www.elsevier.com / locate / bres
Research report
a
-Synuclein-positive structures in cases with sporadic Alzheimer’s
disease: morphology and its relationship to tau aggregation
a a,b ,
*
c c cYasushi Arai , Mineo Yamazaki
, Osamu Mori , Hiromi Muramatsu , Goro Asano ,
a
Yasuo Katayama
a
The Second Department of Internal Medicine, Nippon Medical School, 1-1-5 Sendagi, Bunkyo-ku, Tokyo 113-8602, Japan
b
Department of Neurology, Hatsuishi Hospital, Chiba, Japan
c
The Second Department of Pathology, Nippon Medical School, Tokyo, Japan
Accepted 3 October 2000
Abstract
Alzheimer’s disease (AD) and Parkinson’s disease share common clinical and pathological features. In this study, we examined the relationship between AD pathology anda-synuclein aggregation. The frequency and distribution ofa-synuclein-positive structures were systematically investigated in 27 cases with sporadic AD bya-synuclein immuno-histochemistry. Thirteen (48.2%) of 27 cases had variousa-synuclein-positive structures as well as Lewy bodies. The frequency and density of senile plaques and neurofibrillary tangles were not significantly different between cases witha-synuclein structures and those without.a-Synuclein-positive structures were found most frequently in the amygdala. The a-synuclein-positive inclusions that are different from Lewy bodies were observed at the highest rate in the hippocampus. The discovery of a-synuclein as the constituent of Lewy bodies facilitated the detection of Lewy-related structures even in AD cases with widespread and numerous neurofibrillary tangles. a-Synuclein-positive inclusions except for Lewy bodies are exposed, and the distribution of them indicates that Lewy body formation may be influenced by the degree of tau aggregation. This study also supports the suggestion that cases with AD pathology can be classified into two groups according to the existence or absence of a-synuclein aggregation. 2001 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Degenerative disease: Alzheimer’s – other
Keywords: a-Synuclein; Alzheimer’s disease; Dementia with Lewy bodies; Lewy bodies
1. Introduction having the above histological features were considered to
be a new clinicopathological entity, termed dementia with
There is a significant overlapping in clinical and Lewy bodies (DLB) [21]. At present, DLB has been
pathological features between Alzheimer’s disease (AD) widely recognized as the second most prevalent form of
and Parkinson’s disease (PD), indicating the existence of neurodegenerative dementia following AD.
common pathogenesis between the two diseases. On the Cortical LBs are the main pathological marker of DLB.
other hand, it was reported that there were neurodegenera- They are usually less eosinophilic, less well defined than
tive disorders characterized by dementia and widespread brainstem-type LBs, and generally lack a halo. It is
occurrence of cortical Lewy bodies (LBs) [10,17,24]. difficult to identify cortical LBs by hematoxylin and eosin
These disorders are generally accompanied by varying (HE) staining. Immunostaining with ubiquitin
anti-degrees of AD pathology in addition to the occurrence of body [18] has been recommended for detecting cortical
LBs in the brainstem and cerebral cortex. In 1995, cases LBs accurately. Previous immuno-histochemical studies
showed that LBs and Lewy neurites (LNs) were a
-synu-clein-positive [4,11,27,31] and neurofibrillary tangles
*Corresponding author. Fax:181-3-3822-4865.
E-mail address: [email protected] (M. Yamazaki). (NFTs) are not immunoreactive [28,30], differing from
ubiquitin immunostaining. Therefore, immuno-histoch- pathological criteria for AD [23] as follows: none, sparse,
emistry using anti-a-synuclein antibodies is considered the moderate and frequent. We examined the areas in which
preferred method for detecting LBs and LNs rather than SPs existed in maximum density. NFTs were identified by
ubiquitin immuno-histochemistry. immuno-histochemistry using AT8. The distribution
pat-Recent studies indicated that in the amygdala of early- tern of NFTs and neuropil threads (NTs) was evaluated
onset familial AD patients [19], and Down’s syndrome based on Braak and Braak staging of neurofibrillary
brains with AD [20], numerous a-synuclein-positive LBs changes of AD [5], and was classified into six stages as
and neurites were noted. These observations led us to follows: transentorhinal stage, stages I–II; limbic stage,
determine the relationship between AD pathology and stages III–IV; and isocortical stage, stages V–VI.
a-synuclein-associated pathology.
We examineda-synuclein immunoreactivity in brains of 2.4. Immno-histochemical analysis using anti-a-synuclein
demented patients who were clinically diagnosed as having antibodies
sporadic AD. This study had the following aims: (1) to
investigate the relationship between AD pathology (focus- We examined immunoreactivities ofa-synuclein against
ing on the distribution and frequency of senile plaques two antibodies. One is a monoclonal antibody (LB509),
(SPs) and NFTs) and a-synuclein-positive structures; and raised against purified LBs specifically from DLB brains
(2) to describe the morphology of a-synuclein-positive [4], and recognizes amino acids 115–122 [14], the other is
structures. a polyclonal antibody ([17) [7], raised against a synthetic
peptide corresponding to amino acids 1–10 of human
a-synuclein. For immuno-histochemistry using anti-a
-2. Materials and methods synuclein antibodies, 6-mm thick paraffin sections were obtained from four selected regions of the brain of each
2.1. Cases case: the hippocampus / entorhinal cortex, amygdala,
mid-brain, and cingulate gyrus. The sections were
deparaffin-In the present study, we examined 27 consecutive brains ized and pretreated with hydrolytic autoclaving in citrate
obtained at autopsy from demented patients, who were buffer for 15 min at 1218C to enhance the
immuno-clinically diagnosed as having probable AD based on the reactivity. Thereafter, they were incubated in 0.01% H O2 2
NINCDS-ADRDA criteria [22] in the Hatsuishi Hospital. in methanol at room temperature for 30 min to block
Twenty-seven patients with probable AD were divided into endogenous peroxidase activity and pretreated to block
seven men and 20 women. The average age of death was nonspecific immunoreactivity prior to the addition of
82.0 years (range 60–94 years). The average duration of LB509 (dilution of 1:50) or[17 (dilution of 1:1000) with
illness was 10.2 years (range 2–22 years). The average normal rabbit serum, or normal goat serum respectively.
brain weight was 1022.2 g (range 740–1240 g). This was followed by one-overnight incubation at 48C.
Staining was performed using the avidin–biotin complex
2.2. Tissue sampling and routine staining technique with 3,39-diaminobenzidine (DAB) as the
chromogen and followed by counterstaining with
hemato-All brains examined in the present study were obtained xylin. Sections of the midbrain of PD patients, in which
at autopsy within 24 h after death. The entire brain was typical brainstem-type LBs were detected on HE staining,
fixed in 10% formaldehyde for about 2 weeks and then cut were used as positive controls.
in coronal slices. For overall neuropathologic evaluation, All four sections were immunolabeled with anti-a
-synu-tissue blocks were obtained from selected regions: middle clein antibodies, and were then examined for the presence
frontal, inferior parietal and superior temporal neocortex, ofa-synuclein-positive structures in each case. In cases in
hippocampus / entorhinal cortex, basal ganglia, cerebellum, whicha-synuclein-positive structures could be found in at
midbrain, pons, medulla oblongata. These tissue blocks least one region, five additional regions were selected.
were embedded in paraffin and 4-mm thick sections were These additional regions were the frontal, parietal,
tempo-¨
cut and stained with HE, Kluver-Barrera and Bodian ral neocortex, pons and medulla oblongata, which were
staining. Immuno-histochemical staining of 6-mm thick recommended in the guidelines for brain sampling by the
sections was performed using the anti-phosphorylated tau consortium on DLB international workshop [21], and were
monoclonal antibody (mAb) AT8 (Innogenetics, dilution of immunolabeled in the same manner.
1:3000), anti-human beta-amyloid mAb 6F / 3D (Dako,
dilution of 1:100) and rabbit anti-ubiquitin antibody 2.5. Quantitation ofa-synuclein-positive structures
(DAKO, dilution of 1:100).
Initially, each section was scanned at a magnification of
2.3. Evaluation of neocortical SPs and NFTs 3100 to check whether or not there is any a
-synuclein-positive structures and identify the regions in which the
Neocortical SPs were identified by Bodian staining, and most abundant a-synuclein-positive structures were
a-synuclein-positive intra-cytoplasmic inclusions and a -synuclein-positive neurites. To semiquantitatively evaluate them, a score ranging from 0 to 3 was assigned according to the number of intra-cytoplasmic inclusions and the density of neurites in an area of maximum density for each
region. Intra-cytoplasmic inclusions were defined as a
-synuclein-positive structures including the nucleus in neurons in the plane of sections. Their number was counted in three nonoverlapping fields at a magnification
of 3200 for each section, and the final score was the
average of the three fields as follows: (score 0, 0; score 1,
1–5; score 2, 6–10; score 3, .10). Similarly, the density Fig. 1. Comparison of frequency and density of SPs between two groups.
of a-synuclein-positive neurites was scored as follows: Neuritic plaques were identified in Bodian-stained tissue preparations
according to CERAD criteria for AD. There were no significant
differ-(score 0, none; score 1, a few neurites; score 2, some
ences betweena-synuclein-positive and-negative groups. P;a
-synuclein-neurites; score 3, many neurites).
positive group, N;a-synuclein-negative group.
Additionally, a-synuclepositive intra-cytoplasmic
in-clusions were morphologically subdivided into two groups
according to whether or not they were of the LBs-type, as the positive group. Nine cases in the positive group had
which are revealed as spherical, ellipsoidal, angular and some a-synuclein-positive structures in all four regions,
reniform by staining. The regional rate of thea-synuclein- and the four cases in the positive groups hada
-synuclein-positive intra-cytoplasmic inclusions, which were impos- positive structures in only two or three regions. The
sible to identify as LBs morphologically at a magnification remaining 14 cases did not have a-synuclein-positive
of3400 (5non-LB type), was expressed as the percentage structures in all four regions, and were defined as the
relative to the total a-synuclein-positive intra-cytoplasmic negative group. We compared the positive group with the
inclusions. The quantitation was performed for all cases negative group with respect to age of onset, age of death,
and sections by the same examiner. duration of illness, and brain weight. There was a tendency
that cases of the positive group were slightly older at the
2.6. Confocal microscopy time of death, and had shorter duration of illness, and
lighter brain weight than those of the negative group. The
To assure that a-synuclein-positive intra-cytoplasmic two groups were not found to be significantly different by
inclusions were colocalized with NFTs, sections were the Mann–Whitney U-test.
double-labeled with the anti-a-synuclein ([17) antibody
and anti-tau (AT8) antibody. Then, [17 and AT8 were 3.2. The frequency of SPs and NFTs in two groups
fluorescence-labeled with Alexa 488 (Anti-Rabbit IgG
(H1L)), detected as green, and with Alexa 594 (Anti- The SP density determined based on CERAD
neuro-Mouse IgG (H1L)), detected as red, respectively. Sections pathological criteria for AD [23] did not differ significantly
were observed under a confocal microscopy system (TCS- among the neocortical three regions between the two
SP, Leica, Heidelberg, Germany). groups. The average SP density was the highest in the
temporal cortex, followed by the parietal cortex and by the frontal cortex (Fig. 1). The distribution pattern of NFTs
3. Results and NTs based on Braak and Braak staging [5] showed
that the majority of examined cases were classified into
3.1. Summary of 27 cases (Table 1) stages V–VI except for three cases of the positive group,
which were classified into stage IV (Table 2). In the
In a-synuclein immuno-histochemistry, 13 (48.2%) out positive group, all 13 cases were categorized as having
of 27 cases had a-synuclein-positive structures in at least definite AD based on CERAD diagnostic criteria [23], and
one region of the four selected regions, and were defined diagnostic criteria for the neuropathology of AD by the
Table 1
a
Comparison of characteristics of two groups of study patients
Number Age of onset (years) Age of death (years) Duration (y) Brain weight (g) (Sex; M / F) average (range) average (range) average (range) average (range)
a-synuclein-positive group 13 (4 / 9) 73.5 (61–89) 83.2 (72–93) 9.7 (2–19) 998.3 (840–1220)
a-synuclein-negative group 14 (3 / 11) 72.8 (55–89) 80.9 (60–94) 11.0 (4–22) 1048.2 (720–1270)
a
Table 2 amined. The next frequent occurrence of a
-synuclein-a
Comparison of stages of neurofibrillary changes between two groups positive inclusions was observed in the parahippocampal
Stage I II III IV V VI gyrus, in which 11 (84.6%) out of 13 cases had a
-synuclein-positive inclusions. The parietal cortex, in which
Positive group 0 0 0 3 3 7
Negative group 0 0 0 0 5 9 nine cases (69.2%) had a-synuclein-positive inclusions,
a was the region in which the occurrence of these inclusions
The extent of neurofibrillary changes was evaluated by
immuno-histoch-was the most infrequent among the three neocortices. This
emistry of tau (AT8) in all cases of each group according to classification
of Braak and Braak. Most of the cases in two groups were assigned to an frequency was the same as that observed in the cingulate isocortical stage. gyrus, which is considered as one of the preferred regions
[17]. Moreover, the amygdala showed the highest density
National Institute on Aging, and Regan Institute Working of a-synuclein-positive inclusions, followed by the
Group [1], ten cases as high likelihood and three cases as parahippocampal gyrus.the cingulate gyrus, CA2 / 3 and
intermediate likelihood. In the negative group, 13 cases CA4 regions of hippocampus formation.
categorized as having definite AD or high likelihood, and the remaining one case as having probable AD or
inter-mediate likelihood. 3.3.2. a-Synuclein-positive neurites
The distribution and frequency of a-synuclein-positive
3.3. The frequency and distribution ofa-synuclein- neurites among the positive group are described in Fig. 3.
positive structures a-Synuclein-positive neurites were present not only in CA2 / 3 region of hippocampus formation [8], but also in
In this study, the difference in immunostaining between the other regions. Twelve cases (92.3%) hada
-synuclein-LB509 and[17 was hardly recognized, and the immuno- positive neurites in the amygdala, and ten cases (78.5%)
staining for LB509 was evaluated. had them in CA2 / 3 region of hippocampus formation,
parahippocampal gyrus, temporal cortex, substantia nigra
3.3.1. a-Synuclein-positive intra-cytoplasmic inclusions and locus ceruleus. Five cases (38.5%) had a
-synuclein-Fig. 2 shows the distribution and frequency ofa-synu- positive neurites in the frontal cortex, and six cases
clein-positive intra-cytoplasmic inclusions in brains of the (46.2%) had them in the parietal cortex. In the frontal and
positive group. In 12 (92.3%) out of 13 cases,a-synuclein- parietal cortices, the a-synuclein-positive neurites were
positive inclusions were most frequently observed in the much less dense than in the other regions. The region with
amygdala and temporal cortex among all regions ex- the highest density of positive neurites was the amygdala,
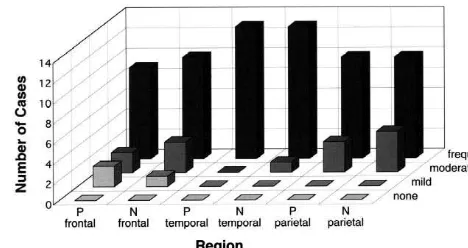
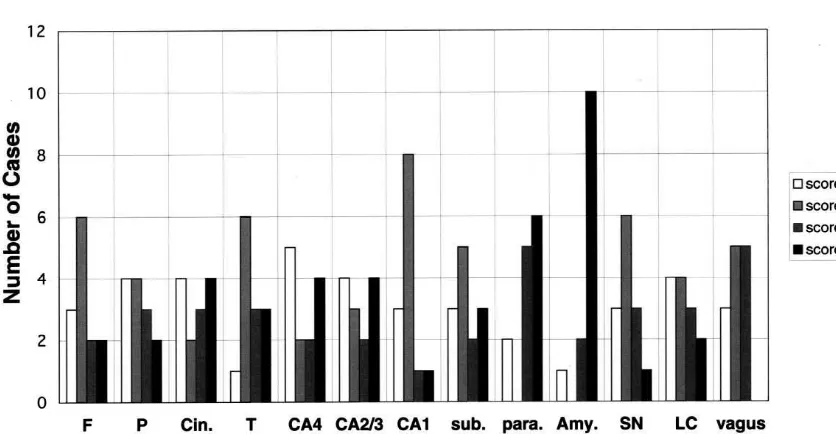
Fig. 2. Frequency and distribution ofa-synuclein-positive intra-cytoplasmic inclusions. The number ofa-synuclein-positive intra-cytoplasmic inclusion was counted in selected regions in the brain of 13 cases ofa-synuclein-positive group. Each score was graded semiquantitatively according to the average number of a-synuclein-positive inclusions per field (2003 magnification); score 050, score 151–5, score 256–10, score 35.10. Note that
Fig. 3. Frequency and distribution ofa-synuclein-positive neurites. The density ofa-synuclein-positive neurites was estimated in selected regions in the brain of 13 cases ofa-synuclein-positive group. Each score was graded semiquantitatively according to the density ofa-synuclein-positive neurites per field (2003magnification); score 0, none; score 1, a few; score 2, some; score 3, many. Note that a-synuclein-positive neurites were observed most frequently and densely in the amygdala. F, middle frontal gyrus; P, inferior parietal lobule; Cin., cingulate gyrus; T, middle temporal gyrus; CA4, CA2 / 3, CA1, CA4, CA2 / 3 and CA1 regions of the hippocampus, respectively; sub., subiculum; para., parahippocampal gyrus; Amy., amygdala; SN, substantia nigra; LC, locus ceruleus; vagus, dorsal motor nucleus of vagus.
followed by CA2 / 3 region of hippocampus and parahip- stem, the percentage was much lower than in the other
pocampal gyrus. regions.
3.4.2. a-Synuclein-positive neurites
3.4. Morphological features ofa-synuclein-positive
In the superficial layer of temporal cortex in particular,
structures by light microscopy
a-synuclein-positive fine neurites that morphologically
resemble NTs, were more dense in some cases. Such cases
3.4.1. a-Synuclein-positive intra-cytoplasmic inclusions had much more numerous a-synuclein-positive structures
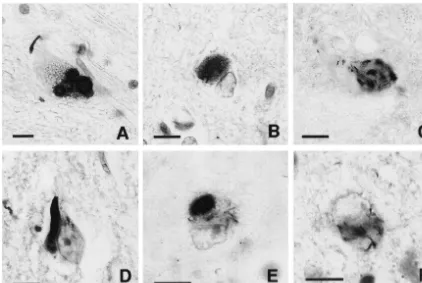
In immuno-cytochemistry using an anti-a-synuclein including LBs in the deep layer, although individual cases
antibody, brainstem-type LBs (Fig. 4A) exhibited spherical showed slight variability.
immunoreactivity with a halo. This pattern of staining was
very similar to that of brainstem-type LBs immunostained 3.5. Double-labeling confocal microscopy ofa-synuclein
with an anti-ubiquitin antibody. On the other hand, in and tau
cerebral cortices, a-synuclein-positive inclusions were
recognized as various shapes. For example, the staining Laser scanning confocal microscopy of sections
double-patterns of cortical a-synuclein-positive inclusions were immunolabeled with antibodies againsta-synuclein ([17)
spot-shaped (Fig. 4C), flame-shaped (Fig. 4D), filamentous and tau (AT8) showed that the respective
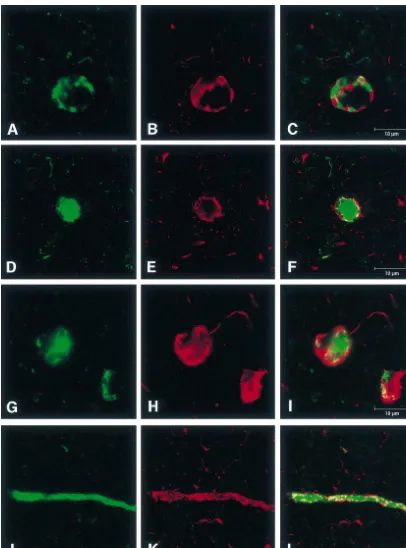
immunoreactivi-in neuronal perikarya (Fig. 4E,F), besides the typical shape ty colocalized in some neurons. These neurons exhibited
of cortical LBs (Fig. 4B). The number of these inclusions mainly two patterns as follows: (1) intermingled with tau
that are different from LBs (5non-LB type) varied from and a-synuclein-positive aggregation in the neuron (Fig.
case to case and their percentages with respect to the total 6A–C); and (2) LBs and a-synuclein-positive masses
number of a-synuclein-positive inclusions also varied surrounded by tau immunoreactivity (Fig. 6D–I). In the
depending on the region (Fig. 5). Namely, in the hip- present study, the latter pattern was observed much more
pocampus formation (especially the CA4 region), the frequently than the former. Conversely, NFTs and
tau-percentage is higher than in the other regions, and the positive masses surrounded by a-synuclein
immuno-temporal cortex has the highest percentage among the reactivity were not observed in the same neuron.
Fig. 4. Photomicrographs of the immuno-histochemical staining with the anti-a-synuclein antibody (LB509). (A) Brainstem-type LBs in the substantia nigra. (B) Typical cortical LBs in the cingulate gyrus. (C) Spot-shapeda-synuclein-positive inclusions in the subiculum. (D) Flame-shapeda -synuclein-positive inclusions in the CA1 region of the hippocampus. (E) Filamentous inclusions in neuronal perikarya in addition to typical cortical LBs in the frontal cortex. (F) Filamentous inclusions in neuronal perikarya in the hippocampus. Scale bar510mm in each panel.
AT8. These neurites appeared to be covered with tau- a-synuclein protein to aggregate. Immuno-histochemistry
positive coarse granular deposits (Fig. 6J–L). of a-synuclein revealed a variety of a-synuclein-positive
inclusions that are different from LBs. These inclusions were considered to represent an early stage of LB
forma-4. Discussion tion in PD and DLB, because they were recognized more
numerously on patients with shorter disease duration [30],
a-Synuclein, also known as the precursor of the non-Ab or their ultrastructural features were similar to LBs [2].
component of AD amyloid (NACP) [29], is a soluble However, in these sporadic AD cases, it is uncertain
protein, and it is predominantly localized to presynaptic whether the so-called non-LB type a-synuclein
aggrega-nerve terminals [13,15] in a normal brain. Some previous tion would be that of LBs or not.
studies have identifieda-synuclein as the major component In this study, the ratio of the number of a
-synuclein-of LBs and LNs. In the present study, the cases with SPs positive inclusions other than LBs to the total number of
and NFTs numerous enough to meet the criteria of AD inclusions was less in the brainstem and the cingulate
could be subdivided into two groups according to whether gyrus, where these are generally considered to be the
a-synuclein aggregation was present or not. The majority preferential regions for LB formation. Meanwhile the
of a-synuclein-positive cases have a-synuclein-positive hippocampus, in which few LBs were usually found [9],
structures in various amounts in almost all the regions had higher ratio than in any other regions. These findings
examined in this study. This finding indicates that the indicate that there are two kinds of regions in the brain:
aggregation ofa-synuclein was distributed more extensive- one is susceptible to LB formation, and the other to
ly in the central nervous system than we had expected. non-LB-type of a-synuclein aggregation. The amount of
Among all the regions examined, the amygdala contained NACP/a-synuclein was not equal across the brain, and
the highest amount ofa-synuclein-positive structures. This a-synuclein was relatively more abundant in the
hip-result is consistent with those of DLBD [25], familial AD pocampus and less in the brainstem in rats [12]. It is
[19], Down’s syndrome with AD [20] and Parkinsonism– uncertain whether the hippocampus is abundant with a
-dementia complex of Guam [33], which implies that the synuclein in humans as well. However, it was indicated
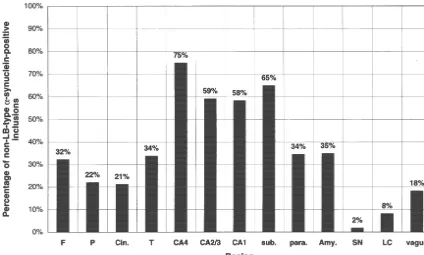
Fig. 5. Percentage of non-LB-type a-synuclein-positive inclusions with respect to the total number of a-synuclein-positive ones in each region.
a-Synuclein-positive inclusions were morphologically subdivided into LBs-type and non-LB-type. In all cases of the positive group, the numbers of non-LB-type in each region was the percentage of the totala-synuclein-positive inclusions. The highest percentage was observed in the hippocampus. On the other hand, the brainstem and cingulate gyrus had lower percentages than the other regions. F, middle frontal gyrus; P, inferior parietal lobule; Cin., cingulate gyrus; T, middle temporal gyrus; CA4, CA2 / 3, CA1, CA4, CA2 / 3 and CA1 regions of the hippocampus, respectively; sub., subiculum; para., parahippocampal gyrus; Amy., amygdala; SN, substantia nigra; LC, locus ceruleus; vagus, dorsal motor nucleus of vagus.
on its concentration and nucleation in vitro [32]. Taking other both in vitro and in vivo. There could be two
these reports into consideration together with our observa- possibilities with respect to non-LB-type a-synuclein
tions, it could be supposed that in the hippocampus, the aggregation: one was that these non-LB-type inclusions
aggregation of a-synuclein could not always lead to LB represent the early stage of LB formation, and the other is
formation and non-LB-typea-synuclein aggregation could that these non-LB-typea-synuclein inclusions are formed
secondarily occur under the influence of tau aggregation. secondary to tau aggregation and never lead to LB
Alternatively, a large number of NFTs could inhibit LB- formation. We could not determine which possibility
type a-synuclein aggregation because tau aggregates and applies. However, we could not usually observe typical
a-synuclein could interact with each other. In the brain- LBs in the hippocampus, and it would be difficult to
stem, tau aggregation was not prominent and a-synuclein conclude that all of these a-synuclein-positive inclusions
could aggregate to form typical LBs. Although the cellular signified the early stage of LB formation.
mechanism underlying the aggregation ofa-synuclein and In summary, the discovery ofa-synuclein as a marker of
LB formation is unknown, there are some reports indicat- LBs facilitated the detection of LB-related structures even
ing the relevance of tau and a-synuclein aggregation. It in AD cases with widespread and numerous NFTs.
More-was reported thata-synuclein binds to tau and stimulated over, a-synuclein-positive inclusions other than LBs were
the protein kinase A-catalyzed tau phosphorylation in vitro observed, and their distribution indicates that LB formation
[16]. Moreover, in some neuropathological reports, it was may be influenced by the degree of tau aggregation. The
indicated that neocortical LBs were significantly more cases with AD might be subdivided into two groups
frequent in cases having both AD and PD lesions than in according to the existence or absence of a-synuclein
cases with either PD or AD lesion alone [6]. It was also aggregation.
reported that in the limbic area [12], particularly in the amygdala [26], LBs and NFTs were colocalized in the same neurons.
A recent immunoelectron microscopical study demon- Acknowledgements
strated that the tau-epitope was occasionally incorporated
even into brainstem-type LBs [3]. The above studies We thank Professor Takeshi Iwatsubo and Ms Minami
Fig. 6. Confocal microscopy images of sections double-stained fora-synuclein ([17) and tau (AT8) immunoreactivity.[17 was labeled with Alexa 488
[15] R. Jakes, M.G. Spillantini, M. Goedert, Identification of two distinct
Tokyo University, for generously providing the anti-a
-synucleins from human brain, FEBS Lett. 345 (1994) 27–32.
synuclein antibodies and for critical comments.
[16] P.H. Jensen, H. Hager, M.S. Nielsen, P. Hojrup, J. Gliemann, R. Jakes, a-Synuclein binds to Tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356, J.
References Biol. Chem. 274 (1999) 25481–25489.
[17] K. Kosaka, Diffuse Lewy body disease in Japan, J. Neurol. 237 (1990) 197–204.
[1] Anonymous, Consensus recommendations for the postmortem
diag-[18] G. Lennox, J. Lowe, K. Morrell, M. Landon, R.J. Mayer, Anti-nosis of Alzheimer’s disease. The National Institute on Aging, and
ubiquitin immunocytochemistry is more sensitive than conventional Reagan Institute Working Group on Diagnostic Criteria for the
techniques in the detection of diffuse Lewy body disease, J. Neurol. Neuropathological Assessment of Alzheimer’s Disease, Neurobiol.
Neurosurg. Psychiatry 52 (1989) 67–71. Aging 18 (Suppl. 4) (1997) S1–S2.
[19] C.F. Lippa, H. Fujiwara, D.M. Mann, B. Giasson, M. Baba, M.L. [2] K. Arima, K. Ueda, N. Sunohara, S. Hirai, Y. Izumiyama, H.
Schmidt, L.E. Nee, B. O’Connell, D.A. Pollen, P. St. George-Tonozuka-Uehara, M. Kawai, Immunoelectron-microscopic
demon-Hyslop, B. Ghetti, D. Nochlin, T.D. Bird, N.J. Cairns, V.M. Lee, T. stration of NACP/a-synuclein-epitopes on the filamentous
com-Iwatsubo, J.Q. Trojanowski, Lewy bodies contain altered a -synu-ponent of Lewy bodies in Parkinson’s disease and in dementia with
clein in brains of many familial Alzheimer’s disease patients with Lewy bodies, Brain Res. 808 (1998) 93–100.
mutations in presenilin and amyloid precursor protein genes, Am. J. [3] K. Arima, S. Hirai, N. Sunohara, K. Aoto, Y. Izumiyama, K. Ueda,
Pathol. 153 (1998) 1365–1370. K. Ikeda, M. Kawai, Cellular co-localization of phosphorylated
tau-[20] C.F. Lippa, M.L. Schmidt, V.M. Lee, J.Q. Trojanowski, Antibodies and NACP/a-synuclein-epitopes in Lewy bodies in sporadic
Parkin-son’s disease and in dementia with Lewy bodies, Brain Res. 843 to a-synuclein detect Lewy bodies in many Down’s syndrome (1999) 53–61. brains with Alzheimer’s disease, Ann. Neurol. 45 (1999) 353–357. [4] M. Baba, S. Nakajo, P.H. Tu, T. Tomita, K. Nakaya, V.M. Lee, J.Q. [21] I.G. McKeith, D. Galasko, K. Kosaka, E.K. Perry, D.W. Dickson, Trojanowski, T. Iwatsubo, Aggregation of a-synuclein in Lewy L.A. Hansen, D.P. Salmon, J. Lowe, S.S. Mirra, E.J. Byrne, G. bodies of sporadic Parkinson’s disease and dementia with Lewy Lennox, N.P. Quinn, J.A. Edwardson, P.G. Ince, C. Bergeron, A. bodies, Am. J. Pathol. 152 (1998) 879–884. Burns, B.L. Miller, S. Lovestone, D. Collerton, E.N.H. Jansen, C. [5] H. Braak, E. Braak, Neuropathological stageing of Alzheimer- Ballard, R.A.I. de Vos, G.K. Wilcock, K.A. Jellinger, R.H. Perry, related changes, Acta Neuropathol. 82 (1991) 239–259. Consensus guidelines for the clinical and pathologic diagnosis of [6] D.F. Brown, M.A. Dababo, E.H. Bigio, R.C. Risser, K.P. Eagan, dementia with Lewy bodies (DLB): report of the consortium on
C.L. Hladik, C.L. White III, Neuropathologic evidence that the DLB international workshop, Neurology 47 (1996) 1113–1124. Lewy body variant of Alzheimer disease represents coexistence of [22] G. McKhann, D. Drachman, M. Folstein, R. Katzman, D. Price, Alzheimer disease and idiopathic Parkinson disease, J. Neuropathol. E.M. Stadlan, Clinical diagnosis of Alzheimer’s disease: Report of Exp. Neurol. 57 (1998) 39–46. the NINCDS-ADRDA Work Group under the auspices of Depart-[7] K.A. Conway, J.D. Harper, P.T. Lansbury, Accelerated in vitro fibril ment of Health and Human Services Task Force on Alzheimer’s
formation by a mutanta-synuclein linked to early-onset Parkinson Disease, Neurology 34 (1984) 939–944.
disease, Nat. Med. 4 (1998) 1318–1320. [23] S.S. Mirra, A. Heyman, D. McKeel, S.M. Sumi, B.J. Crain, L.M. [8] D.W. Dickson, D. Ruan, H. Crystal, M.H. Mark, P. Davies, Y. Kress, Brownlee, F.S. Vogel, J.P. Hughes, G. van Belle, L. Berg, The S.H. Yen, Hippocampal degeneration differentiates diffuse Lewy Consortium to Establish a Registry for Alzheimer’s Disease body disease (DLBD) from Alzheimer’s disease: light and electron (CERAD). Part II. Standardization of the neuropathologic assess-microscopic immunocytochemistry of CA2-3 neurites specific to ment of Alzheimer’s disease, Neurology 41 (1991) 479–486. DLBD, Neurology 41 (1991) 1402–1409. [24] R.H. Perry, D. Irving, G. Blessed, A. Fairbairn, E.K. Perry, Senile [9] M.M. Esiri, B.T. Hyman, K. Beyreuther, C.L. Masters, Parkinson’s dementia of Lewy body type. A clinically and neuropathologically disease and dementia, in: D.I. Graham, P.L. Lantos (Eds.), 6th distinct form of Lewy body dementia in the elderly, J. Neurol. Sci. Edition, Greenfield’s Neuropathology, Vol. 2, Arnold, London, 1997, 95 (1990) 119–139.
pp. 188–192. [25] P. Rezaie, N.J. Cairns, A. Chadwick, P.L. Lantos, Lewy bodies are [10] L. Hansen, D. Salmon, D. Galasko, E. Masliah, R. Katzman, R. located preferentially in limbic areas in diffuse Lewy body disease,
DeTeresa, L. Thal, M.M. Pay, R. Hofstetter, M. Klauber, V. Rice, N. Neurosci. Lett. 212 (1996) 111–114.
Butters, M. Alford, The Lewy body variant of Alzheimer’s disease: [26] M.L. Schmidt, J.A. Martin, V.M. Lee, J.Q. Trojanowski, Conver-a clinicConver-al Conver-and pConver-athologic entity, Neurology 40 (1990) 1–8. gence of Lewy bodies and neurofibrillary tangles in amygdala [11] M.C. Irizarry, W. Growdon, T. Gomez-Isla, K. Newell, J.M. George, neurons of Alzheimer’s disease and Lewy body disorders, Acta
D.F. Clayton, B.T. Hyman, Nigral and cortical Lewy bodies and Neuropathol. 91 (1996) 475–481.
dystrophic nigral neurites in Parkinson’s disease and cortical Lewy [27] M.G. Spillantini, M.L. Schmidt, V.M. Lee, J.Q. Trojanowski, R. body disease containa-synuclein immunoreactivity, J. Neuropathol. Jakes, M. Goedert,a-Synuclein in Lewy bodies, Nature 388 (1997)
Exp. Neurol. 57 (1998) 334–337. 839–840.
[12] E. Iseki, W. Marui, K. Kosaka, K. Ueda, Frequent coexistence of [28] A. Takeda, M. Mallory, M. Sundsmo, W. Honer, L. Hansen, E. Lewy bodies and neurofibrillary tangles in the same neurons of Masliah, Abnormal accumulation of NACP/a-synuclein in neurode-patients with diffuse Lewy body disease, Neurosci. Lett. 265 (1999) generative disorders, Am. J. Pathol. 152 (1998) 367–372. 9–12. [29] K. Ueda, H. Fukushima, E. Masliah, Y. Xia, A. Iwai, M. Yoshimoto, [13] A. Iwai, E. Masliah, M. Yoshimoto, N. Ge, L. Flanagan, H.A.R. de D.A.C. Otero, J. Kondo, Y. Ihara, T. Saitoh, Molecular cloning of Silva, A. Kittel, T. Saitoh, The precursor protein of non-Ab cDNA encoding an unrecognized component of amyloid in Al-component of Alzheimer’s disease amyloid is a presynaptic protein zheimer disease, Proc. Natl. Acad. Sci. USA 90 (1993) 11282– of the central nervous system, Neuron 14 (1995) 467–475. 11286.
[14] R. Jakes, R.A. Crowther, V.M. Lee, J.Q. Trojanowski, T. Iwatsubo, [30] K. Wakabayashi, S. Hayashi, A. Kakita, M. Yamada, Y. Toyoshima, M. Goedert, Epitope mapping of LB509, a monoclonal antibody M. Yoshimoto, H. Takahashi, Accumulation ofa-synuclein / NACP directed against human a-synuclein, Neurosci. Lett. 269 (1999) is a cytopathological feature common to Lewy body disease and
[31] K. Wakabayashi, K. Matsumoto, K. Takayama, M. Yoshimoto, H. [33] M. Yamazaki, Y. Arai, M. Baba, T. Iwatsubo, O. Mori, Y. Katayama, Takahashi, NACP, a presynaptic protein, immunoreactivity in Lewy K. Oyanagi, a-Synuclein inclusions in amygdala in the brains of bodies in Parkinson’s disease, Neurosci. Lett. 239 (1997) 45–48. patients with the Parkinsonism–dementia complex of Guam, J. [32] S.J. Wood, J. Wypych, S. Steavenson, J.C. Louis, M. Citron, A.L. Neuropathol. Exp. Neurol. 59 (2000) 585–591.