www.elsevier.com / locate / bres
Short communication
Post-ictal analgesia: involvement of opioid, serotoninergic and
cholinergic mechanisms
a ,
*
a a a aN.C. Coimbra
, C. Castro-Souza , E.N. Segato , J.E.P. Nora , C.F.P.S. Herrero ,
a b
W. Tedeschi-Filho , N. Garcia-Cairasco
a
˜ ´
Laboratorio de Neuroanatomia e Neuropsicobiologia, Departamento de Farmacologia, Faculdade de Medicina de Ribeirao Preto,
˜ ˜
Universidade de Sao Paulo(USP), 14049-900, Ribeirao Preto (SP), Brazil
b
˜ ´
Laboratorio de Neurofisiologia e Neuroetologia Experimental, Departamento de Fisiologia, FMRP-USP, 14049-900, Ribeirao Preto (SP), Brazil Accepted 10 October 2000
Abstract
The neural mechanisms involved in post-ictal analgesia remain to be elucidated. Pentylenetetrazol (PTZ) is used experimentally to
2
induce seizure in animal subjects. This non-competitive antagonist blocks GABA-mediated Cl flux. The aim of this work is to study the neurochemical basis of the antinociception induced by convulsions elicited by peripheral administration of PTZ (64 mg / kg). The analgesia was measured by the tail-flick test, in eight rats per group. Convulsions were followed by significant increase in the tail-flick latencies (TFL), at least for 30 min of the post-ictal period. Peripheral administration of naloxone (5 mg / kg and 10 mg / kg), atropine (1 mg / kg and 5 mg / kg), methysergide (1 mg / kg and 5 mg / kg) and ketanserine (1 mg / kg and 2 mg / kg) caused a significant decrease in the TFL in seizing animals, as compared to controls. However, while naloxone antagonized analgesia 15 and 25 min post convulsions, the other drugs caused a blockade of the post-ictal analgesia in a relatively greater period of time. These results indicate that endogenous opioids, serotonin and acetylcholine may be involved in post-ictal analgesia. 2001 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters and receptors
Topic: GABA
Keywords: Post-ictal analgesia; Pentylenetetrazol; GABA receptor; 5-HT receptor; Endogenous opioid; Muscarinic receptor; Tail-flick testA 2
A recent finding in the literature, demonstrates that in duced by stimulation of structures where it is also possible patients with temporal lobe epilepsy, the nociception to induce convulsive seizures [1,2,5,14,20].
threshold seems to be spontaneously high but is not In this work we tried to demonstrate whether or not antagonized by pretreatment with naloxone [13]. However, seizures induced by drugs, such as PTZ, might be followed the analgesia that follows experimentally induced convul- by antinociception. After the detection of post-ictal analge-sive seizures still needs to be clarified. PTZ is a GABAer- sia, the correlated neurochemistry was investigated using gic non-competitive antagonist that does not interact some non-specific pharmacological antagonists, such as directly with GABA receptors, but blocks the GABA- naloxone and methysergide, and specific ones, such as
2
mediated Cl influx. The intraperitoneal (i.p.) injection of ketanserin and atropine. Thus, the involvement of some PTZ, in rats, causes tonic–clonic seizures [8,19]. receptors subtypes such as cholinergic muscarinic and The study of GABAergic, opioid, serotonergic and those from the serotonergic 5-HT2 subfamily, were also cholinergic mechanisms can offer elucidative data about considered.
the neurochemistry of the post-ictal analgesia. In fact, Wistar albino rats, weighing between 200 and 250 g, endogenous opioids, serotonin and acetylcholine seem to four in a cage, had free access to food and water. All be critically implicated in antinociceptive processes in- protocols were used according to the rules for animal experimentation of the Brazilian Society for Neuroscience and Behavior.
*Corresponding author.
E-mail address: [email protected] (N.C. Coimbra). All the rats had their nociception thresholds compared,
using the tail flick test. Each animal was placed in a Two other groups received either PTZ or saline intraper-restraining apparatus (Stoelting) with acrylic walls, and its itoneally (i.p.). After receiving the drug, animals were tail was placed in a heating sensor (tail-flick Analgesia placed in the arena, until the end of seizures (PTZ), or 15 Instrument; Stoelting), whose calorimetric progressive min after saline, i.p., when their tail-flick latencies were elevation was automatically interrupted, as long as the determined.
animal took out his tail of the apparatus. A small current Independent groups of animals received peripheral intensity adjustment could be done, if necessary, in the administrations of naloxone, atropine, methysergide, ketan-beginning of the experiment, aiming to obtain three serin or saline followed by, after 10 min, i.p. PTZ consecutive tail-flick latencies (TFL), between 2.5 s and administration. The nociceptive responses (threshold) were 3.5 s. If the animal did not remove his tail out of the heater measured immediately after seizures and after 5, 15, 25 within 6 s, the apparatus would be turned off in order to and 30 min following the convulsion.
prevent damage to the skin. All the tail flick latencies were Aiming to investigate occasional motor alterations that normalized in an index of analgesia (IA) using the could interfere in the motor reflexes, another group of formula: animals were injected with either PTZ (i.p.) or its vehicle,
]] the animals were placed in the open-field, and observed up
(TFLtest)2(TFLcontrol)
]]]]]]]
IA5 ]] to 30 min for the following behaviors: crossing (entrance
62(TFLcontrol) of the four paws in one of 12 compartments of the
open-field), turnings (3608 rotations), rearings (rearing of Three baselines of control tail-flick latencies were taken
at 5-min intervals. Tail-flick latencies were also measured the front paws, supporting in the arena’s walls), limbic following seizures elicited by peripheral administrations of behavior (smelling out and chewing movements, with PTZ. lateral or vertical head movements), and body cleaning Behavioral tests were made in the interior of a circular (grooming). This ethological analysis was made in a 30 arena, whose walls, made with transparent acrylic, mea- min period of time.
sured 60 cm diameter and 50 cm height, and the floor was The motor performance of the animals was also investi-divided in 12 equal sections. This arena was located in an gated by means of the rota-rod test. After receiving i.p. experimental compartment and illuminated by a fluorescent administrations of PTZ or saline, animals were taken to the lamp (350 lux at the arena floor level). rota-rod, where was recorded the permanence time after The evaluation of the effects of drugs administration seizures (or 1 min after saline administration) and 5, 15, (PTZ, naloxone, methysergide, ketanserine, atropine and 25, 30 min after the drug. Putting animals in dorsal saline) as well as the ethogram (analysis of motor behavior decubit, the righting reflexes were evaluated in both in the open-field), and the recording of the righting experimental groups immediately after seizures or 1 min reflexes, were made in the interior of this apparatus. after saline administration. Following that, the time spent
In the post-ictal period, the motor performance of each in the righting operation was recorded.
animal, in another group, was evaluated using the rota-rod PTZ (Sigma), naloxone (Sigma), atropine (Sigma), test. Animals were taken out of the arena and placed in the methysergide (RBI) and ketanserin (RBI) were each rota-rod apparatus (Ugo Basile 7750 model) that consists dissolved in physiological saline (NaCl; 0.9%) shortly in an acrylic cylinder, divided in equal compartments, before use. Physiological saline also served as vehicle placed 40 cm away from a semi-suspended plaque. This control.
interrupted a digital counter when activated by the rat’s Drugs were administered in the following doses: nalox-weight, during its fall in the cylinder. Latencies of the one (5 and 10 mg / kg), atropine (1 and 5 mg / kg), permanence of each animal in the cylinder in movement methysergide (1 and 5 mg / kg), ketanserin (1 and 2 mg / could be recorded after peripheral administration of either kg) and PTZ (64 mg / kg).
PTZ or its vehicle. The data were analyzed using the Mann–Whitney U-During the tests, animals were gently placed above a test, because the patterns did not follow a normal dis-cylinder that was immediately started. Following that, the tribution and the groups were independent. A level of permanence time in the cylinder in movement was also P,0.05 was used to confirm statistically significant differ-recorded. Immediately after this recording, animals were ences.
removed away and placed again in the arena, in order to PTZ induced severe tonic–clonic seizures in all animals. continue the ethological analysis. Convulsions were not preceded by wild running, and lasted
First of all, a baseline of the tail-flick test was made in from 20 s up to 80 s.
every animal of each group. Animals of the control group Control animals submitted to tail-flick test and placed were taken out of the box and placed in the open-field, also in the experimental situation for 10 min, without without receiving drug injection and observed in a 15-min receiving any type of drugs afterwards, did not display time window. After this period, animals were taken out of significant changes in the nociceptive threshold at any time the arena and had their nociception threshold standardized studied (P.0.05 in all cases).
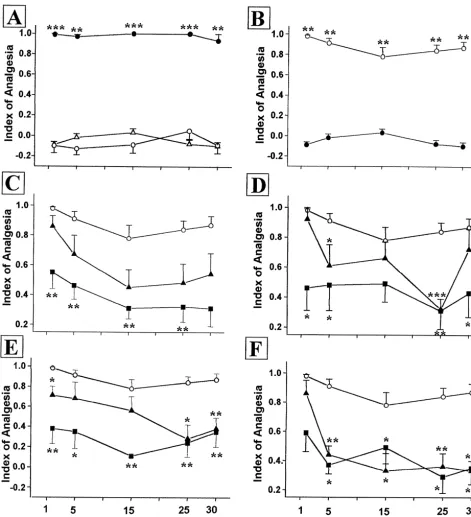
group, also did not induce any significant change in the was a tendency to induce antagonism immediately after nociceptive threshold during the time observed, 1 min after convulsive reactions (Mann–Whitney: U524; P.0.05) at saline administration (Mann–Whitney: U523.5; P.0.05), 15 (Mann–Whitney: U526; P.0.05) and 30 min (Mann– 5 (Mann–Whitney: U514; P.0.05), 15 (Mann–Whitney: Whitney: U528.5; P.0.05), this effect was not
statisti-U515.5; P.0.05), 25 (Mann–Whitney: U515.5; P. cally significant.
0.05) and 30 min (Mann–Whitney: U524; P.0.05) after Methysergide pre-treatment (5 mg / kg), was capable of the drug, when compared to the control group. antagonizing the post-ictal antinociception immediately However, after the generalized tonic–clonic seizures (Mann–Whitney: U510; P,0.05), 5 (Mann–Whitney: induced by PTZ we observed a strong analgesia, supported U514; P,0.05), 25 (Mann–Whitney: U56.5; P,0.01) by increased tail-flick latencies, recorded immediately after and 30 min (Mann–Whitney: U510.5; P,0.05) after the end of seizures (Mann–Whitney: U50.0; P,0.001), 5 seizures. At 15 min of the post-ictal period, however, there (Mann–Whitney: U50.0; P,0.01), 15 (Mann–Whitney: was a tendency to antagonism, but this effect was not
U50.0; P,0.001), 25 (Mann–Whitney: U50.0; P, statistically significant (Mann–Whitney: U510.5; P.
0.001) and 30 min (Mann–Whitney: U50.0; P,0.01) 0.05). These data follow a dose-dependent effect.
after seizures. This effect was also highly significant Pre-treatment with ketanserin (1 mg / kg), a specific 5 statistically after the saline pre-treatment, followed, after HT / 5HT2 1C antagonist, was effective in antagonizing the 10 min, by PTZ. Also in this experiment, which was used post-ictal analgesia immediately after seizures (Mann– as a control for the neurochemistry study, it was detected Whitney: U515; P,0.05), 25 (Mann–Whitney: U59; an expressive post-ictal analgesia (Mann–Whitney: U5 P,0.05) and 30 min (Mann–Whitney: U57.5; P,0.01) 0.0; P,0.001 in all post-ictal periods studied). after seizures. However there was a tendency to antagon-Immediately (Whitney: U520; P.0.05), and 5 min ism at 5 (Mann–Whitney: U524; P.0.05) and 15 min (Mann–Whitney: U519.5; P.0.05) after seizures, al- (Mann–Whitney: U524; P.0.05) post-convulsion, but though there was a tendency to be antagonized by nalox- these differences were not statistically significant.
one, this effect was not statistically significant. However, Kentanserin pre-treatment (2 mg / kg) was effective also naloxone pre-treatment (5 mg / kg) was effective in an- in antagonizing the post-ictal antinociception in the differ-tagonizing the post-ictal analgesia recorded at 15 (Mann– ent stages of this antinociceptive process. This effect was Whitney: U513; P,0.05) and 25 (Mann–Whitney: U5 extremely consistent immediately after seizures (Mann– 13; P,0.05) min after convulsion. Although there was Whitney: U55; P,0.01) and at 5 (Mann–Whitney: U59; also a tendency to antagonism in the 30 min post-seizure, P,0.05), 15 (Mann–Whitney: U52; P,0.01), 25 this difference was not statistically significant (Mann– (Mann–Whitney: U57; P,0.01) or 30 min (Mann–Whit-Whitney: U513.5; P50.051). Nevertheless, the pre-treat- ney: U54; P,0.01) after convulsion. These data follow ment, with naloxone (10 mg / kg), was effective in an- clearly a dose–effect curve. All psychopharmacological tagonizing the post-ictal analgesia recorded immediately effects were presented in Fig. 1.
(Mann–Whitney: U55; P,0.01), 5 (Mann–Whitney: U5 Righting reflexes were evaluated in animals that re-3; P,0.01), 15 (Mann–Whitney: U55; P,0.01), 25 ceived saline (recorded in the first minute after drug) or (Mann–Whitney: U52; P,0.01) and 30 min (Mann– PTZ (recorded immediately after seizures), detected in Whitney: U54; P,0.01) after seizures. These data fol- 100% of the animals studied in the present work; there was lowed a dose-dependent effect. no statistically significant difference in their latencies, When the involvement of others transmitters in the when compared to controls (data not shown). The evalua-post-ictal analgesia were studied, we observed that, the tion of the motor performance of animals submitted to the pre-treatment with atropine (1 mg / kg and 5 mg / kg), was rota-rod test showed no statistically significant differences capable of antagonizing the post-ictal antinociception, between latencies observed immediately after seizures mainly at 5 (Mann–Whitney: U51 and 11; P,0.001, (Mann–Whitney: U515.5; P.0.05), at 5
(Mann–Whit-P,0.05, respectively), 15 (Mann–Whitney: U58 and 9; ney: U515; P.0.05), 15 (Mann–Whitney: U517.5; P. P,0.05 and P,0.02, respectively), 25 (Mann–Whitney: 0.05), 25 (Mann–Whitney: U58.5; P.0.05) and 30 min
U52 and 13; P,0.01 and P,0.05, respectively) and 30 (Mann–Whitney: U513.5; P.0.05) of the post-ictal min (Mann–Whitney: U51 and 3, respectively; P,0.01 period. These data are presented in Fig. 2A.
Fig. 1. (A) Effects of administration of PTZ (d–d) (64 mg / kg; i.p.) or saline (s–s) on nociceptive threshold (n57; **P,0.01, ***P,0.001 according to Mann–Whitney’s U-test, as compared to the control). Control group (n–n): animals were placed in the open-field, without any pharmacological treatment, used as general control, as well as to evaluate the aversive character of either open-field or contention cylinder. (B) Effect of administration of PTZ (64 mg / kg), preceded by saline (s–s) administration (NaCl, 0.9%) on nociceptive threshold. (n58; ***P,0.001, according to Mann–Whitney’s
liculus [17] have been involved in epileptogenic activity, a consequent morpho-functional situation will be that neural substrates responsible for the expression of post-ictal analgesia may be also placed in the mesencephalic tectum [3]. In support of this hypothesis, we also recently de-scribed the presence of neurochemical mechanisms respon-sible for antinociceptive processes in the mesencephalic tectum [5,6].
Our results suggest that, although there is no evidence of opiate involvement in the initial stage of post-ictal analge-sia, these neurotransmitters seem to be recruited in a later stage. Furthermore, at higher doses, naloxone caused a statistically significant antagonism of post-ictal analgesia, in all periods studied. It is possible that some opioid receptor subtypes, such as mu-type, are involved in later stages of post-ictal analgesia, while other types of opioid receptors, such as kappa or delta, could be recruited in the immediate post-ictal period. Naloxone, at higher doses, may be reaching other receptors, antagonizing, in that form, the post-ictal analgesia in the first steps. Corroborat-ing these data, several works have suggested that opioid mechanisms are involved in convulsive reactions [2,8,21]. However, a possible dissociation between endogenous opioid effects in both analgesia and epilepsy has been discussed elsewhere [15]. Our current results additionally point to a cholinergic mechanism recruited in the post-ictal analgesia. Acetylcholine seems to be crucially involved in the initial and later stages of antinociception that follows convulsive reactions. Corroborating these data,
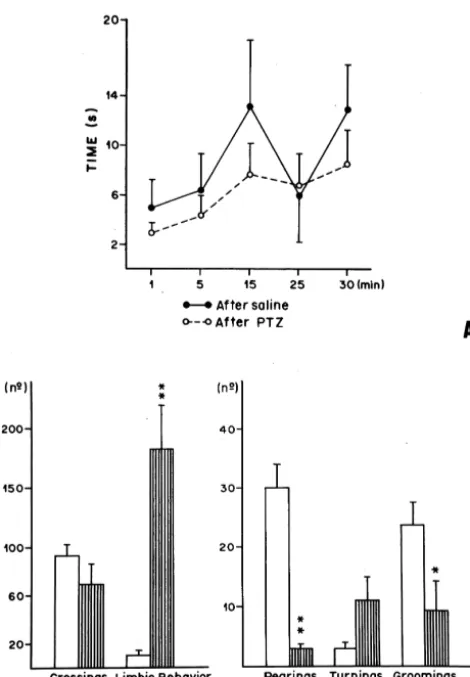
acetylcho-Fig. 2. (A) Lack of effect of pretreatment with PTZ or saline (NaCl,
line has been recently implicated in antinociceptive
pro-0.9%) on motor performance of the animals submitted to the rota-rod test.
cesses either caused by drugs that competitively inhibit
(n58; P.0,05, when compared with control, according to Mann–
acetylcholinesterase [16] or elicited by amygdaloid
com-Whitney’s U-test.) Time: recorded window in which animals were on the
cylinder in movement. (B) Effect of the administration of PTZ (64 plex stimulation [14], a structure also related to seizures mg / kg) or saline (NaCl, 0.9%) on the motor performance of the animals [12]. The lack of effect of the pre-treatment with opioid in the open-field test. The ethogram was done by observing diverse
and cholinergic antagonists on tail-flick latencies recorded
behaviors elicited in the arena after drug’s administration, in a 30-min
immediately after seizures can not be related to a sudden
period of time. The columns represent mean, and the bars, the S.E.M.
motor deficit caused by post-ictal depression, because the
(n58; *P,0.05; **P,0.01, when compared with control, according to
Mann–Whitney’s U-test.) The open columns represent animals which study of the motor’s performance of the animal in the received i.p. administration of saline; the hatched, those with PTZ. rota-rod test (with previous treatment with saline or PTZ)
denies a possible motor deficit that could alter spinal are grateful to J.R. Espreafico, M.T. Castania, J.A.C. de reflexes. Oliveira and I.R.Violante for expert technical assistance. Naloxone and atropine are opiate and cholinergic com- J.R. Espreafico was the recipient of a fellowship from petitive antagonists, respectively, and it is possible that FAPESP (proc. 97 / 10440-3).
their lack of effect, at lower doses, in antagonizing the post-ictal analgesia immediately after seizures, is related to
a massive discharge of these neurotransmitters at the post- References ictal period. They could displace blockers from their sites,
and consequently reduce their pharmacological efficiency. [1] S.H. Cardoso, N.C. Coimbra, M.L. Brandao, Defensive reactions˜ In addition, at least for naloxone, its efficiency in an- evoked by activation of NMDA receptors in distinct sites of the
inferior colliculus, Behav. Brain Res. 63 (1994) 17–24.
tagonizing the post-ictal analgesia, at a lower dose (5
˜
[2] S.H. Cardoso, L.L. Mello, N.C. Coimbra, M.L. Brandao, Opposite
mg / kg), does not seem to relate to post-ictal depression
effects of low and high doses of morphine on neural substrates of
effects, because it has been already demonstrated that
aversion in the inferior colliculus, Behav. Pharmacol. 3 (1992)
naloxone has a property to attenuate it or to abolish it 489–495.
completely [10]. We should consider that during post-ictal [3] N.C. Coimbra, C. Castro-Souza, N. Garcia-Cairasco, Neuroanatomi-cal and neurophysiologiNeuroanatomi-cal study of post-ictal antinociceptive
pro-analgesia, muscarinic cholinergic mechanisms would be
cesses in experimental models of epilepsy, Arquivos de
Neuro-initially recruited, while endogenous opiates such as
Psiquiatria Resumo /Abstracts 56 (1998) 22.
enkephalin and endorphin, would have a place in a later
˜
[4] N.C. Coimbra, M.L. Brandao, GABAergic nigro-collicular pathways
stage of post-seizure antinociception. Meanwhile, mu modulate the defensive behavior elicited by midbrain tectum stimu-opioid receptors may be then involved in this delayed stage lation, Behav. Brain Res. 59 (1993) 131–139.
˜
of the post-ictal analgesia. [5] N.C. Coimbra, M.L. Brandao, Effects of 5-HT receptors blockade2
on fear-induced analgesia elicited by electrical stimulation of the
However, we cannot discard the possibility of
in-deep layers of the superior colliculus and dorsal periaqueductal gray,
volvement of other neurotransmitters in this process, such
Behav. Brain Res. 87 (1997) 97–103.
as serotonin. Serotoninergic circuits are widely cited in [6] N.C. Coimbra, C. Tomaz, M.L. Brandao, Evidence for the in-˜ literature as responsible for neurochemistry of some an- volvement of serotonin in the antinociception induced by electrical
tinociceptive processes [5,6], and as participants in the or chemical stimulation of the mesencephalic tectum, Behav. Brain Res. 50 (1992) 77–83.
endogenous system of pain inhibition [9,18].
Methyser-[7] N.C. Coimbra, M.C. Kawasaki, J.G. Ciscato Jr., S.H. Cardoso,
gide, a non-specific blocker of serotoninergic receptors,
ˆ
S.A.L. Correa, Nigro-tectal pathway: neuroanatomy and role on
was effective in antagonizing the post-ictal analgesia at defensive behaviour elicited by midbrain tectum stimulation, Soc. different steps of this antinociceptive process. Corroborat- Neurosci. Abstr. 24 (1998) 1930.
ing these data, peripheral administration of ketanserin, a [8] T.C. De Lima, G. A Rae, Effects of cold-restraint and swim stress on convulsions induced by penthylenetetrazol and electroshock:
specific blocker of 5-HT / 5HT2 1C receptors, was equally
influence of naloxone pretreatment, Pharmacol. Biochem. Behav. 40
effective in antagonizing the post-ictal analgesia in all
(1991) 297–300.
post-convulsive periods studied. It is necessary to consider [9] H.L. Fields, A.I. Basbaum, Endogenous pain control mechanisms, the possibility of muscarinic cholinergic and serotoninergic in: P.D. Wall, R. Melzack (Eds.), Textbook of Pain, Churchill
mechanisms involvement in the immediately post-ictal Livingstone, Edinburgh, 1989, pp. 206–217.
[10] H. Frenk, B.C. MacCarty, J.C. Liebeskind, Different brain areas
period, while opioid mechanisms, mediated by mu
re-mediate the analgesic and epileptic properties of enkephalin, Science
ceptors, would be recruited later on. Serotoninergic
re-200 (1978) 335–337.
ceptors in the 5-HT subfamily can be negligible in this2 [11] N. Garcia-Cairasco, R.M. E Sabbatini, Possible interaction between
earlier stage of the post-ictal analgesia; although there is a the inferior colliculus and the substantia nigra in audiogenic seizures
clear involvement of these receptors in other post-ictal in rats, Physiol. Behav. 50 (1991) 421–427.
[12] N. Garcia-Cairasco, H. Wakamatsu, J.A.C. Oliveira, E.L.T. Gomes,
analgesia stages. We should still consider the possibility of
E. A Del Bel, L.E.A. M Mello, Neuroethological and morphological
other serotoninergic receptors’ involvement in this
an-(neo-Timm staining) correlates of limbic recruitment during the
tinociceptive process, as well as other neurotransmitters. development of audiogenic kindling in seizures susceptible Wistar Studies that give support to this possibility are being rats, Epilepsy Res. 26 (1996) 177–192.
conducted in our laboratory. [13] R. Guieu, E. Mesdjian, J. Roger, P. Dano, J. Pouget, G. Serratice, Nociceptive threshold in patients with epilepsy, Epilepsy Res. 12 (1992) 57–61.
[14] M.A. Oliveira, W. A Prado, Antinociception and behavioral mani-Acknowledgements festations induced by intracerebroventricular or intra-amygdaloid administration of cholinergic agonist in the rat, Pain 57 (1994)
This work was supported by FAPESP (proc. 96 / 8574-9 383–391.
[15] F. Pavone, C. Castellano, A. Oliverio, Stram-dependent effects of
and 95 / 3604-4). C. Castro-Souza (proc. 96 / 5464-8) and
shock-induced release of opioids: dissociation between analgesia
E.N. Segato (proc. 98 / 07416-6) were the recipients of
and behavioral seizures, Brain Res. 366 (1986) 326–328.
fellowships from FAPESP. N.C. Coimbra and N. Garcia- [16] M.E. Pereira, J.B.T. Rocha, I. Izquierdo, Atropine reverses an-Cairasco are recipients of Research Fellowships from tinociception induced by 2,5-hexanedione in rats, Brain Res. 77
[17] C.E. Ribak, A.L. Manio, M.S. Navetta, C. M Gall, In situ hybridiza- [19] P.R. Schofield, Sequence and functional expression of the GABA-A tion for c-fos mRNA reveals the involvement of the superior receptor shows a ligand-gated receptor super-family, Nature 328 colliculus in the propagation of seizure activity in genetically (1987) 221–227.
epilepsy-prone rats, Epilepsy Res. 26 (1997) 397–406. [20] V.C. Terra, N. Garcia-Cairasco, NMDA-dependent audiogenic sei-[18] M.L.N.M. Rosa, M.A. Oliveira, R.B. Valente, N.C. Coimbra, W.A. zures are differentially regulated by inferior colliculus subnuclei,
Prado, Pharmacological and neuroanatomical evidence for the Behav. Brain Res. 62 (1994) 129–139.
involvement of the anterior pretectal nucleus in the antinociception [21] B.C. Yoburn, K. Lutfy, V. Sierra, F.C. Tortella, Tolerance develops induced by stimulation of the dorsal raphe nucleus in rats, Pain 74 to spinal morphine analgesia but not morphine-induced convulsions,