)-immersion tail-flick tests after injection of morphine (s.c.) at 1
in tail-flick latency (ca. 1–2 s) still occurred in all three test mg / kg (.), 1 mg / kg (s) and 10 mg / kg (d). Note marked increase in
groups and all showed return to baseline latencies when
magnitude and duration of antinociception after treatment with 10 mg / kg
(d) vs. 1 mg / kg (s) (similar to dose-dependent effects of morphine in retested 3 h after the initial test.
previous assays using 558C water bath [31]. By contrast, note significant
onset of hyperalgesic effects (manifested by decreases in tail-flick 3.2. Ultra-low-dose NTX blocks low-dose-morphine-latency) within 30 min after injection of 1mg / kg morphine (.) (not
induced hyperalgesia and unmasks potent opioid detected in previous tests at 558C [34]). Furthermore, cotreatment of a 4th
analgesia group of mice with 0.1 ng / kg NTX blocks 1mg / kg morphine-induced
hyperalgesia and unmasks a remarkable degree of opioid analgesia
comparable to that elicited by a 1000-fold higher dose of morphine alone Cotreatment with 1mg / kg morphine plus a 10,000-fold (s). (B) Cotreatment with 1 pg / kg NTX unmasks the potent
antinocicep-lower dose of NTX, 0.1 ng / kg resulted in a remarkable
tive effects of a still lower dose of morphine, 0.1mg / kg (,), whereas the
conversion of the hyperalgesic effects elicited by this low
potential analgesic effect of a much higher dose of morphine alone, 30
dose of morphine alone (Fig. 1A: .) to a significant mg / kg is almost completely masked (.) by concomitant excitatory
opioid receptor-mediated hyperalgesia. A control tail-flick assay carried degree of analgesia which lasted for .3 h after dosing
out without drug injection (d) shows that sequential tail-immersion in a (Fig. 1A: ,). The antinociceptive effect of cotreatment
528C water bath does not result in significant alterations in baseline with 1 mg / kg morphine plus 0.1 ng / kg NTX is compar-tail-flick latency, in contrast to the typical hyperalgesic effects that are
able in magnitude and much longer in duration than that
elicited after injection of a low dose of morphine, 0.1mg / kg (s) or 1
elicited by a 1,000-fold higher dose of morphine alone mg / kg morphine (A:.). Note: in this and all subsequent figures, n58 for
blocked by cotreatment with ultra-low dose NTX, unmask-ing potent opioid analgesia ([14] and Crain and Shen, in preparation).
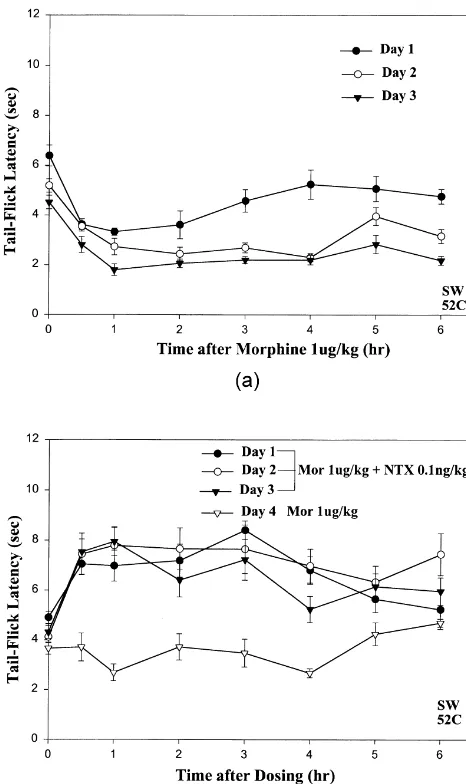
3.3. Acute hyperalgesic effects elicited by low doses of morphine do not show desensitization during chronic opioid treatment
Daily injections of low 1mg / kg doses of morphine for 3 days resulted in maintenance of similar or slightly larger acute hyperalgesic effects (Fig. 2A), whereas daily in-jections of high doses of morphine result in progressive decreases in analgesia, i.e., tolerance [10,13,34]. Interest-ingly, the baseline tail-flick latency showed sequential decreases on the 2nd and 3rd days (Fig. 2A). Furthermore, chronic cotreatment of mice with daily injections of 1
mg / kg morphine plus 0.1 ng / kg NTX for 3 days resulted in sustained block of hyperalgesia and maintenance of prominent analgesic effects (Fig. 2B: d, s and .) [in contrast to the marked tolerance observed after three daily doses of 4 mg / kg morphine alone ([13]: Fig. 3)]. Acute low-dose morphine-induced hyperalgesia reappeared when the NTX was deleted on the 4th day of drug treatment (Fig. 2B:,).
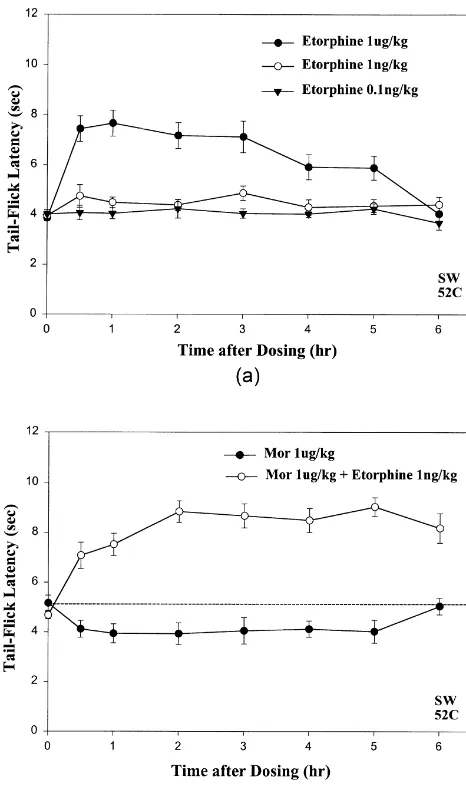
3.4. In contrast to morphine low subanalgesic doses of etorphine do not elicit hyperalgesic effects in mice
Administration of the super-potent opioid analgesic, etorphine [3], at 1mg / kg resulted in marked antinocicep-tion (Fig. 3A:d) comparable to the effects of.1,000-fold higher doses of morphine (Fig. 1A, B). However, subanalgesic doses of etorphine (ca.1 pg / kg–1 ng / kg) did
Fig. 2. Acute hyperalgesic effects elicited by daily injections of low
not elicit hyperalgesic effects (Fig. 3A:s,.; see Section doses (1mg / kg) of morphine do not show desensitization during chronic
4.1), in contrast to the prominent hyperalgesia evoked by
treatment, whereas sustained analgesia during chronic cotreatment with
ultra-low-dose NTX results in acute tolerance after withdrawal of NTX. subanalgesic doses of morphine (Fig. 1). The antinocicep-(A) Daily injections of low 1mg / kg doses of morphine for three days tive effects of 1mg / kg morphine that were unmasked by result in maintenance of similar or slightly larger acute hyperalgesic
cotreatment with 1 ng / kg etorphine were even larger in
effects. Note the sequential decreases in the baseline tail-flick latency on
magnitude and longer-lasting than the antinociception
successive days. (B) Chronic cotreatment of mice with daily injections of
evoked by a 1,000-fold higher dose of etorphine alone (cf.
1mg / kg morphine plus 0.1 ng / kg NTX for 3 days results in maintenance
of prominent analgesic effects, as shown by the time–effect curves on Fig. 3B:s vs. Fig. 3A:d).
days 1, 2 and 3. By contrast, injection of 1mg / kg morphine without NTX Interestingly, in a comparative test carried out on three on day 4 results in almost complete loss of analgesia and occurrence of
additional groups of mice, the magnitude and the duration
small hyperalgesic effects.
of the antinociceptive effects elicited by cotreatment with 1
mg / kg morphine plus 0.1 ng / kg NTX were remarkably similar to those elicited by 1mg / kg etorphine (Fig. 4).
a 10-fold lower dose of morphine (0.1 mg / kg) was
blocked by a much lower dose of NTX (1 pg / kg), 3.5. Low doses of morphine elicit analgesia rather than unmasking prominent opioid analgesic effects (Fig. 1B: hyperalgesia in 129 /SvEvTac vs. SW mice
,).
Fig. 4. Antinociception elicited in mice by cotreatment with a low, hyperalgesic dose of morphine (d) plus ultra-low-dose NTX (s) is comparable to that of the super-potent analgesic, etorphine (.).
mice with 1 mg / kg morphine plus 0.1 ng / kg NTX attenuated morphine’s antinociceptive effects (Fig. 5: s), in sharp contrast to the marked enhancement by low-dose NTX of morphine analgesia in SW mice (Fig. 1: ,).
Fig. 3. Low subanalgesic doses of etorphine do not elicit hyperalgesic effects in mice and they block low-dose morphine-induced hyperalgesia. (A) The super-potent opioid analgesic, etorphine elicits prominent analge-sic effects at 1mg / kg. Lower subanalgesic doses of etorphine, 0.1–1 ng / kg do not elicit significant hyperalgesic effects (nor did 1 pg / kg etorphine; data not shown), in sharp contrast to the hyperalgesia observed after low doses of morphine (Fig. 1:s). (B) Hyperalgesia elicited by low-dose morphine (1mg / kg) is blocked by cotreatment with ultra-low-dose etorphine (1 ng / kg) (s) unmasking potent opioid analgesia (d), as occurs after cotreatment with ultra-low-dose NTX. Note: dashed line has been inserted in B and the following figures to facilitate visualization of
the hyperalgesic effects (cf. saline control in Fig. 1B:d). Fig. 5. Low doses of morphine (1mg / kg) elicit analgesia in the 129 / SvEvTac strain of mice (d), in contrast to the hyperalgesia elicited in
SW mice (cf. Fig. 1:.). Furthermore, cotreatment of 129 / SvEvTac mice
with 1 mg / kg morphine plus 0.1 ng / kg NTX does not enhance, but instead attenuates, the antinociceptive effects of morphine (s), in contrast Administration of a low 1 mg / kg dose of morphine to the marked low-dose-NTX enhancement of morphine analgesia in SW resulted in analgesic effects in 129 / SvEvTac mice in mice (Fig. 1:,; see text). Note also the unusually potent antinociceptive contrast to the hyperalgesia elicited in SW mice (cf. Fig. 5: effects of 1mg / kg morphine alone in 129 / SvEv mice (.), which are
greater than the effects of 1 mg / kg morphine in SW mice (Fig. 1:s) and d vs. Fig. 1: .). In these assays at 528C, morphine
comparable to the effects of cotreatment of 1mg / kg morphine plus 0.1
showed remarkably potent antinociceptive effects in 129 /
4. Discussion studies suggest that two other agents can attenuate acute opioid hyperalgesia by interfering either with GM1 gan-4.1. Acute hyperalgesia elicited by low-dose morphine glioside-regulation or Gs-coupling of excitatory opioid may be mediated by activation of high-efficacy excitatory receptor functions. (1) Cotreatment of mice with low doses opioid receptor functions of cholera toxin-B subunit (ca. 10 mg / kg, i.p.), which binds selectively to a putative allosteric GM1 regulatory The present study demonstrates that extremely low site on excitatory opioid receptors [12,30,31,37], blocks doses of morphine, ,1 mg / kg, can elicit acute thermal opioid-induced hyperalgesia and unmasks potent opioid hyperalgesia in normal, naive mice. Acute low-dose mor- analgesia (Shen and Crain, in preparation), comparable to phine-induced hyperalgesia appears to be mediated by the effects of ultra-low-dose NTX. (2) Downregulation of selective activation of excitatory opioid receptor functions the Gsa regulatory protein by intrathecal injection of because it can be blocked by ultra-low-dose NTX. The antisense oligonucleotides in mice also blocks low-dose mechanism underlying the efficacy of extremely low doses morphine-induced hyperalgesia [15] (see also Section 4.2). of NTX in selectively antagonizing excitatory opioid Furthermore, the maintenance of prominent hyperalgesic receptor-mediated hyperalgesia is unknown. We have responses to 1mg / kg morphine during daily injections for suggested that NTX may have higher binding affinity for 3 days (Fig. 2A) is consonant with evidence that chronic excitatory vs. inhibitory opioid receptors following con- opioid treatment of DRG neurons in culture results in formational changes induced by GM1 ganglioside in the progressive sensitization of excitatory opioid receptor Gs-coupled receptor [11,12]. functions [7,11,31]. This is in contrast to characteristic The present demonstration that morphine can elicit desensitization of inhibitory opioid receptors [1,5,19] and hyperalgesic effects at doses.1,000-fold lower than those most other G protein-coupled receptors during sustained required to elicit analgesic effects provides strong support agonist exposure (see review in [11]).
for our hypothesis that nociceptive neurons contain a small We have carried out preliminary assays with NMDA fraction of Gs-coupled excitatory opioid receptors which receptor antagonists which show that these agents can also have much higher efficacy than the more abundant fraction block acute low-dose morphine-induced hyperalgesia. Our of Gi / Go-coupled inhibitory opioid receptors [11]. Fur- analyses suggest that the acute hyperalgesic effects of thermore, cotreatment of mice with 1 mg / kg morphine morphine, as well as other mu and specific kappa opioid plus ultra-low-dose NTX (0.1 ng / kg) results in a remark- agonists, can be most clearly accounted for by a primary able enhancement of morphine’s antinociceptive potency action on excitatory Gs-coupled, GM1-regulated opioid (Fig. 4). These in vivo results are consonant with our receptor functions which are modulated by complex inter-studies showing that cotreatment of mouse DRG neurons actions with NMDA- and related excitatory amino acid-in culture with very low (pM) concentrations of naloxone receptor functions (Crain and Shen, in preparation). Simi-or NTX selectively antagonizes excitatSimi-ory, Gs-coupled lar issues have been discussed by Crain and Shen [13] opioid receptor-mediated (‘hyperalgesic’) effects elicited regarding ‘‘possible interrelationships between excitatory by very low concentrations (fM–nM) of morphine and Gs-coupled opioid receptor functions and excitatory unmasks potent inhibitory, Gi / Go-coupled opioid receptor- NMDA-receptor functions that may account for the mediated (‘analgesic’) effects of these low morphine anomalous effects of opioids on 129 / SvEv mice’’. concentrations [10].
The absence of acute hyperalgesia after administration 4.2. Related studies of acute opioid-induced of etorphine at doses .1,000-fold lower than analgesic hyperalgesia
levels (1 pg / kg–1 ng / kg) (Fig. 3A) is consistent with our
Two brief earlier reports also indicated that low doses of morphine (Fig. 1:d,s) is in good agreement with recent morphine or other opioid agonists can elicit acute hy- reports that administration of high doses of heroin (2.5 peralgesia in normal, naive animals. Kiyatkin [22] showed mg / kg) or fentanyl (0.1–0.4 mg / kg) in rats results in that a relatively low dose of morphine (0.2 mg / kg) elicited long-lasting hyperalgesia, for hours or even several days significant hyperalgesic effects in freely moving (but not in after the initial 2–5 h analgesia [6,24]. Furthermore, many restrained) rats assayed by decreases in the vocalization studies have shown that chronic treatment of rodents with threshold in response to a painful electric stimulus to the high doses of opioid agonists results in the development of tail [22]. This unexpected decrease in vocalization thres- hyperalgesia in association with tolerance to opioid analge-hold lasted for 3 h after drug administration. By contrast, sic effects (e.g., [25,27,28]). All of these hyperalgesic higher doses of morphine (0.6–6 mg / kg) resulted in dose- effects that develop progressively after initial high-dose dependent increases in pain threshold. Furthermore, Apfel opioid analgesia may involve much more complex mecha-et al. [2] demonstrated that a remarkably low dose of the nisms than those that underlie the acute low-dose mor-kappa opioid peptide, dynorphin (0.5mg / kg, s.c.) injected phine-induced hyperalgesia demonstrated in the present into normal, naive mice resulted in thermal hyperalgesia study. Further work is required to determine the degree to measured by a significant decrease in tail-flick latency at which sustained activation of excitatory Gs-coupled opioid 90 min after administration, whereas a 100-fold higher receptor functions may mediate some of these complex dose (50 mg / kg) elicited analgesia. tolerance and hyperalgesia effects during chronic opioid Interestingly, in an animal model of persistent pain exposure [7,11,13,31], in addition to the significant roles of (arthritic rats) Kayser et al. [21] found that ‘‘exceedingly excitatory NMDA-receptor functions [25–28].
low doses of morphine [3–10 mg / kg, i.v.] . . . elicit a
naloxone-reversible paradoxical hyperalgesia [whereas in- 4.4. Clinical implications creased doses are] highly effective in producing
analge-sia’’. However, no significant modification of the pain The results of the present study suggest that the clinical threshold (measured by the vocalization threshold induced use of high mg / kg doses of morphine to inhibit pain are by mechanical paw pressure) was detected in normal rats required merely to overcome concomitant activation of treated with these low doses of morphine [21]. The low- excitatory opioid receptor-mediated hyperalgesic effects. dose morphine-induced hyperalgesia observed in the pres- Selective blockade of the latter effects by cotreatment with ent study is also consonant with single-unit recordings an ultra-low-dose of an opioid antagonist may result in from dorsal-horn neurons in normal rats showing that significant analgesia utilizing remarkably lower mg / kg application of low concentrations of mu- or kappa-opioid doses of morphine. This novel mode of pain treatment may receptor agonists to the spinal cord produced facilitation of markedly reduce tolerance, dependence and other aversive C-fiber-evoked nociceptive responses, whereas higher con- side-effects of morphine and similar bimodally-acting centrations resulted in inhibition [23]. opioid analgesics that often require .1,000-fold doses Paradoxical hyperalgesia has also been observed within higher when administered alone [29]. The feasibility of 1 h after i.v. injection of relatively low doses of the kappa treatment of some types of pain with such a low mg / kg opioid agonists, nalbuphine (5 mg) or butorphanol (2 mg) dose of morphine plus ultra-low-dose NTX (as suggested following tooth-extraction surgery in male patients [16,17]. by the data in Fig. 4) is supported by the potent analgesic This result is in contrast to moderate analgesia elicited by effects elicited by 1 mg / kg etorphine in chronic pain similar opioid doses in female patients. Furthermore, Gear patients [4] (see review [9,33,38]).
et al. [18] recently demonstrated that this low-dose kappa opioid-induced hyperalgesia is blocked by cotreatment with 0.5 mg naloxone, unmasking prominent opioid
anal-Acknowledgements
gesia. Our preclinical studies suggest that low doses of these bimodally-acting kappa opioid agonists may
pref-This study was supported by a research grant from Pain erentially activate excitatory vs. inhibitory opioid receptor
Therapeutics Inc., CA. functions in pain patients (as occurs with low
concen-trations of dynorphin on DRG neurons [7,11,32] and low doses of dynorphin in mice [2]), thereby resulting in acute
hyperalgesia similar to that elicited in mice by low doses References
of the mu opioid agonist, morphine in the present study.
[1] G.K. Aghajanian, Tolerance of locus coeruleus neurons to morphine and suppression of withdrawal response by clonidine, Nature 276
4.3. Opioid hyperalgesia after acute or chronic
(1978) 186–188. treatment with high doses of opioid agonists
[2] S.C. Apfel, M. Newel, C. Dormia, J.A. Kessler, Kappa opioid receptors participate in nerve growth factor-induced hyperalgesia,
The delayed onset of hyperalgesia observed in the Neuroscience 68 (1995) 1199–1206.
etorphine hydrochloride (M99): a potent morphine-like agent, Brit. [21] V. Kayser, J.M. Besson, G. Guilbaud, Paradoxical hyperalgesic J. Pharmacol. Chemother. 30 (1967) 11–22. effect of exceedingly low doses of systemic morphine in an animal [4] G.F. Blane, D.S. Robbie, Trial of etorphine hydrochloride (M99 model of persistent pain (Freund’s adjuvant-induced arthritis rats),
Reckitt) in carcinoma pain, preliminary report, Proc. Brit. Phar- Brain Res. 414 (1987) 155–157.
macol. Soc. 20 (1970) 252P–253P. [22] E.A. Kiyatkin, Morphine: some puzzles of well-known substance, [5] L.M. Bohn, R.J. Lefkowitz, R.R. Gainetdinov, K. Peppel, M.G. Int. J. Neurosci. 45 (1989) 231–246.
Caron, F.-T. Lin, Enhanced morphine analgesia in mice lacking [23] R.J. Knox, A.H. Dickenson, Effects of selective and non-selective
b-arrestin 2, Science 286 (1999) 2495–2498. kappa-opioid receptor agonists on cutaneous C-fibevoked re-[6] E. Celerier, C. Rivat, Y. Jun, J.-P. Laulin, A. Larcher, P. Reynier, G. sponses of rat dorsal horn neurones, Brain Res. 415 (1987) 21–29. Simonnet, Long-lasting hyperalgesia induced by fentanyl in rats, [24] J.P. Laulin, A. Larcher, E. Celerier, M. Le Moal, G. Simonnet, Anesthesiology 92 (2000) 465–472. Long-lasting increased pain sensitivity in rat following exposure to [7] S.M. Crain, K.-F. Shen, After chronic opioid exposure sensory heroin for the first time, Eur. J. Neurosci. 10 (1998) 782–785.
neurons become supersensitive to the excitatory effects of opioid [25] J.P. Laulin, E. Celerier, A. Larcher, M. Le Moal, G. Simonnet, agonists and antagonists as occurs after acute elevation of GM1 Opiate tolerance to daily heroin administration: an apparent phe-ganglioside, Brain Res. 575 (1992) 13–24. nomenon associated with enhanced pain sensitivity, Neuroscience [8] S.M. Crain, K.-F. Shen, After GM1 ganglioside treatment of sensory 89 (1999) 631–636.
neurons naloxone paradoxically prolongs the action potential but [26] B.H. Manning, J. Mao, H. Frenk, D.D. Price, D.J. Mayer, Continu-still antagonizes opioid inhibition, J. Pharmacol. Exp. Ther. 260 ous co-administration of dextromethorphan or MK-801 with mor-(1992) 182–186. phine: attenuation of morphine dependence and naloxone-reversible [9] S.M. Shen, K.-F. Crain, Etorphine elicits unique inhibitory-agonist attenuation of morphine tolerance, Pain 67 (1996) 79–88.
and excitatory-antagonist actions at opioid receptors on sensory [27] J. Mao, D.D. Price, D.J. Mayer, Thermal hyperalgesia in association neurons: new rationale for improved clinical analgesia and treatment with the development of morphine tolerance in rats: roles of of opiate addiction, in: R.S. Repaka, H. Sorer (Eds.), Discovery of excitatory amino acid receptors and protein kinase C, J. Neurosci. 14 Novel Opioid Medications, NIDA Monograph, U.S. Govt. Print. (1994) 2301–2312.
Office, Washington, DC, 1995, pp. 234–268. [28] J. Mao, D.D. Price, D.J. Mayer, Mechanisms of hyperalgesia and [10] S.M. Crain, K.-F. Shen, Ultra-low concentrations of naloxone morphine tolerance: a current view of their possible interactions,
selectively antagonize excitatory effects of morphine on sensory Pain 62 (1995) 259–274.
neurons, thereby increasing its antinociceptive potency and attenuat- [29] R.K. Portenoy, R. Payne, Acute and chronic pain, in: J.H. Lowinson, ing tolerance / dependence during chronic cotreatment, Proc. Natl. P. Ruiz, R.B. Millman, J.G. Langrod (Eds.), Substance Abuse: A Acad. Sci. USA 92 (1995) 10540–10544. Comprehensive Textbook, 3rd Edition, Williams and Wilkins, [11] S.M. Crain, K.-F. Shen, Modulation of opioid analgesia, tolerance Baltimore, 1997, pp. 563–589.
and dependence by Gs-coupled, GM1 ganglioside-regulated opioid [30] K.-F. Shen, S.M. Crain, Cholera toxin-B subunit blocks excitatory receptor functions, Trends Pharmacol. Sci. 19 (1998) 358–365. effects of opioids on sensory neuron action potentials indicating that [12] S.M. Crain, K.-F. Shen, GM1 ganglioside-induced modulation of GM1 ganglioside may regulate Gs-linked opioid receptor functions,
opioid receptor-mediated functions, Ann. N.Y. Acad. Sci. 845 (1998) Brain Res. 531 (1990) 1–7.
106–125. [31] K.-F. Shen, S.M. Crain, Chronic selective activation of excitatory [13] S.M. Crain, K.-F. Shen, Enhanced analgesic potency and reduced opioid receptor functions in sensory neurons results in opioid
tolerance of morphine in 129 / SvEv mice: evidence for a deficiency ‘dependence’ without tolerance, Brain Res. 597 (1992) 74–83. in GM1 ganglioside-regulated excitatory opioid receptor functions, [32] K.-F. Shen, S.M. Crain, Nerve growth factor rapidly prolongs the Brain Res. 856 (2000) 227–235. action potential of mature sensory neurons in culture and this effect [14] S.M. Crain, K.-F. Shen, Acute thermal hyperalgesia elicited by low requires activation of Gs-coupled excitatory kappa opioid receptors
doses of morphine or tramadol in normal mice is blocked by on these cells, J. Neurosci. 14 (1994) 5570–5579.
ultra-low-dose naltrexone, unmasking potent opioid analgesia, [33] K.-F. Shen, S.M. Crain, Antagonists at excitatory opioid receptors Amer. Pain Soc. Abstr., 2000, p. 217. on sensory neurons in culture increase potency and specificity of [15] R.A. Cruciani, G.W. Pasternak, Blockade of hyperalgesia to mor- opiate analgesics and attenuate development of tolerance /
depen-phine by antisense oligodeoxynucleotides to G , in: Internationalsa dence, Brain Res. 636 (1994) 286–297.
Narcot. Res. Conf. Abstr., 2000, p. 59. [34] K.-F. Shen, S.M. Crain, Ultra-low doses of naltrexone or etorphine [16] R.W. Gear, C. Miaskowski, N.C. Gordon, S.M. Paul, P.H. Heller, increase morphine’s antinociceptive potency and attenuate
toler-J.D. Levine, Kappa-opioids produce significantly greater analgesia ance / dependence in mice, Brain Res. 757 (1997) 176–190. in women than in men, Nature Med. 2 (1996) 1248–1250. [35] R.J. Tallarida, R.B. Murray, Manual of Pharmacologic Calculations [17] R.W. Gear, C. Miaskowski, N.C. Gordon, S.M. Paul, P.H. Heller, With Computer Programs, 2nd Edition, Springer–Verlag, New York,
J.D. Levine, The kappa opioid nalbuphine produces gender- and 1986.
dose-dependent analgesia and antianalgesia in patients with post- [36] G. Wu, Z.-H. Lu, R.W. Ledeen, Interaction ofd-opioid receptor with operative pain, Pain 83 (1999) 339–345. GM1 ganglioside: conversion from inhibitory to excitatory mode, [18] R.W. Gear, C. Miaskowski, N.C. Gordon, S.M. Paul, P.H. Heller, Molec. Brain Res. 44 (1997) 341–346.
J.D. Levine, Action of naloxone on gender-dependent analgesic and [37] G. Wu, Z.-H. Lu, T.J. Wei, R.D. Howells, K. Christoffers, R.W. antianalgesic effects of nalbuphine in humans, J. Pain 1 (2000) Ledeen, The role of GM1 ganglioside in regulating excitatory opioid
122–127. effects, Ann. N.Y. Acad. Sci. 845 (1998) 161–175.
[19] G.C. Harris, J.T. Williams, Transient homologousm-opioid receptor [38] S.M. Crain, K.-F. Shen, Antagonists of excitatory opioid receptor desensitization in rat locus coeruleus neurons, J. Neurosci. 11 functions enhance morphine’s analgesic potency and attenuate (1991) 7025–7029. tolerance / dependence liability, Pain 84 (2000) 121–131.
[20] J.S. Heyman, S.A. Mulvaney, H.I. Mosberg, F. Porreca, Opioidd