Elsevier, and the attached copy is provided by Elsevier for the
author’s benefit and for the benefit of the author’s institution, for
non-commercial research and educational use including without
limitation use in instruction at your institution, sending it to specific
colleagues that you know, and providing a copy to your institution’s
administrator.
All other uses, reproduction and distribution, including without
limitation commercial reprints, selling or licensing copies or access,
or posting on open internet sites, your personal or institution’s
website or repository, are prohibited. For exceptions, permission
may be sought for such use through Elsevier’s permissions site at:
Author's personal copy
Journal of Colloid and Interface Science 300 (2006) 543–552www.elsevier.com/locate/jcis
Design of an NO photoinduced releaser xerogel based on the controlled
nitric oxide donor
trans
-[Ru(NO)Cl(cyclam)](PF
6
)
2
(cyclam
=
1,4,8,11-tetraazacyclotetradecane)
✩Kleber Queiroz Ferreira
a, José F. Schneider
b, Pedro A.P. Nascente
c,
Ubirajara Pereira Rodrigues-Filho
d, Elia Tfouni
a,∗aDepartamento de Química, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Av. dos Bandeirantes 3900,
14040-901 Ribeirão Preto, SP, Brazil
bInsituto de Física de São Carlos, Universidade de São Paulo, CP 369, 13560-970 São Carlos, SP, Brazil cDepartamento de Engenharia de Materiais, Universidade Federal de São Carlos, 13565-905 São Carlos, SP, Brazil
dInstituto de Química de São Carlos, Universidade de São Paulo, CP 780, 13560-970 São Carlos, SP, Brazil
Received 21 February 2006; accepted 28 March 2006
Available online 6 April 2006
Abstract
The immobilization and properties of the nitric oxide donortrans-[Ru(NO)Cl(cyclam)](PF6)2, Ru–NO, entrapped in a silica matrix by the sol– gel process is reported herein. The entrapped nitrosyl complex was characterized by spectroscopic (UV–vis, infrared (IR), X-ray photoelectron, and13C and29Si MAS NMR) and electrochemical techniques. The entrapped species exhibit one characteristic absorption band in the UV–vis region of the electronic spectrum at 354 nm and one IRνNOstretching band at 1865 cm−1, as does the Ru–NO species in aqueous solution. Our results show thattrans-[Ru(NO)Cl(cyclam)](PF6)2can be entrapped in a SiO2matrix with preservation of the molecular structure. However, in a SiO2/SiNH2matrix, the complex undergoes a nucleophilic attack by the amine group at the nitrosonium. Irradiation of the complex, entrapped in the SiO2matrix, with light of 334 nm, resulted in NO release. The material was regenerated to its initial nitrosyl form by reaction with nitric oxide.
2006 Elsevier Inc. All rights reserved.
Keywords:Nitric oxide; Sol–gel; Xerogel; Ruthenium; Nitrosyl; Controlled; Photochemistry; Cyclam; Silica; Aminopropylsilica; Nucleophilic; Attack
1. Introduction
In mammalian species, nitric oxide (NO) plays key roles in almost every function[1], where high or low NO concentrations can be either beneficial or harmful and could accompany nu-merous pathological states[1]. For this reason, there has been a growing interest in NO donors and scavengers aiming at thera-peutic applications[2–17]. Ruthenium nitrosyl complexes have shown to be very promising NO donors[5,6,9,10,12,14,17–25], and some of them have shown biological activity[6,22,24–27].
✩ Taken in part from K.Q. Ferreira, Ph.D. thesis, Departamento de Química da Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, 2004.
* Corresponding author. Fax: +55 16 3602 4838.
E-mail address:eltfouni@usp.br(E. Tfouni).
In these quite stable complexes, the coordinated NO has a ni-trosonium character, and it can be released photochemically or via one-electron reduction[3,12,17–20,22,26,28].
Our laboratories have directed efforts toward the synthesis of ruthenium complexes as NO donors and scavengers[6,14,23, 24,26]. Thetrans-[Ru(NO)Cl(cyclam)](PF6)2complex releases NO photochemically or upon reduction [14,29,30], and it is less toxic than nitroprusside, a well-known vasodilator[6,9,14]. Furthermore, it is as effective at reducing blood pressure as ni-troprusside, but with a longer effect [6]. The blood pressure effects were interpreted in terms of the reactivities of the com-plexes involved in NO release. The longer blood pressure re-duction effect oftrans-[Ru(NO)Cl(cyclam)]2+was interpreted as a result of the much lower rate of NO release from trans-[Ru(NO)Cl(cyclam)]2+
than from similar tetraammine nitrosyl
Author's personal copy
ruthenium complexes[6,9,14]. Aiming at extending the rangeof potential applications, there is an interest in designing carri-ers or supportcarri-ers for such complexes. Conceivably, the immo-bilization of these complexes could result in materials that may be used in association with optical fibbers to provide the op-portunity for controlled NO release at specific target sites using laser photoexcitation[31,32].
In this regard, novel strategies using NO donors other than metal nitrosyl complexes have also been investigated. Nitric oxide-releasing diazeniumdiolates are successfully being im-mobilized in polymers, silica-gel, and metal surfaces, aiming at biological applications[33]. Recently, the preparation, char-acterization, and preliminary biomedical application of various nitric oxide (NO)-releasing fumed silica particles with amine groups has been reported[34]. These amine groups were then converted into the corresponding N-diazeniumdiolate groups via reaction with NO(g) at high pressure in the presence of methoxide bases. The N-diazeniumdiolate moieties attached to the silica surface underwent a primarily proton-driven dissoci-ation to NO under physiological conditions, and they also un-derwent slow thermal dissociation to NO. These resulting NO-releasing fumed silica particles could be embedded into poly-mer films to create thromboresistant coatings, via NO release at fluxes that mimic healthy endothelial cells (EC), making them a very interesting system. The NO-addition efficiency for this direct reaction, however, was found to be 12% in an acetonitrile suspension of Sil-2N[6]particles. This NO-loading capacity is lower than the one observed for various free amines, which lead to a typical yield of 30–90%[35]. The immobilization of diaze-niumdiolates in sol–gel to yield NO releasing materials has also been reported[36]. More recently, a ruthenium salen nitrosyl complex has been copolymerized with ethyleneglycol dimethy-lacrylate to form a material which is photolabile for NO release
[37]. Toma et al. have also recently reported ruthenium hexaac-etate clusters incorporated in polyvinyl alcohol films that are sensitive to daylight[38].
The use of ruthenium nitrosyl immobilized species as NO donors has some advantages in relation to other species, since ruthenium complexes can deliver and recover NO at milder con-ditions than those associated with the diazeniumdiolate system. Moreover, it should be noted that the NO delivery from ruthe-nium amine (or ammine) nitrosyl complexes can be tuned pho-tochemically, through their different UV–vis spectra or by their different reduction potentials [3,12,14,17,20,21,24,26,39,40], and thus, the choice of complex can be made based on the target and conditions. These NO donors attached to solid state matrices can be achieved by two different approaches: (a) graft-ing or physical adsorption of the complex on a matrix already prepared; (b) occlusion, where the complex is mixed with pre-cursors of the matrix which is formed around the complex. The matrix must be inert toward the complex and the NO released, and it should also be chemically stable. Furthermore, in order to keep the photochemical NO release, the matrix should not absorb in the same wavelength range of the complex. Although an organic matrix[37]can be envisaged, a xerogel matrix has the advantage of exhibiting higher chemical and physical iner-tia, as well as displaying lower or zero absorbance in the near
UV and visible region. Sanchez et al. have already reviewed the advantages and challenges of using hybrid xerogels for opti-cal applications, and they clearly showed the feasibility of their use in such applications[41]. The first attempt to immobilize a ruthenium complex with potential ability to act as NO donor was achieved by Franco et al. They used the chemisorption oftrans-[Ru(NH3)4(SO2)(H2O)]2+on 3-(L-imidazolyl)propyl organomodified silica gel to prepare a ruthenium complex modified silica gel [–Si(CH2)3imN-Ru(NH3)4SO2][42]. This method has the advantage of leading to a chemical bond be-tween the ruthenium complex and the silica gel, possibly lead-ing to a more stable material from the recycllead-ing point of view. However, the ruthenium loading in these materials is low even for relatively small complexes like the ruthenium ammine com-pounds because they are not able to diffuse inside the inner silica pores. Therefore, other methodologies should be used for the preparation of heavily loaded ruthenium nitrosyl silicas.
As suggested before, the immobilization by sol–gel entrap-ment/occlusion in silica matrices can be a better choice[43–46]. The mild characteristics offered by the sol–gel process allow the introduction of inorganic complexes inside an inorganic net-work[47]. The sol–gel methodology has so far been used in the context of inorganic catalysts, as part of the matrix[48], as supports for dispersed metal particles[49], and for copoly-merization with suitable silicon-containing ligands [50]. The introduction of a host molecule is obtained by adding its so-lution to the polymerizing mixture. When the polymerization is complete, the dopant molecules are entangled in the inorganic polymeric network. The nature of the entrapment is still not fully understood, and it is really remarkable to see how many applications of the entrapment have been developed, without a full understanding of the process at the molecular level[51]. The entrapment of ruthenium nitrosyls can conceivably lead to changes in kinetic properties, such as rate of release of NO, which would probably lower than in solution. Similarly, Avnir and Frenkel-Mullerad[52]recently studied the unusual stabi-lization of alkaline and acid phosphatases occluded in xerogels. Remarkably, the enzymes kept their activity even at pH as low as 0.9. The explanation proposed for such a high stability took into consideration the porous microenvironment in xerogels at a molecular level. The restricted space inside these pores seems to challenge the classical meaning of thermodynamic parameters like pH, and a nanoscopic view of the interactions inside the pores among the surface groups, i.e., silanols, adsorbed water and entrapped species, seems to be more appropriate. There-fore, the study of chemical reaction on largely restricted media (LRM) needs a different approach from that used in bulk solu-tion chemistry.
In this context and considering that ruthenium nitrosyl com-plexes allow the possibility of tuning the NO donor properties
Author's personal copy
K.Q. Ferreira et al. / Journal of Colloid and Interface Science 300 (2006) 543–552 545
In this paper, we describe the immobilization and character-ization of the controlled NO donor trans-[Ru(NO)Cl(cyclam)]-(PF6)2entrapped in xerogels containing tetraethylorthosilicate (TEOS), and 3-aminopropyltriethoxysilane (3-APTS), by the sol–gel process. The reaction of 3-aminopropyltriethoxysilane with the coordinated nitrosonium of the complex, the photo-chemical release of NO from the SiO2 material, and the re-generation of the ruthenium nitrosyl complex entrapped in the matrix are also described.
2. Experimental
2.1. Chemicals and reagents
Ruthenium trichloride (RuCl3·nH2O) (Strem) was the start-ing material for the synthesis of the ruthenium complexes. Acetone, acrylonitrile, chloroform, and ethanol were purified according to procedures published in the literature[54]. Dou-bly distilled water was used throughout. Tetraethylorthosilicate (TEOS) and 3-aminopropyltriethoxysilane (3-APTS) (Aldrich) were used without further purification. All other materials were reagent grade and were used without further purification.
2.2. Complex syntheses
Trans-[RuCl(tfms)(cyclam)](tfms) (Rutfms), trans-[RuCl2 -(cyclam)]Cl (RuCl), and trans-[Ru(NO)Cl(cyclam)](PF6)2 (RuNO) were synthesized by using a published procedure[14].
2.2.1. Preparation of the entrapped complex trans-[Ru(NO)Cl(cyclam)](PF6)2
The ruthenium complex (25 mg; 3.8×10−5mol) was dis-solved in a hydrolytic solution containing TEOS (2.5 mL) in ethanol:water (4:1 v/v) and 0.1 M HCl (0.4 mL). TEOS hydrol-ysis resulted in the formation of silanol groups (Si–OH)[51]. These silanol moieties reacted further among them to form siloxane (Si–O–Si) oligomers in a condensation reaction, lead-ing to the formation of a colloidal suspension (sol). Finally, the solvents were removed from the interconnected porous net-work during a three-day drying process at 50◦C, leading to 2.7 g of a dry vitreous material, the xerogel SiO2/RuNO. The xerogel with amino groups, SiO2/SiNH2/RuNO, was obtained using the desired amount of Ru complex and TEOS (1.7 mL, 8.5 mmol) mixed with 3-APTS (0.83 mL, 4.0 mmol) instead of the pure TEOS solution. The xerogels were characterized by optical and scanning electronic microscopy; X-ray photo-electron, UV–visible, infrared, and 29Si and13C MAS NMR spectroscopies; and electrochemical techniques.
2.2.2. Reaction of trans-[Ru(NO)Cl(cyclam)](PF6)2with 3-aminopropyltrietoxysilane
The reaction of the complex with aminopropyltrietoxysilane was monitored by three spectroscopic techniques: (1) diffuse reflectance infrared spectroscopy, using the decrease of the in-tensity of theνNO band at 1865 cm−1, and theνNO/νSiOpeaks areas ratios; (2) electronic, using the increase of the absorbance of the absorption band at 484 nm; (3)13C NMR, using various
proportions of complex, aminopropyltrietoxysilane and tetrae-toxysilane. The 13C NMR spectroscopy studies were carried out in the solid state and in solution by bubbling NO(g) in aminopropyltrietoxysilane dissolved in CDCl3. Also,13C NMR spectra of the free complex and in the presence of aminopropyl-trietoxysilane were obtained.
2.2.2.1. Electrochemical measurements Cyclic voltammetry and differential pulse voltammetry experiments were taken with a model 273 PARC potentiostat/galvanostat, using a conven-tional three-electrode cell consisting of a modified carbon paste, an Ag/AgCl, and a platinum wire as the working, reference and auxiliary electrodes, respectively. The voltammetric spectra of the complex on the matrix (carbon paste mixture) was hindered by the work potential range of the carbon paste (−1.1 to 1.1 V versus Ag/AgCl)[55]. The measurements were carried out at 25◦C.E′
1/2values for the redox process of the nitrosyl ligand in the immobilized complex were determined. TheE′
1/2values were the arithmetic means of theEpaandEpcvalues.
2.2.2.2. Electronic, vibrational, and NMR spectra Electronic absorption spectra of immobilized complex were recorded us-ing a Hewlett–Packard model 8452A recordus-ing spectropho-tometer. Since the refraction index of carbon tetrachloride and the silica matrix are nearly the same[56], the supported com-plex on the silica gel surface was immersed in a spectra grade carbon tetrachloride and the spectra of the suspension were obtained using a quartz cell of 1 mm path length. Diffuse re-flectance infrared spectra were obtained on a MB-102 Bomem spectrophotometer.
13C and29Si solid-state high resolution NMR spectra were
obtained on a Varian Unity INOVA 400 (9.4 Tesla) spectrometer and a CP/MAS 7 mm Varian probe. The magic angle spinning technique (MAS) was used with a spinning frequency of 5 kHz. 29Si NMR spectra were measured by applying a single radio
frequency π/2 pulse of 4.5 µs. A recycle time of 400 s was sufficient to ensure complete magnetization recovery for all the silicon species. For the13C spectra, the cross-polarization (CP) 1H–13C NMR technique was applied with aπ/2 1H pulse of 4 µs, contact time of 1 ms and recycle time of 5 s. Tetramethyl-silane was used as reference for the obtention of13C and29Si chemical shifts.
2.2.2.3. Scanning electron microscopy The xerogel was spread over a double-side carbon tape and the sample was coated with a carbon film using a BALTECMed 020 sputter unit. Scanning electron microscopy of the xerogel was recorded using a LEO 440 microscopy equipped with an Oxford EDS de-tector.
2.2.2.4. X-ray photoelectron spectroscopy The powder sam-ples were prepared by spreading the powder over a carbon double-side adhesive tape. The charge correction was done us-ing the internal C–HxC 1s peak at 285 eV and the Si 2p peak
Author's personal copy
(a) (b)
Fig. 1. (a) Topographic, backscattered electron, image oftrans -[Ru(NO)Cl-(cyclam)](PF6)2entrapped in the SiO2matrix. (b) EDAX mapping based on the SiκαX-ray emission line.
(1253.6 eV; 180 W), as the X-ray power source, and a Kratos XSAM spectrometer were used. The Xp-spectra were fitted to a Gaussian–Lorentzian set of peaks as described before[58].
2.2.2.5. Photochemistry Monochromatic irradiations at 334 nm were carried out using a 150 W Xenon lamp in a model 6253, Oriel Universal Arc Lamp Source. The irradiation wave-length was selected with an Oriel interference filter for photol-ysis at the appropriate wavelengths. The interference filters had an average band pass of 10 nm and the collimated beam inten-sities ranged from 1×10−9to 4×10−8 einstein−1cm−2, as determined by ferrioxalate actinometry. A sample of the xero-gel in CCl4was placed in the cuvette and deaerated by bubbling argon before irradiation. The progress of the photoreactions was monitored spectrophotometrically on a MB Bomem 102 FTIR Spectrometer, using a ZnSe ATR crystal, or on HP8452A diode array spectrophotometers, in the cases of in situ vibrational and electronic spectroscopy, respectively.
3. Results and discussion
3.1. Characterization of the material
The material containing trans-[Ru(NO)Cl(cyclam)](PF6)2 entrapped in the SiO2 matrix exhibits a homogeneous color and smooth surface as judged by optical microscopy. The ho-mogeneous distribution of the Ru–NO complex was verified by scanning electronic spectroscopy (SEM) using the EDS de-tector to generate an elemental mapping of Si, and Cl X-ray emission. Unfortunately, it was not possible to collect enough signal to study the distribution oftrans-[Ru(NO)Cl(cyclam)]2+, so we had to use the Clκαline to do so. The topographic im-age and EDAX Si and Cl mapping of the SiO2/RuNO xerogel, which is similar to that of SiO2/SiNH2/RuNO, are shown in
Fig. 1. The Si mapping (Fig. 1b) confirms the siliceous nature of the xerogel. The Cl mapping, not shown, shows an homoge-neous distribution for the Ru complex in the material.
3.2. Elemental analysis
The concentration of ruthenium in the xerogels, as esti-mated from the Cl X-ray emission, is 0.6×10−2 mmol g−1, corresponding to 43% of the theoretical maximum value of
Fig. 2. Electronic spectrum in CCl4 oftrans-[Ru(NO)Cl(cyclam)](PF6)2 en-trapped in the SiO2matrix (100 mg of SiO2/RuNO in 10 mL).
Fig. 3. Electronic spectrum in CCl4 oftrans-[Ru(NO)Cl(cyclam)](PF6)2 en-trapped in the SiO2/SiNH2matrix (100 mg of SiO2/SiNH2/RuNO in 10 mL). Solid line: experimental. Dotted line: spectrum fitted with Gaussian compo-nents.
1.4×10−2 mmol g−1. It was not possible to estimate the N content by EDS.
3.3. Electronic and infrared spectra
Figs. 2–4 show the electronic and vibrational spectra of trans-[Ru(NO)Cl(cyclam)](PF6)2 entrapped in the SiO2 and SiO2/SiNH2matrices.
Author's personal copy
K.Q. Ferreira et al. / Journal of Colloid and Interface Science 300 (2006) 543–552 547
Fig. 4. Vibrational spectrum oftrans-[Ru(NO)Cl(cyclam)](PF6)2entrapped in the SiO2matrix (SiO2/RuNO).
is barely seen in the matrices, and by fitting the spectra an ab-sorption band can be located at 484 nm for the SiO2/SiNH2 matrix. The absence of the former band and the presence of the latter indicate the absence of the NO+group. However, it has been observed that nitrosonium can undergo nucleophilic attack from an amine in solution[59–61]. As a matter of fact, a rela-tively more intense band appears at 484 nm in the spectrum of a mixture of the complex and 3-aminopropyltrietoxysilane during gel formation as a result of such reaction (see ahead). Thus, the relatively weak absorption at 484 nm in the SiO2/SiNH2 ma-trix may indicate that there is also some amount of a modified complex in the matrix. This is consistent with the color change from yellow to orange in this matrix and with the infrared re-sults.
The νNO band in the infrared spectra of ruthenium nitrosyl complexes is medium-dependent and can split as a result of solid-state effects [30]. For trans-[Ru(NO)Cl(cyclam)](PF6)2, this band appears at 1875 cm−1in KBr pellet[14,26,30]. In nu-jol mulls this band is split into two bands, 1881 and 1867 cm−1, and in acetonitrile and water it appears as a single absorption at 1889 and 1899 cm−1, respectively[30]. For the complex en-trapped in the SiO2 and SiO2/SiNH2 matrices, the νNO band appears at 1870 and 1854 cm−1, respectively. These differences could also be conceivably due to possible different microenvi-ronments in the matrix, as already observed for other systems
[62–64]. The infrared spectrum of pure silica displays a weak band near 1870 cm−1assigned to a combination of fundamen-tal silica skeleton vibrations, which may also contribute to ab-sorption in this region in the entrapped complex. However, for SiO2/SiNH2, theνNO band decreases in intensity and there is an increase in the absorption at 1600 cm−1. These changes are consistent with a nucleophilic attack of the –NH2group on the coordinated nitrosonium.
3.4. Magic angle spinning nuclear magnetic resonance
3.4.1. 13C and29Si solid-state NMR
The29Si NMR spectrum of the SiO2/RuNO matrix is shown inFig. 5. Three broad NMR peaks centered at−111.0 ppm,
−102.0 ppm and −91.5 ppm can be observed. They can be
Fig. 5.29Si NMR spectrum oftrans-[Ru(NO)Cl(cyclam)](PF6)2entrapped in the SiO2matrix, SiO2/RuNO. Dotted curves: least-square fittings to the ob-served peaks.
readily attributed to silicon species with different condensa-tion degrees:Q4,Q3andQ2, respectively[65]. As usual,Qn
indicates the degree of condensation of a given SiO4 tetrahe-dron, being n the number of O involved in Si–O–Si bridges. The silicon species ratios can be obtained from the integrated intensities of the NMR lines. A least square fitting of Gauss (Q4) and Lorentz (Q3 and Q2) functions was carried out to quantify these intensities. From these results, we can conclude that 55% of the silicon present in the matrix correspond to
Q4species, where the polymerization occurred in three dimen-sions, not leaving bound silanol groups. Also, 43% correspond toQ3(only one silanol group) and less than 2% toQ2 (gemi-nated silanol).
These observations have led us to think that the condensa-tion of the hydrolyzed tetraethylorthosilicate was not hindered by the presence of the complex in the sol medium, resulting in a highly interconnected three-dimensional network of SiO2. This is very important for the final properties of the mater-ial, since the presence of large numbers of geminal groups or even of hydrolyzed tetraethylorthosilicate would mean a loss in the thermal and mechanical stability of the xerogel or would even prevent its formation. Also, the presence of 43% of iso-late silanol groups (Q3) is typical of high surface energy and high polar surface. So, we could expect a polar environment surrounding the complex, similar to that felt by the complex in a polar solvent like ethanol[66]. This polar environment could stabilize charge dissociation reaction pathways and facilitate in-duced NO dissociation from the entrapped complex. A similar matrix effect was observed for pentacyanoferrates anchored on organomodified silica gel[67]and for molybdenum carbonyls in the intrazeolyte cavity of the NaY zeolite[68].
pos-Author's personal copy
Fig. 6. (a) Representative13C CP-MAS NMR spectrum oftrans-[Ru(NO)Cl-(cyclam)](PF6)2entrapped in the SiO2matrix, SiO2/RuNO. (b)13C CP-MAS NMR spectrum oftrans-[Ru(NO)Cl(cyclam)](PF6)2 entrapped in the SiO2/ SiNH2matrix.
Fig. 7. In situ electronic spectrum of a mixture of trans -[Ru(NO)-Cl(cyclam)](PF6)2 (1 ×10−3 mol L−1) and 3-aminopropyltrietoxysilane (0.1 mol L−1) in acetonitrile. Spectra taken after 5, 10, 15, 60, 90, 100 and 130 min of reaction.
sible nucleophilic attack of the –NH2group on the coordinated nitrosonium[59–61]. This process occurs in the ammino mod-ified xerogel but not in the SiO2xerogel, because of the nucle-ophilic character of the –NH2groups.
3.4.2. Nucleophilic attack of 3-aminopropyltrietoxysilane to trans-[Ru(NO)Cl(cyclam)](PF6)2
Figs. 7 and 8 show the in situ UV–vis and infrared ab-sorption spectra of the reaction between the trans-[Ru(NO)Cl-(cyclam)]2+ complex and 3-aminopropyltrietoxysilane. The spectroscopic monitoring of this reaction shows alterations in the electronic and vibrational spectra of the complex as indi-cated by an increase in the absorbance of the bands at 350 and 484 nm (Fig. 7) due to the reaction products, and a de-crease in the intensity of the stretching band of the coordi-nated NO at 1875 cm−1, accompanied by an increase around 1600 cm−1. These spectral changes can be attributed to the nu-cleophilic attack of the –NH2 group to the NO+ coordinated
Fig. 8. In situ vibrational spectrum of a mixture of trans -[Ru(NO)-Cl(cyclam)](PF6)2 (1× 10−3 mol L−1) and 3-aminopropyltrietoxysilane (0.1 mol L−1) in acetonitrile. Time between successive spectra is 5 min.
to the ruthenium ion, with an estimated half-life of 10 min. Furthermore, the13C NMR spectrum ind3-acetonitrile of the product of the reaction oftrans-[Ru(NO)Cl(cyclam)](PF6)2and 3-aminopropyltrietoxysilane displays a peak at 180 ppm, which is not present in the13C NMR spectrum obtained in solution by bubbling NO(g) in 3-aminopropyltrietoxysilane dissolved in CDCl3 or in the 13C NMR spectrum of the free complex in acetonitrile-d3. These results are consistent with the reac-tion betweentrans-[Ru(NO)Cl(cyclam)](PF6)2 and the amine group, and also with the infrared and UV–visible spectral data of the complex immobilized in the SiO2/SiNH2 matrix. The residual absorption peak at 1875 cm−1 in the infrared spec-trum can be attributed to an incomplete reaction. However, the reaction product of this reaction is not clear. The reac-tion of some nitrosyl complexes with aromatic amines has been reported to be a diazotation reaction [59–61], result-ing in infrared bands around 2000 cm−1 and UV–vis bands around 300 nm in the spectra of the products. In the case of trans-[Ru(NO)Cl(cyclam)](PF6)2, there are no infrared bands at∼2000 cm−1in the xerogel and the band at 484 nm present in the electronic spectrum of the free complex in solution is not observed in the xerogel either, possibly because only a partial reaction occurs.
3.5. X-ray photoelectron spectroscopy
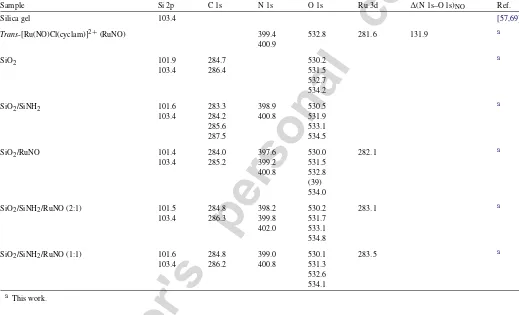
In order to study the xerogels by XPS, it was necessary to first characterize the non-entrapped Ru-cyclam compounds by the same spectroscopy. The Ru 2p3/2, N 1s, Cl 2p3/2, and the separation between N 1s (NO) and O 1s (NO) of the pure trans-[Ru(NO)Cl(cyclam)](PF6)2andtrans-[RuCl2(cyclam)]Cl com-pounds are shown inTable 1. Those of the entrapped complexes are shown inTable 2. The(N–O) difference observed for the complex is about the same as that observed in other M–NO complexes[69,70].
trans-Author's personal copy
K.Q. Ferreira et al. / Journal of Colloid and Interface Science 300 (2006) 543–552 549
Table 1
XPS binding energies in eV for the ruthenium nitrosyl complexes synthesized herein and for complexes reported in the literature
Sample Ru 3p3/2 N 1s
(cyclam)
N 1s (NO)
Cl 2p3/2 (bound)
(N 1s–O 1s) Ref.
Trans-[RuCl2(cyclam)]+ 463.1 398.7 196.5 a
Trans-[RuCl(NO)(cyclam)]2+ 462.5 399.4 400.9 198.6 131.9 a
[Ru(NO)2(Pφ3)2]4+ 464.5 400.6 132.0 [69]
[Ru(NO)Cl5]2− 464.6 402.8 [70]
a This work.
Table 2
XPS binding energies in eV for the xerogel matrices (SiO2and SiO2/SiNH2) and for the xerogel matrices containing the entrapped complexes (SiO2/RuNO, SiO2/SiNH2/RuNO)
Sample Si 2p C 1s N 1s O 1s Ru 3d (N 1s–O 1s)NO Ref.
Silica gel 103.4 [57,69]
Trans-[Ru(NO)Cl(cyclam)]2+(RuNO) 399.4 532.8 281.6 131.9 a
400.9
SiO2 101.9 284.7 530.2 a
103.4 286.4 531.5
532.7 534.2
SiO2/SiNH2 101.6 283.3 398.9 530.5 a
103.4 284.2 400.8 531.9
285.6 533.1
287.5 534.5
SiO2/RuNO 101.4 284.0 397.6 530.0 282.1 a
103.4 285.2 399.2 531.5
400.8 532.8 (39) 534.0
SiO2/SiNH2/RuNO (2:1) 101.5 284.8 398.2 530.2 283.1 a
103.4 286.3 399.8 531.7
402.0 533.1 534.8
SiO2/SiNH2/RuNO (1:1) 101.6 284.8 399.0 530.1 283.5 a
103.4 286.2 400.8 531.3
532.6 534.1 a This work.
[Ru(NO)Cl(cyclam)](PF6)2. This higher binding energy can be interpreted as a result of a lower electronic density on the Ru–Cl complex. This is in agreement with the oxidation state of the Ru atoms in the different complexes. In Ru–Cl the Ru atom is formally+3, and in thetrans-[Ru(NO)Cl(cyclam)](PF6)2 com-plex it is+2. It is also possible to see that the Cl 2p3/2 and N 1s (cyclam) peaks in thetrans-[Ru(NO)Cl(cyclam)](PF6)2 complex are at higher binding energies than those for the trans-[RuCl2(cyclam)]Cl complex. Therefore, it seems that the coor-dinated nitrosonium, NO+
, is drifting electronic density from the chloro ligand in trans and from the nitrogen of the cy-clam. This explanation is in agreement with the previously reported decrease in the pKaof the coordinated water in trans-[Ru(NH3)4(NO)(H2O)]3+ compared to [Ru(NH3)5(H2O)]3+
[23,26]. Indeed, the authors explained this decrease in pKa in terms of electronic density drifting from water to NO through the H2O–Ru–NO+axis[23,26]. So, the chloro ligand in
trans-[Ru(NO)Cl(cyclam)](PF6)2 is probably acting as aσ- andπ -Lewis base toward Ru and, therefore, it transfers electronic density to the Ru–NO moiety.
The Ru 3d5/2and N 1s XP-peaks of SiO2/RuNO are quite similar to those of the bulk trans-[Ru(NO)Cl(cyclam)]2+, as shown inTable 2. These findings agree with the FT-IR results.
The N 1s XP-peaks of SiO2/SiNH2display an asymmetric peak fitted with 2 Gaussian–Lorentzian components assigned to NH2and NH+3 species at 398.9 and 400.8 eV, respectively[71]. The ratio between these species is ca. 3:1.
Author's personal copy
and 283.5 eV for the xerogel made with 3-APTS andtrans-[Ru(NO)Cl(cyclam)](PF6)2 complex with molar ratios of 2:1 and 1:1. This higher shift seems to corroborate the UV–vis, FTIR, and13C NMR results for these xerogels.
3.6. Electrochemistry
The immobilization oftrans-[Ru(NO)Cl(cyclam)](PF6)2in the SiO2 matrix does not significantly affect the redox po-tentials, as in the case of trans-[Ru(NH3)4(imN)(SO2)] [42]. In the potential range studied (−1.0–1.0 V), where the mate-rials are not electro-active, the cyclic voltammetry of trans-[Ru(NO)Cl(cyclam)](PF6)2entrapped in SiO2shows the occur-rence of two electrochemical processes (vs Ag/AgCl):Epc1 at −0.39 V,Epc2 at −0.30 V (E′
1/2= −0.34 V; E=0.09 V;
Ipa/Ipc=0.8). TheEpc1 value is close to that of the complex in solution (−0.33 V vs Ag/AgCl)[14], and is assigned like-wise to the reduction of the nitrosonium ligand (NO+/0) in the immobilized complex.
3.7. Photochemical studies
In view of the previous results, we focused our attention on SiO2/RuNO. Exposure of SiO2/RuNO, in CCl4, in deaer-ated conditions, to irradiation with light of 334 nm results in decrease in the intensity of theνNO band at 1870 cm−1, (Fig. 9). This decrease undoubtedly suggests the photochem-ical labilization of NO. Similar changes in the infrared spec-trum had already been observed with a 10−4 mol L−1 trans-[RuCl(NO)(cyclam)](PF6)2 solution at pH 7 (0.1 mol L−1, phosphate buffer)[29]. Furthermore, after photolysis, the color of the solid material changed from yellow to green, with a broad absorption increase in the 300–340 nm region of the UV–vis spectrum, which is consistent with the formation of a RuIII(cyclam) complex photoproduct in the intra-pores of the xerogel, thus yielding an NO depleted material, SiO2/Ru (Fig. 10), as observed for trans-[RuCl(NO)(cyclam)](PF6)2
Fig. 9. Vibrational spectra, in the mid-infrared region, of SiO2/RuNO under irradiation at 334 nm. Arrows indicate the decrease in the NO peak as a function of the irradiation time. Elapsed time between each line is 60 min.
[41] and other [Ru(NH3)4(X)(NO)]n+ [8,20,72] and trans-[RuCl(NO)([15]ane)]2+[73]complexes in solution, which ren-ders the respective Ru(III) aqua species and NO.
Despite the undoubted infrared results, confirmation of the photolabilization of NO from the entrapped complex in the case of SiO2/RuNO was achieved by a trapping technique us-ing a quartz cuvette topped with a glass reservoir. The xerogel was placed in the cuvette and 3 mL of a 7.4×10−5 mol L−1 solution of the trapping agent,trans-[RuCl(cyclam)(OH2)]2+, was placed in the upper reservoir under argon atmosphere. The trans-[RuCl(cyclam)(OH2)]2+complex reacts with NO (kon= 0.2 M−1s−1at pH 1 and 25◦C)[29]to form the corresponding nitrosyl complextrans-[RuCl(NO)(cyclam)]2+
. Upon photol-ysis of the solid material in the cuvette, the released NO was thus trapped by trans-[RuCl(cyclam)(OH2)]2+ to give trans-[RuCl(NO)(cyclam)]2+ as evidenced by the new absorption band at 266 nm, typical of the latter species (Fig. 11).
Fig. 10. UV–vis spectra of SiO2/RuNO (a) before irradiation at 334 nm and (b) after 60 min of irradiation.
Author's personal copy
K.Q. Ferreira et al. / Journal of Colloid and Interface Science 300 (2006) 543–552 551
Fig. 12. Infrared spectral changes during reaction between the photolysis prod-uct, at 334 nm, oftrans-[Ru(NO)Cl(cyclam)](PF6)2 entrapped in SiO2 and NO(g). (a) Before reaction (in Web version green solid) and (b) after reaction (in Web version yellow solid).
The NO depleted xerogel (SiO2/Ru) could be reloaded to the nitrosyl form, SiO2/RuNO, by reaction with bubbling NO(g), at 25◦C, for 60 min in deaerated conditions. The regeneration of SiO2/RuNO was confirmed by observing the reappearance of its typical yellow color and the νNO stretching band at 1870 cm−1in the FTIR spectrum (Fig. 12). Therefore, a load-ing–depleting–reloading cycle can be delineated as illustrated inFig. 13.
4. Summary
Our results show that the controlled NO donor trans-[Ru(NO)Cl(cyclam)](PF6)2can be entrapped in a SiO2matrix with preservation of its molecular structure and properties. In a SiO2/SiNH2 matrix, the complex undergoes a nucleophilic
attack by the amine group at the nitrosonium, thus indicating that the use of amine functionalized silicas for metal nitrosyl complexes should be avoided. Like the complex in solution, ir-radiation of the complex entrapped in a SiO2matrix with light of 334 nm results in the release of NO, as evidenced by changes in the UV–vis and IR spectra of the solid as well as by trapping the released NO. This material was regenerated to the nitro-syl form by reaction with nitric oxide. Thus, this system has the potential to serve as a model to design regenerable pre-cursors for photochemical NO delivery to various biological targets, and it also contributes to the design of materials coat-ings such as stents. Experiments concerning further materials characteristics as well as quantitative data concerning chemical and photochemical release of NO and chemical regeneration of the material are under schedule in our lab.
Acknowledgments
The authors thank the Brazilian agencies FAPESP, CNPq, and CAPES for financial support, Prof. Thiery Gacoin for helpful suggestions and Dr. Cynthia Maria de Campos Prado Manso, for the English revision of the manuscript.
Supplementary material
The online version of this article contains additional supple-mentary material.
Please visitDOI: 10.1016/j.jcis.2006.03.081.
References
[1] M.J. Ignarro, Nitric Oxide: Biology and Pathobiology, Academic Press, New York, 2000.
[2] H. Kunkely, A. Vogler, Inorg. Chim. Acta 346 (2003) 275.
[3] M.G. Sauaia, R.G. de Lima, A.C. Tedesco, R.S. da Silva, J. Am. Chem. Soc. 125 (2003) 14718.
Author's personal copy
[4] Y. Chen, R.E. Shepherd, J. Inorg. Biochem. 68 (1997) 183.[5] A.S. Torsoni, B.F. de Barros, J.C. Toledo, M. Haun, M.H. Krieger, E. Tfouni, D.W. Franco, Nitric Oxide Biol. Chem. 6 (2002) 247.
[6] F.G. Marcondes, A.A. Ferro, A. Souza-Torsoni, M. Sumitani, M.J. Clarke, D.W. Franco, E. Tfouni, M.H. Krieger, Life Sci. 70 (2002) 2735. [7] P.C. Ford, I.M. Lorkovic, Chem. Rev. 102 (2002) 993.
[8] K. Szacilowski, W. Macyk, G. Stochel, Z. Stasicka, S. Sostero, O. Tra-verso, Coord. Chem. Rev. 208 (2000) 297.
[9] E. Tfouni, M. Krieger, B.R. McGarvey, D.W. Franco, Coord. Chem. Rev. 236 (2003) 57.
[10] A.K. Patra, M.J. Rose, K.A. Murphy, M.M. Olmstead, P.K. Mascharak, Inorg. Chem. 43 (2004) 4487.
[11] S. Wecksler, A. Mikhailovsky, P.C. Ford, J. Am. Chem. Soc. 126 (2004) 13566.
[12] R.G. de Lima, M.G. Sauaia, D. Bonaventura, A.C. Tedesco, R.F.V. Lopez, L.M. Bendhack, R.S. da Silva, Inorg. Chim. Acta 358 (2005) 2643. [13] P.G. Wang, M. Xian, X.P. Tang, X.J. Wu, Z. Wen, T.W. Cai, A.J. Janczuk,
Chem. Rev. 102 (2002) 1091.
[14] D.R. Lang, J.A. Davis, L.G.F. Lopes, A.A. Ferro, L.C.G. Vasconcellos, D.W. Franco, E. Tfouni, A. Wieraszko, M.J. Clarke, Inorg. Chem. 39 (2000) 2294.
[15] S.P. Fricker, E. Slade, N.A. Powell, O.J. Vaughan, G.R. Henderson, B.A. Murrer, I.L. Megson, S.K. Bisland, F.W. Flitney, Br. J. Pharmacol. 122 (1997) 1441.
[16] J. Bourassa, W. DeGraff, S. Kudo, D.A. Wink, J.B. Mitchell, P.C. Ford, J. Am. Chem. Soc. 119 (1997) 2853.
[17] M.G. Sauaia, F.D.S. Oliveira, R.G. de Lima, A.D.L. Cacciari, E. Tfouni, R.S. da Silva, Inorg. Chem. Commun. 8 (2005) 347.
[18] J. Bordini, D.L. Hughes, J.D.D. Neto, C.J. da Cunha, Inorg. Chem. 41 (2002) 5410.
[19] C.F. Works, C.J. Jocher, G.D. Bart, X.H. Bu, P.C. Ford, Inorg. Chem. 41 (2002) 3728.
[20] R.M. Carlos, A.A. Ferro, H.A.S. Silva, M.G. Gomes, S.S.S. Borges, P.C. Ford, E. Tfouni, D.W. Franco, Inorg. Chim. Acta 357 (2004) 1381. [21] F.D. Oliveira, V. Togniolo, T.T. Pupo, A.C. Tedesco, R.S. da Silva, Inorg.
Chem. Commun. 7 (2004) 160.
[22] J.C. Toledo, L.G.D. Lopes, A.A. Alves, L.P. da Silva, D.W. Franco, J. Inorg. Biochem. 89 (2002) 267.
[23] C.W.B. Bezerra, S.C. da Silva, M.T.P. Gambardella, R.H.A. Santos, L.M.A. Plicas, E. Tfouni, D.W. Franco, Inorg. Chem. 38 (1999) 5660. [24] B.F. de Barros, J.C. Toledo, D.W. Franco, E. Tfouni, M.H. Krieger, Nitric
Oxide Biol. Chem. 7 (2002) 50.
[25] J.A. Silva, J.C. Toledo, M.E.R. Guzzi, D.W. Franco, D. Wilhelm, R.C. Pedrosa, T.B. Creczynski-Pasa, Free Radical Biol. Med. 33 (2002) S244. [26] B.R. McGarvey, A.A. Ferro, E. Tfouni, C.W.B. Bezerra, I. Bagatin, D.W.
Franco, Inorg. Chem. 39 (2000) 3577.
[27] D. Bonaventura, F.S. Oliveira, R.S. da Silva, L.M. Bendhack, Clin. Exp. Pharmacol. Physiol. 32 (2005) 478.
[28] V. Mori, J.C. Toledo, H.A.S. Silva, D.W. Franco, M. Bertotti, J. Elec-troanal. Chem. 547 (2003) 9.
[29] K.Q. Ferreira, Ph.D. thesis, Universidade de São Paulo, 2004.
[30] E. Tfouni, K.Q. Ferreira, F.G. Doro, R.S. da Silva, Z.N. da Rocha, Coord. Chem. Rev. 249 (2005) 405.
[31] G. Stochel, A. Wanat, E. Kulis, Z. Stasicka, Coord. Chem. Rev. 171 (1998) 203.
[32] P.C. Ford, J. Bourassa, K. Miranda, B. Lee, I. Lorkovic, S. Boggs, S. Kudo, L. Laverman, Coord. Chem. Rev. 171 (1998) 185.
[33] L.K. Keefer, Annu. Rev. Pharmacol. Toxicol. 43 (2003) 585.
[34] H.P. Zhang, G.M. Annich, J. Miskulin, K. Stankiewicz, K. Osterholzer, S.I. Merz, R.H. Bartlett, M.E. Meyerhoff, J. Am. Chem. Soc. 125 (2003) 5015.
[35] J.A. Hrabie, J.R. Klose, D.A. Wink, L.K. Keefer, J. Org. Chem. 58 (1993) 1472.
[36] S.M. Marxer, A.R. Rothrock, B.J. Nablo, M.E. Robbins, M.H. Schoen-fisch, Chem. Mater. 15 (2003) 4193.
[37] J.T. Mitchell-Koch, T.M. Reed, A.S. Borovik, Angew. Chem. Int. Ed. 43 (2004) 2806.
[38] H.E. Toma, A.M. Alexiou, A.L.B. Formiga, M. Nakamura, S. Dovi-dauskas, M.N. Eberlin, D.M. Tomazela, Inorg. Chim. Acta 358 (2005) 2891.
[39] J.C. Toledo, H.A.S. Silva, M. Scarpellini, V. Mori, A.J. Camargo, M. Bertotti, D.W. Franco, Eur. J. Inorg. Chem. 9 (2004) 1879.
[40] J.M. Slocik, M.S. Ward, R.E. Shepherd, Inorg. Chim. Acta 317 (2001) 290.
[41] C. Sanchez, B. Lebeau, F. Chaput, J.P. Boilot, Adv. Mater. 15 (2003) 1969. [42] S.M.C. Neiva, J.A.V. Santos, J.C. Moreira, Y. Gushikem, H. Vargas, D.W.
Franco, Langmuir 9 (1993) 2982.
[43] J. Blum, A. Rosenfeld, N. Polak, O. Israelson, H. Schumann, D. Avnir, J. Mol. Catal. A Chem. 107 (1996) 217.
[44] Y. Kurusu, D.C. Neckers, J. Org. Chem. 56 (1991) 1981. [45] A. Rosenfeld, J. Blum, D. Avnir, J. Catal. 164 (1996) 363. [46] C. Sanchez, F. Ribot, B. Lebeau, J. Mater. Chem. 9 (1999) 35. [47] H. Schmidt, A. Kaiser, H. Patzelt, H. Scholze, J. Phys. 43 (1982) 275. [48] D. Bianchi, M. Lacroix, G.M. Pajonk, S.J. Teichner, J. Catal. 68 (1981)
411.
[49] T. Lopez, P. Bosch, M. Asomoza, R. Gomez, J. Catal. 133 (1992) 247. [50] U. Schubert, New J. Chem. 18 (1994) 1049.
[51] D. Avnir, Acc. Chem. Res. 28 (1995) 328.
[52] H. Frenkel-Mullerad, D. Avnir, J. Am. Chem. Soc. 127 (2005) 8077. [53] J. Bordini, P.C. Ford, E. Tfouni, Chem. Commun. (2005) 4169. [54] A.I. Vogel, Química Orgânica, Ao Livro Técnico, Rio de Janeiro, 1980. [55] K. Kalcher, J.M. Kauffmann, J. Wang, I. Svancara, K. Vytras, C. Neuhold,
Z. Yang, Electroanalysis 7 (1995) 5.
[56] Y. Gushikem, C.R.M. Peixoto, U.P. Rodrigues, L.T. Kubota, E. Stadler, J. Colloid Interface Sci. 184 (1996) 236.
[57] S.S. Chao, Y. Takagi, G. Lucovsky, P. Pai, R.C. Custer, J.E. Tyler, J.E. Keem, Appl. Surf. Sci. 26 (1986) 575.
[58] A. Proctor, D.M. Hercules, Appl. Spectrosc. 38 (1984) 505.
[59] S. Sinha, A.K. Banerjee, B.K. Ghosh, Trans. Met. Chem. 23 (1998) 151. [60] F. Doctorovich, C. Trápani, Tetrahedron Lett. 40 (1999) 4635.
[61] W.L. Bowden, W.F. Little, T.J. Meyer, J. Am. Chem. Soc. 99 (1977) 4340. [62] Z.J. Wu, H. Joo, I.S. Ahn, J.H. Kim, C.K. Kim, K. Lee, J. Non-Cryst.
Solids 342 (2004) 46.
[63] M.S. Ahola, E.S. Sailynoja, M.H. Raitavuo, M.M. Vaahtio, J.I. Salonen, A.U.O. Yli-Urpo, Biomaterials 22 (2001) 2163.
[64] M. Zayat, R. Pardo, D. Levy, J. Mater. Chem. 13 (2003) 2899.
[65] G. Engelhardt, E.D. Michel, High Resolution Solid-State NMR of Sili-cates and Zeolites, Wiley, Norwich, 1987.
[66] C. Rottman, G. Grader, D. Avnir, Chem. Mater. 13 (2001) 3631. [67] U.P. Rodrigues, Y. Gushikem, F.Y. Fujiwara, E. Stadler, V. Drago, Struct.
Chem. 5 (1994) 129.
[68] H.O. Pastore, G.A. Ozin, A.J. Pöe, J. Am. Chem. Soc. 115 (1993) 1215. [69] D.T. Clark, I.S. Woolsey, S.D. Robinson, K.R. Laing, J.N. Wingfield,
In-org. Chem. 16 (1977) 1201.
[70] V.I. Nefedov, Y.V. Salyn, A.P. Sadovsky, L. Beyer, J. Electron Spectrosc. Relat. Phenom. 12 (1977) 121.
[71] J.L. Magalhaes, L.M. Moreira, U.P. Rodrigues, M.J. Giz, M.A. Pereira da Silva, R. Landers, R.C.G. Vinhas, P.A.P. Nascente, Surf. Interface Anal. 33 (2002) 293.
[72] E. Tfouni, Coord. Chem. Rev. 196 (2000) 281.
Cl-6)2 entrapped in the SiO2 matrix](https://thumb-ap.123doks.com/thumbv2/123dok/2063821.1604913/5.595.312.542.94.256/topographic-backscattered-electron-ofauthor-personal-copythe-entrapped-matrix.webp)
2 entrapped inthe SiO2 matrix (SiO2/RuNO).](https://thumb-ap.123doks.com/thumbv2/123dok/2063821.1604913/6.595.58.280.90.257/vibrational-spectrum-ofauthor-personal-cyclam-entrapped-matrix-runo.webp)
2 (1 × 10−3 molL−1) and 3-aminopropyltrietoxysilane(0.1 mol L−1) in acetonitrile](https://thumb-ap.123doks.com/thumbv2/123dok/2063821.1604913/7.595.45.277.78.280/electronic-spectrum-mixture-ofauthor-personal-cyclam-aminopropyltrietoxysilane-acetonitrile.webp)
![Fig. 11. UV–vis spectra of the NO sequestering complex, trans-[RuCl(cyclam)-(OH2)]2+, during the NO trapping experiment, as a function of time of irradi-ation of SiO2/RuNO.](https://thumb-ap.123doks.com/thumbv2/123dok/2063821.1604913/9.595.310.545.545.736/spectra-sequestering-complex-cyclam-trapping-experiment-function-irradi.webp)
2 entrapped in SiO2 andNO(g)](https://thumb-ap.123doks.com/thumbv2/123dok/2063821.1604913/10.595.86.522.520.744/infrared-spectral-changes-reaction-photolysis-author-personal-entrapped.webp)