www.elsevier.com / locate / bres
Research report
Description of a short-term Taxol -induced nociceptive neuropathy in
rats
a b c d a ,
*
Nicolas Authier , Jean-Pierre Gillet , Joseph Fialip , Alain Eschalier , Franc¸ois Coudore
a
´ ´
Laboratoire de Toxicologie, Faculte de Pharmacie, Equipe NeuroPsychoPharmacologie Universite d’Auvergne (INSERM EMI 9904), 28 Place H. Dunant, BP 38, 63001 Clermont-Ferrand, France
b
Centre de Recherche, 63200 Riom, France
c
´ ´
Laboratoire de Pharmacologie, Faculte de Pharmacie, Equipe NeuroPsychoPharmacologie Universite d’Auvergne (INSERM EMI 9904), 63001 Clermont-Ferrand, France
d
´ ´ ´ ´
Laboratoire de Pharmacologie Medicale, Faculte de Medecine, Equipe NeuroPsychoPharmacologie Universite d’Auvergne (INSERM EMI 9904), 63001 Clermont-Ferrand, France
Accepted 22 August 2000
Abstract
This work describes a new animal model of neuropathic pain produced by the single intraperitoneal administration of Taxol (32 mg / kg) to male Sprague–Dawley rats. During the course of the experiment, the clinical status of the rats remained satisfactory and motor function was not altered. A number of classical behavioural tests of nociception as well as histological and electrophysiological
investigations were performed. Taxol administration produced an important and rapidly developing mechanical hyperalgesia, a thermal hypoalgesia but no mechanical or thermal allodynia. Degenerative changes were observed in the sciatic nerve, the nerve fibres in the paw
subcutaneous tissue and in the lumbar spinal cord. When Taxol or vehicle (a mix of Cremophor and ethanol) were repeatedly injected once a week for 5 weeks, similar nociceptive disorders were observed in addition to a decrease in peripheral nerve conduction velocity.
The selective dysfunction of high-diameter myelinated fibres observed after one single administration of Taxol (32 mg / kg) may be attributable to paclitaxel-induced neuropathy, however other mechanisms causing neurochemical dysfunction must also be involved.
2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Neurotoxicology
Keywords: Hyperalgesia; Hypoalgesia; Neurotoxicity; Axonopathy
1. Introduction models of neuropathic pain. Among these anti-cancer
agents, Taxol i.e. paclitaxel, widely used in cancer Studying the mechanisms eliciting neuropathic pain chemotherapy because of its ability to increase the stability necessitates reliable animal models reproducing as accu- of tubulin polymers [15,28], is one of the compounds most rately as possible the symptoms observed in patients. frequently implicated in the onset of painful peripheral However, the animal models of nerve injury actually neuropathy.
available are few and are produced by either trauma [4,21] The change in the sensory nervous system produced by
or metabolic changes [14]. Considering that anti-neoplastic Taxol explains the high incidence (50–90%) of patients agents frequently produce painful peripheral neuropathies, developing painful peripheral neuropathies after a single
2
it may be possible to use these agents to create new animal dose of 250 mg / m ; Taxol , or after repeated administra-tion of lower doses [30]. These well-documented neuro-toxic effects are, in frequency, the primary adverse effects *Corresponding author. Tel.: 133-473-608-000; fax: 133-473-274-
occurring in patients treated with Taxol . Clinically, these 621.
E-mail address: [email protected] (F. Coudore). peripheral neuropathies can cause painful complaints, 0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
numbness and paraesthesia of the extremities [23,30,36], before administration to a final concentration of between an increase in the vibratory threshold and the loss of deep 0.9 to 1.3 mg / ml, depending on the animal weight and tendon reflexes [30]. Nerve conduction velocities and ensuring that volumes of less than 5 ml would be injected amplitudes of the sensory nerve action potentials are into the peritoneal cavity.
always reduced [11,23,30]. Moreover, there is no really
effective therapy for the neuropathy-induced pain. 2.2.2. Control groups
Previous studies of Taxol -induced neuropathy in ani- The vehicle, made up of Cremophor EL (Sigma, L’Isle mals have described electrophysiological and histological d’Abeau, France) and absolute ethanol (Merck, Darmstadt, changes [1,7–9,12] but none have fully described all types Germany) in equal parts, was diluted at the time of of nociception in animals after single dose or chronic injection with saline (NaCl 0.9%), in the same proportion
Taxol administration. The intraperitoneal route of ad- as in the Taxol -treated groups.
ministration, previously proposed by Apfel et al. [1], Injected volumes of saline (NaCl 0.9%) were calculated Hamers et al. [18] and Cavaletti et al. [9] is particularly according to the weight of the rat.
suited to increasing the intratumoral disposition of the
active principle of Taxol , leading to high drug levels in 2.3. Experimental procedures liver, colon, pancreas and ovaries. Previously we have
observed that five intraperitoneal injections, one per week, Taxol was administered intraperitoneously according
do not modify the elimination kinetics of Taxol [13] and to two different schedules:
are of too short a duration of administration to induce Taxol multiple injection groups: one injection of 16
ascites [9]. Consequently, this schedule of administration mg / kg Taxol per week for five consecutive weeks, giving
was used initially. a cumulative dose of 80 mg / kg / rat.
To fully describe the animal model of Taxol -induced Taxol single injection groups: a single injection of 16
painful neuropathy in terms of pain, we have used the mg / kg or 32 mg / kg Taxol . common battery of behavioural pain tests using noxious or
non-noxious mechanical or thermal stimuli and the animal 2.4. Assessment of general toxicity strain commonly used for pain studies, i.e., the male
Sprague–Dawley rat. The schedule of administration was Body weights were measured before each injection, adapted in order to preserve a satisfactory clinical status in every 3 days for 3 weeks after the fifth administration, and the animals. In addition, electrophysiological and neuro- for 15 days following the single injection. Motor activity pathological studies were performed using both light and was monitored every 4 days using an actimeter Actisystem electron microscopy in order to describe possible changes (Apelex, Passy, France). In addition, all rats were ex-in nervous structures, to evaluate the functionality of the amined every day to detect clinical signs such as piloerec-peripheral nervous fibres, and to determine the site of the tion or hindlimb weakness. Body temperature was assessed
neurotoxic action of Taxol . at regular intervals.
2.5. Behavioural assessment 2. Materials and methods
All behavioural tests were conducted before each in-
2.1. Animals jection of Taxol . The guidelines of the IASP Committee
for Research and Ethical Issues were followed for all Two hundred and fifty two male Sprague–Dawley rats experiments [40].
`
(Charles River, St-Aubin-Les-Elbeuf, France) weighing
180 to 200 g at the beginning of the induction were used 2.5.1. Grip strength test [25]
(nine rats / test / dose) and housed in plastic cages on a 12-h After strain gauge was zeroed, the rat was placed with light / 12-h dark cycle with access to water an food ad both forepaws inside the front grip grid. When the rat libitum. Room temperature was maintained at 228C and gripped the grid, it was steadily pulled backwards by the relative humidity was usually between 50 and 60%. tail until its grip was broken. Reading on strain gauge was Animals were allowed a 1-week acclimatisation period recorded, the strain gauge was zeroed, and the rat retested before use in experiments. until readings on three successive trials were obtained.
2.2. Drugs 2.5.2. Paw pressure test
The paw pressure test has been previously described by 2.2.1. Taxol-treated groups Randall and Selitto [29]. Nociceptive thresholds, expressed
the nociceptive threshold was the vocalization of the placed between two electrodes separated by 2.5 cm. After animal. Rats were habituated to the testing procedures and estimating the depolarization threshold of the nerve, the handling by the investigator during the week prior to the nerve was stimulated 50 times with a supramaximal experiment. Experiments were performed until two similar stimulus. Nerve conduction velocity was directly calcu-consecutive pressure values were obtained. The cut-off lated using a computer and a locally-made software. All
pressure was 450 g. measurements were performed under constant conditions
in a Faraday cage. 2.5.3. Von Frey hair test [10]
To assess changes in mechanical nociceptive thresholds,
2.7. Microscopic examination rats were placed on a plastic mesh floor covered by
transparent Plexiglas cages. On the week before the first
administration of Taxol or saline, rats were placed in the
2.7.1. Fixation of tissues test environment each day for 15 min. For testing, rats
At the time of sacrifice, based on satisfactory results of were allowed to acclimatise for 10 min. Successively
behavioural tests, selected rats were deeply anesthetized by greater diameter von Frey nylon monofilaments (Stoelting,
an intraperitoneal injection of a 6% aqueous pentobarbital Wood Dale, IL, USA) (pressures 1.202, 1.479, 2.041,
solution (0.5 ml / 100 g body weight). After opening the 3.630, 5.495, 8.511, 11.749, 15.136 and 28.840 g) were
thoracic cavity, the vascular system was intracardiacally applied to the medial plantar surface of both hind paws.
perfused using a phosphate buffer solution (pH57.2–7.4, For each filament, a series of five stimuli were applied
200 ml), followed by a 4% paraformaldehyde11% within a 2–3 s period per paw. Paw withdrawal threshold
glutaraldehyde (200 ml) solution. was defined as the minimum pressure required to elicit a
Samples of the lumbar spinal cord, sciatic nerve and withdrawal reflex of the paw.
skin with the subcutaneous tissue of the paws were stored in the fixative solution as above, until trimmed for 2.5.4. Plantar test [19]
histology and examination of the ultrastructure. The rats were placed on a glass plate under a Plexiglas
cage for 15 min before each experiment. On the week
before the first administration of Taxol or saline, rats 2.7.2. Histology
were placed in the test environment each day for 15 min. A Samples of lumbar spinal cord, sciatic nerve and skin radiant heat stimulus was applied to the plantar surface of with subcutaneous tissue of the paws were paraffin embed-each hind paw by aiming a beam of light produced by a ded, cut at 4 mm thick, and stained by the Haematoxylin 50-W projection lamp (Apelex, Passy, France). When the and Eosin method before examination using light micro-rat lifted the paw, the light beam was turned off auto- scopy.
matically allowing measurement of the time between the start of the light stimulus and the foot lift. This time was
defined as the withdrawal latency. Heat stimuli were 2.7.3. Electron microscopy
stopped at 20.8 s to avoid skin injury. Three trials were Blocks of lumbar spinal cord, sciatic nerve and skin with conducted on each hind paw, with 5 min between each subcutaneous tissue of the paws of approximatively 2323
trial. 1 mm were postfixed in a 1% osmium tetroxide solution,
and embedded in Epon after graded dehydration in alcohol. 2.5.5. Tail immersion test [27] Semithin and ultrathin sections were obtained using a The tail of the rat was immersed in water maintained at Reichert Ultracut E ultramicrotome. Semithin sections 428C or 488C, until the tail was withdrawn. The duration of were stained with Toluidine Blue before light microscopy. immersion was recorded and a cut-off time of 15 s was Ultrathin sections were contrasted by the uranyl acetate– used. Rats were habituated to the testing procedures and to lead citrate method before examination with a Hitachi HU handling by the investigator during the week prior to the 12A electron microscope at 75 KV.
experiment.
2.6. Electrophysiological study 2.8. Statistical analysis
Five rats per batch at 7 and 21 days after the last Treatments were randomised within each cage of six
injection of Taxol In the repeated Taxol -injection group (five injection study) or four (single injection study) rats.
3. Results
3.1. Assessment of general toxicity
In the five Taxol -injection group, two rats died, one
after the first and one after the fourth Taxol injection, after a substantial decrease in body weight and piloerection were observed. All other animals survived until the end of the study and received the five injections as scheduled in the protocol.
A significantly lower body weight gain was observed from the fourth injection to the end of the study (215% compared to both control groups; P,0.01) in the multiple
Taxol -treated group. Therefore, these rats showed a lower decrease in motor activity, however this was not significant compared to the control groups. No difference in cutaneous temperature was noted in the treated rats and both control groups.
There was no mortality in the single Taxol injection groups. A significant decrease in body weight (maximal decrease: 217%, P,0.001 and 210%, P,0.01 after 3
days, for 32 and 16 mg / kg, respectively) was observed, Fig. 1. Mechanical hyperalgesia (five injections) — time-course of pain
with a total recovery within 2 weeks after the injection. withdrawal thresholds in treated (y, Taxol 16 mg / kg, n59) and control (d, saline, n59) (m, vehicle, n59) rats submitted to an increasing No rat in the control groups died throughout the course
pressure on the hind paw. Each rat received one i.p. injection (arrow) per of the experiment and no alterations in motor activity,
week for 5 weeks. Scores were determined before each injection. The cutaneous temperature or clinical signs were observed. bars represent S.E.M. A significant difference (*P,0.05, **P,0.01; Body weight gain was similar for both saline and vehicle analysis of variance followed by Student’s t-test in unpaired series) was groups. observed versus the saline group from the first injection to 7 days after
the last injection for the Taxol 16 mg / kg group, and from the 7th to the 21st days after the last injection for the vehicle group.
3.2. Behavioural examinations
3.2.1. Grip strength test
Whatever the treatment, the dose and the schedule of were lower than in the Taxol group for the last injection administration, we observed a normal increase in the motor (214.0610.4 g and 230.6620.5 g for vehicle and Taxol , force without any significative difference compared to respectively). No modification of the vocalisation
thres-saline control group. holds was observed at any time in saline-treated group.
In the single Taxol -injection group, vocalisation thres-3.2.2. Randall and Selitto test holds decreased both in the 16 and 32 mg / kg groups, from In all experiments, mean vocalisation thresholds were the 4th to the 9th day (16 mg / kg) and to the 14th day (32 not significantly different between control and drug-treated mg / kg) after the injection, with a maximal decrease on the groups before the first injection. 7th day after injection, i.e., 225% (P,0.01; in 80% of
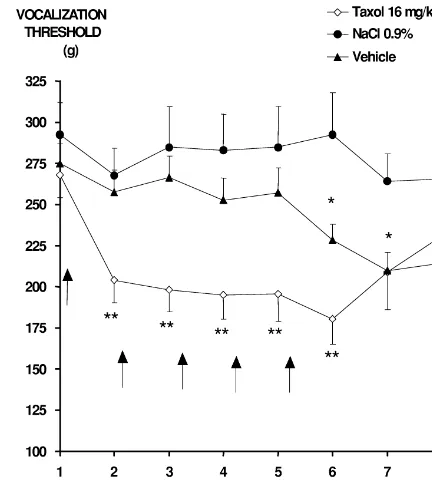
In the multiple Taxol -injection group, a significant rats) and230% (P,0.01; in 90% of rats), respectively. No decrease in vocalisation threshold (80% of rats) compared decrease in vocalization threshold was observed in the to the baseline and control groups, was observed from the control groups during the course of the experiment (Fig. first to the fifth injection (P,0.01) (Fig. 1). The maximum 2).
decrease in vocalisation threshold (maximal decrease:
233%, P,0.01) was observed after the fifth injection. 3.2.3. Von Frey hairs test
From 2 weeks after the fifth injection, vocalisation thres- A significant reduction (P,0.01) in the withdrawal holds increased progressively but up to 21 days after the threshold to von Frey filaments (287% and 281% com-last injection remained significantly lower to baseline pared to baseline values) was observed in two rats (14%)
values (P,0.05). repeatedly treated with Taxol from the fourth injection to
In the multiple vehicle-injection group, a decrease in the end of the experiment. No significant difference in vocalisation threshold compared to baseline values (57% mean withdrawal threshold was observed in treated rats of rats) was noted from the fifth injection to the end of the compared to control groups.
Fig. 3. Thermal hypoalgesia (five injections) — paw withdrawal latencies to a radiant heat stimulus applied to the plantar surface of the hindpaw of Fig. 2. Mechanical hyperalgesia (one injection) — time-course of pain
treated (y, Taxol 16 mg / kg, n59) and control (d, saline, n59) (m,
withdrawal thresholds in treated (y, Taxol 16 mg / kg, n59) (h, Taxol
vehicle, n59) rats. Each rat received one i.p. injection (arrow) per week 32 mg / kg, n59) and control (d, saline, n59) (m, vehicle, n59) rats
for 5 weeks. Scores were determined before each injection. The bars submitted to increasing pressure on a hind paw. Each rat received one i.p.
represent S.E.M. A significant difference (*P,0.05, **P,0.01, analysis injection (arrow). Scores were determined on days 2, 4, 7, 9 and 14 after
of variance followed by Student’s t-test in unpaired series) was observed injection. The bars represent S.E.M. A significant difference (*P,0.05,
versus control groups from the 2nd to the 5th injection for the Taxol 16 **P,0.01; analysis of variance followed by Student’s t-test in unpaired
mg / kg group. series) was observed versus control groups from days 4 to 9 for the
Taxol 16 mg / kg group, and from days 4 to 14 for the Taxol 32 mg / kg.
control groups, withdrawal thresholds were not decreased at any time.
3.2.4. Plantar test
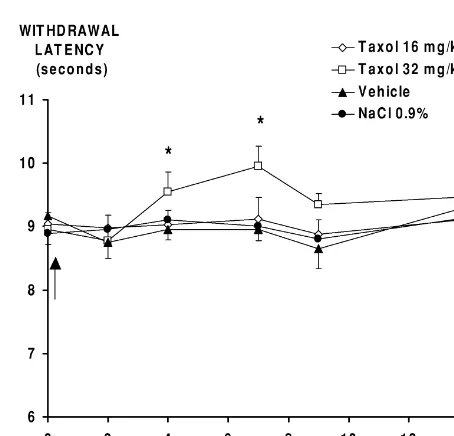
In repeated Taxol -injected rats, compared to control groups, significant increases in paw withdrawal times from the second injection (P,0.01) to 7 days after the last injection (P,0.05), were observed. The maximal increase was 131% compared to baseline (P,0.01). This thermal hypoalgesia affected 57% of treated rats (Fig. 3).
After one single Taxol injection at 32 mg / kg, com-pared to control groups, a significant increase in withdraw-al latency threshold was noted 4 and 7 days after the injection, with a maximum of 117% on day 7 (P,0.05). No modification was noted after administration of 16 mg / kg (Fig. 4).
Whatever the administration schedule, there was no alteration in the withdrawal latencies of the control groups.
Fig. 4. Thermal hypoalgesia (one injection) — paw withdrawal latencies to a radiant heat stimulus applied to the plantar surface of the hindpaw of
treated (y, Taxol 16 mg / kg, n59) (h, Taxol 32 mg / kg, n59) and 3.2.5. Tail immersion test
control (d, saline, n59) (m, vehicle, n59) rats. Each rat has received When thermal allodynia was assessed, tail withdrawal
one i.p. injection (arrow). Scores were determined on days 2, 4, 7, 9 and times did not increase at any time in either the control or 14. The bars represent S.E.M. A significant difference (analysis of
the Taxol -treated rats, whatever the schedule of adminis- variance followed by Student’s t-test in unpaired series) was observed
difference was observed between the Taxol and the control groups.
3.4. Morphological examination
Results of the morphological examination (light and electron microscopy) are summarised in Table 1.
3.4.1. Light microscopy
Degenerative changes were observed on both paraffin and semithin sections, in the sciatic nerve, the nerve fibres in the paw subcutaneous tissue, and in the lumbar spinal
cord. In the five injection Taxol or vehicle groups, at the 7 or 21 day necropsies after the last intraperitoneal injection, lesions were found in all organs examined. In the
single Taxol injection group, a few changes were found in the subcutaneous nerves (16 and 32 mg / kg) and in the sciatic nerve (32 mg / kg). In the single vehicle injection group, and in all saline control groups, no lesion could be
detected. In the five injection Taxol or vehicle groups no
Fig. 5. Peripheral nerve conduction velocity decrease — sciatic nerve major differences could be found between the Taxol or
conduction velocity in paclitaxel-treated (h, Taxol 16 mg / kg, n56) and
the vehicle-treated groups, and between the 7 versus 21 control (j, saline, n56) (9, vehicle, n56) rats measured after five
days post-injection groups. injections and 21 days after the last injection. Each rat received one i.p.
injection (arrow) per week for 5 weeks. The bars represent S.E.M. For the
Taxol 16 mg / kg group, a significant difference (*P,0.05; analysis of 3.4.1.1. Sciatic nerve (Figs. 6 and 7). In the longitudinal variance followed by Student’s t-test in unpaired series) was observed paraffin sections rare Wallerian degeneration and axonal versus the control groups 7 days after the 5th injection.
swellings (giant axons) were observed. In Epon semithin sections, degenerative changes were obvious, with a zonal 3.3. Electrophysiological study pattern in the most severe cases. In the affected area, in myelinated fibres identified as large diameter fibres, the
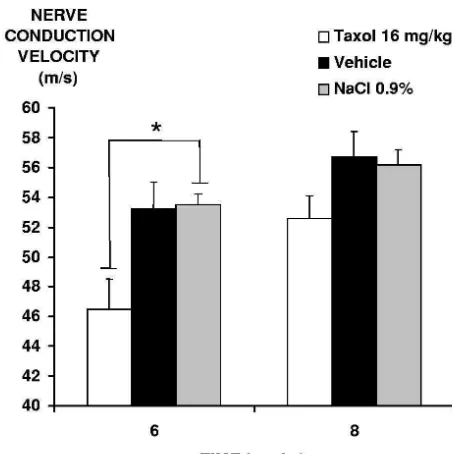
Taxol induced a significant decrease (P,0.05) in axonal clear centre of the fibre was replaced by dark peripheral nerve conduction velocity compared to the myelinic debris, as confirmed by electron microscopy. control groups, 7 days after the last injection. Three weeks
after the end of the last injection, differences between 3.4.1.2. Spinal cord. Degenerative changes, similar to the treated and control groups were no longer apparent (Fig. lesions described above in the sciatic nerve, were ob-5). Seven days after the single injection, no significant served, scattered in the white matter of the lumbar spinal
Table 1
a
Summary of histological examinations after one or five Taxol injections in rats
Nerve of paw subcutaneous tissue Sciatic nerve Lumbar spinal cord (white matter)
Taxol 5316 mg / kg 111 11 1
Sac 7 days PI (Ax; Myel; Schwann) (Ax; Myel) (Ax; Myel)
Vehicle 5 injections 111 11 1
Sac 7 days PI (Ax; Myel; Schwann) (Ax; Myel) (Ax; Myel)
Taxol 5316 mg / kg 111 11 1
Sac 21 days PI (Ax; Myel; Schwann) (Ax; Myel) (Ax; Myel)
Vehicle 5 injections 111 11 1
Sac 21 days PI (Ax; Myel; Schwann) (Ax; Myel) (Ax; Myel)
Vehicle 1 injection 0 0 0
Sac 7 days PI
a
Fig. 6. Swollen degenerated axon — longitudinal paraffin section of the Fig. 8. Degenerated subcutaneous fibres — semithin transversal section
sciatic nerve from a Taxol -treated rat (five injections at 16 mg / kg) of fine subcutaneous nerve fibres in the paw of a Taxol -treated rat (five showing a swollen degenerated axon (arrow). Haematoxylin and Eosin injections at 16 mg / kg) showing numerous degenerative lesions (arrows).
3300. Toluidine Blue31200.
cord. Neurons in the gray matter remained unaffected in all
the light microscope, there were no major ultrastructural groups.
differences between the Taxol and the vehicle-treated groups. The most conspicuous changes were found in the 3.4.1.3. Subcutaneous tissue (Fig. 8). In the fine
subcuta-paw subcutaneous nerve fibres. neous nerves from the paws, degenerative changes affected
almost all nerve fibres, including those of both large and
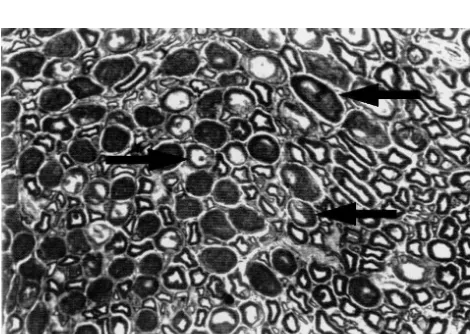
3.5.1. Subcutaneous nerve fibres (Figs. 9 and 10) small diameter. Lesions were similar to those noted for the
Axons were degenerated. They were darkened, with sciatic nerve, and phagocytosis of degenerative myelinic
organelle accumulation, mostly neurotubules and / or vesi-debris was clearly detected at high magnification.
cles, and often seemed compressed by Schwann cells changes as described below. Vesicles were empty or filled 3.5. Ultrastructure
with lamellar debris. Most myelinated and a few non-myelinated axons were affected.
At the ultrastructural level, axonal and / or Schwann cells
Schwann cells and myelin sheaths: vacuolation of changes were found in all Taxol or vehicle groups, except
enlarged abaxonal and adaxonal parts of the Schwann cells in the single injection vehicle group. No changes were
was frequent, with accumulation of various debris, giving noted in saline control groups.
the axon an angular shape. In the most severe cases, In the Taxol and vehicle five injection groups, as under
myelin sheaths were also affected by partial delamination of collapsed myelin sheaths (ovoids) or complete
destruc-Fig. 7. Degenerated large fibres — semithin transversal section of the Fig. 9. Normal subcutaneous fibres — ultrathin section of fine
subcuta-
sciatic nerve from a Taxol -treated rat (five injections at 16 mg / kg) neous nerve fibres in the paw from a control (saline) rat showing several showing numerous degenerated large nerve fibres (arrows). Toluidine normal myelinated (arrows) and non-myelinated (arrowheads) axons.
Fig. 10. Degenerative subcutaneous nervous lesions — ultrathin section Fig. 12. Axonal degenerative changes — ultrathin section of fine
of fine subcutaneous nerve fibres in the paw of a Taxol -treated rat (five subcutaneous nerve fibres in the paw of a Taxol -treated rat (a single injections at 16 mg / kg) showing several degenerated myelinated axons injection at 32 mg / kg) showing axonal degenerative changes (stars) while (arrows), beside few normal nerve fibres (star). Degenerative changes are myelin sheaths remain within normal limits (arrows). Uranyl acetate and also found in non-myelinated schwann cells (arrowheads). Uranyl acetate lead citrate38700.
and lead citrate35000.
organelles and vacuoles in the sciatic nerve (32 mg / kg) and subcutaneous tissue (16 and 32 mg / kg). Schwann tion and subsequent phagocytosis. Non-myelinated
cells and myelin sheaths remained normal and no change Schwann cells were also affected and showed various
was detected in the spinal white matter. No ultrastructural degrees of degeneration, mostly vacuolisation.
change was found in the corresponding vehicle group (Fig. 13).
3.5.2. Sciatic nerve (Fig. 11) and spinal cord
In the affected areas of transverse sections of the sciatic nerve, the nervous fibres showed the various degrees of alteration described above for subcutaneous fibres, with the
4. Discussion same decreased incidence in the semithin sections from the
nerve to the spinal cord. It is also of note that
non-This study demonstrates that either one single or five
myelinated fibres remained unaffected, in contrast to the
repeated intraperitoneal Taxol injections induce a subcutaneous tissue. Neurons of the spinal gray matter had
nociceptive peripheral neuropathy in rats, associated at the a normal ultrastructural aspect, confirming the observations
highest cumulative doses with electrophysiological and made with light microscopy.
histological alterations. Despite the long-standing
knowl-In the Taxol single injection group (Fig. 12), some
edge of the side-effects of Taxol and the widespread use degenerated axons were found with accumulation of
Fig. 11. Increased density of neurotubules — ultrathin section of the Fig. 13. Vehicle-induced nervous lesions — ultrathin section of fine
sciatic nerve from a Taxol -treated rat (five injections at 16 mg / kg) subcutaneous nerve fibres in the paw of a vehicle-treated rat (a single showing increased density of neurotubules. Uranyl acetate and lead citrate injection of Cremophor) showing axons (stars) and myelin sheaths
of this effective drug in cancer therapy, no complete Concurrent to this mechanical hyperalgesia, the applica-description of the associated nociceptive signs could be tion of a non-noxious, mechanical or thermal, stimulus did found in the literature. Moreover, it must be emphasised not modify nociceptive thresholds, irrespective of the
that such a description is necessary firstly, to understand Taxol dose and the number of injections. These results the neurotoxic mechanisms involved in the genesis and show that allodynia is absent and are important because
transmission of the pain syndromes observed after Taxol mechanical and thermal non-noxious stimuli have not chemotherapy and secondly, to develop new and more previously been assessed in the published literature.
efficient pain treatments in these neuropathies which will Thermal hypoalgesia was observed after two Taxol be based on a better knowledge of their pathophysiological injections at 16 mg / kg or after one injection at 32 mg / kg.
mechanisms. A few studies [8,9,12,18], mainly describing A higher Taxol dose was needed to produce thermal electrophysiological and / or morphological modifications hypoalgesia than mechanical hyperalgesia suggesting that
induced by Taxol are available, but in these publications, thermal hypoalgesia requires a more substantial nervous all behavioural results were obtained with animals whose system injury than mechanical hyperalgesia. As regards to strain or sex were not routinely used in studies assessing the literature, only thermal hypoalgesia status has been nociception in animals. A strain, like the Sprague–Dawley previously described in experiments carried out using the rat, with a weak stress seems to be more adapted to realise tail flick test in CD1 mice [1] and Wistar rats [7,8]. behavioural tests [39]. Thus these results published previ- However Cliffer et al. [12] did not observed a decrease in a ously cannot be used to describe the nociceptive symptoms heat nociception test with a cumulative dose of 72 mg / kg
generated by peripheral Taxol neuropathy in the rat. Taxol . Nociceptive signs observed after one Taxol
It should be noted that the Taxol single and multiple injection in rats were well correlated with the disorders of doses used in our study are similar to those administered sensation observed in patients treated by one course of
either intraperitoneally by Cavaletti et al. [9] and Hamers Taxol , such as numbness, tingling and hypoaesthesia et al. [18], (single dose: 8 and 16 mg / kg / week, 6, 9 and 12 [30]. Moreover negative symptoms such as loss of sen-mg / kg / week, respectively; cumulative dose: 40 and 80 sitivity, and positive symptoms, such as pain and dyses-mg / kg, 54 and 66 dyses-mg / kg, respectively) or intravenously thesia, are frequently associated with human sensory by Cavaletti et al. [8] and Cliffer et al. [12] (single dose: 5 neuropathies [26,38].
mg / kg / day and 18 mg / kg / week, respectively; cumulative Electrophysiological ex vivo study of the sciatic nerves
dose: 25 mg / kg, 72 mg / kg, respectively). The schedule of of rats treated with repeated Taxol , vehicle or saline repeated administration, one injection per week for 5 injections showed a significant decrease in the nerve
weeks, was previously used by Cavaletti et al. [9] in conduction velocity after five injections of Taxol com-female Wistar rats. This dose is a good compromise pared to the control groups, but no modification after one between the appearance of nociceptive disorders and the single injection. These results may be due to the low preservation of good clinical status. It was important to sensitivity of electrophysiological studies to detect early avoid severe health alterations in the rats since they may signs of neuropathy. The results of the present study agree severely compromise the predictability of behavioural pain with those published previously in the literature [7–9,12]. tests. No significant change in body weight was observed An increase in the nerve conduction velocity was observed
until the fourth Taxol injection and the loss of weight in control rats, probably due to the degree of maturation of never exceeded 15%. As expected, no significant decrease the nervous system in these relatively young animals [9]. in motor activity and motor force was observed whatever With regard to morphological examination, all nervous
the schedule of Taxol administration. structures, including axons, Schwann cells and myelin With regard to the behavioural assessments, the modi- sheaths, were affected after the fifth 16 mg / kg Taxol
fications observed after five Taxol injections were me- injection or in the vehicle groups at the 7 or 21 day chanical hyperalgesia in 86% of rats and thermal hypo- necropsies after the last intraperitoneal injection. These algesia in 80% of rats. However, only mechanical hy- findings are consistent with previous publications in which peralgesia appeared 1 week after the first administration Wallerian degeneration, spheroids / giant axon formation and persisted until the end of the treatment. We also and microtubule accumulation [8,9,12,31,34], as well as observed mechanical hyperalgesia after five weekly in- changes in Schwann cells (microtubules increased in jections of the vehicle which is a mixture of Cremophor number, debris accumulation and vacuolation by cisternae EL and ethanol. This observation led us to assess the dilatation), were regularly described after various protocols
kinetics of the pain disorders produced after a single 16 or of Taxol administration. The recovery observed after the
32 mg / kg dose Taxol injection. Each dose induced a seventh week in the paw pressure test, 21 days after the state of hyperalgesia of similar intensity to that after the last injection, might be explained by some regeneration five injections which lasted for five and 10 days respective- process which was rarely observed in our samples. Re-ly. These results encouraged us to perform pain tests after generation is not a commonly described process in this
a single injection of Taxol thereby avoiding the vehicle area of publication, and only Roytta has mentioned re-
DMSO as the vehicle), latter than last sampling time of the In conclusion, one single intraperitoneal administration
present experiment [31]. of a 32 mg / kg Taxol dose can be used as a good
In subcutaneous tissue, after five intraperitoneal injec- validated animal model predictive of the painful conditions
tions, all nervous fibres were affected, irrespective of their observed in human peripheral Taxol neuropathy without diameter and the presence of myelin sheath, but in the revealing the neurotoxicity of the vehicle (Cremophor EL). sciatic nerve only large myelinated fibres were affected. A This animal model of peripheral neuropathy is of interest reason for this discrepancy could be that the fine terminal as the neuropathy is easily induced in animals, it is of fibres are ramifications of the large upper sciatic fibres, and quick onset and the nociceptive symptoms are quantitative-that subcutaneous fine non-myelinated fibres may be ly substantial while a good clinical status is observed, and myelinated at an upper level. This highlights the unsolved the recovery of the neuropathy is possible as observed in question of why in the sciatic nerve entirely non-myeli- man.
nated fibres are not degenerated: an explanation could be
the potentiation of Taxol toxicity by the lipophilic
Cremophor, which is known to block multidrug resistance Acknowledgements and to induce neurotoxic side effects [5,6,16,30,37]. Such
an action could be enhanced in the myelin sheath which is This work was supported by the local committee of the rich in lipid. Fiber alterations have already been revealed Ligue Contre le Cancer. We wish thank Dr P. Duprat and in subcutaneous tissues of patients suffering from sensory Dr Sylvain Molon–Noblot for advice and support, Dr A.S.
neuropathy [20,22]. Gross for comments on the manuscript, C. Grauliere for`
In the single Taxol injection group only axonal technical assistance as well as A. Fraysse, J. Payen and M. changes were found, and no lesions were found in the Oron from the Electron Microscopy Department (Professor single injection vehicle group. This suggests that the L. Bourges) of the University of Auvergne,
Clermont-
primary lesion is axonal and due to Taxol alone and not Ferrand. the vehicle. Myelin sheaths could be affected later as a
consequence of axonal degeneration, but predominantly by the direct delayed toxicity of the vehicle. This secondary
References myelinic toxicity occurred after repeated injections in the
case of the five injection groups, and is consistent with the
[1] S.C. Apfel, R.B. Lipton, J.C. Arezzo, J.A. Kessler, Nerve growth findings of human sural nerve biopsies after Taxol
factor prevents toxic neuropathy in mice, Ann. Neurol. 29 (1991) therapy [32] in which the loss of large myelinated and of 87–90.
unmyelinated fibres was noted. Furthermore, the absence [2] R. Baron, C. Maier, Painful neuropathy: C-nociceptor activity may not be necessary to maintain central mechanisms accounting for of neuronal body changes in the spinal cord, and the
dynamic mechanical allodynia, Clin. J. Pain 11 (1995) 63–69. decreased severity of lesions from the subcutaneous
ner-[3] G.J. Bennett, Neuropathic pain, in: P.D. Wall, R. Melzack (Eds.), vous fibres to the spinal cord indicate that the primary
Textbook of Pain, Churchill Livingstone, London, 1994, pp. 201– lesion is axonal and retrograde, most probably progressing 220.
from the terminal fibres to the cellular body. A study of the [4] G.J. Bennett, Y.K. Xie, A peripheral mononeuropathy in rat that spinal ganglions remains to be done to further explore the produces disorders of pain sensation like those seen in man [see
comments], Pain 33 (1988) 87–107.
retrograde toxicity of Taxol .
[5] W. Boogerd, W.W. Bokkel Huinink, O. Dalesio, W.J. Hoppenbrouw-An important issue is to link the alterations in
nocicep-ers, J.J. van der Sande, Cisplatin induced neuropathy: central, tive behaviour observed, i.e., mechanical hyperalgesia and peripheral and autonomic nerve involvement, J. Neurooncol. 9 thermal hypoalgesia, with axonal changes. Mechanical (1990) 255–263.
hyperalgesia could be related to the injury of large fibres [6] D.J. Brat, A.J. Windebank, S. Brimijoin, Emulsifier for intravenous cyclosporin inhibits neurite outgrowth, causes deficits in rapid which normally exert an inhibitory feedback on the small
axonal transport and leads to structural abnormalities in differentiat-fibres [24,35] which convey mechanical hyperalgesia. This
ing N1E.115 neuroblastoma, J. Pharmacol. Exp. Ther. 261 (1992) inhibition may be increased and the noxious impulses 803–810.
conveyed by the small Ad fibres may be amplified. [7] W.M. Campana, N. Eskeland, N.A. Calcutt, R. Misasi, R.R. Myers, However, the absence of allodynia associated with an J.S. O’Brien, Prosaptide prevents paclitaxel neurotoxicity,
Neuro-toxicology 19 (1998) 237–244. alteration of large fibres seems paradoxical as these fibres
[8] G. Cavaletti, E. Cavalletti, P. Montaguti, N. Oggioni, O. De Negri, would take an active part in the occurrence of this type of
G. Tredici, Effect on the peripheral nervous system of the short-term sensitivity disorder by the appearance of ectopic dis- intravenous administration of paclitaxel in the rat, Neurotoxicology charges, the formation of ephapses with small gauge fibres 18 (1997) 137–145.
and abnormal connections to convergent neurons convey- [9] G. Cavaletti, G. Tredici, M. Braga, S. Tazzari, Experimental peripheral neuropathy induced in adult rats by repeated intraperi-ing specific noxious impulses [2,3,17,33,38]. Additional
toneal administration of taxol, Exp. Neurol. 133 (1995) 64–72. studies regarding spinal pain mediator levels will add
[10] S.R. Chaplan, F.W. Bach, J.W. Pogrel, J.M. Chung, T.L. Yaksh, further information about the mechanisms involved in the Quantitative assessment of tactile allodynia in the rat paw, J. pathophysiology of pain, thereby allowing the behavioural Neurosci. Meth. 53 (1994) 55–63.
Cornblath, Peripheral neuropathy from taxol and cisplatin combina- [25] O.A. Meyer, H.A. Tilson, W.C. Byrd, M.T. Riley, A method for the tion chemotherapy: clinical and electrophysiological studies, Ann. routine assessment of fore- and hindlimb grip strength of rats and Neurol. 35 (1994) 304–311. mice, Neurobehav. Toxicol. 1 (1979) 233–236.
[12] K.D. Cliffer, J.A. Siuciak, S.R. Carson, H.E. Radley, J.S. Park, D.R. [26] H. Mitsumoto, A.J. Wilbourn, Causes and diagnosis of sensory Lewis, E. Zlotchenko, T. Nguyen, K. Garcia, J.R. Tonra, N. neuropathies: a review, J. Clin. Neurophysiol. 11 (1994) 553–567. Stambler, J.M. Cedarbaum, S.C. Bodine, R.M. Lindsay, P.S. DiS- [27] R. Necker, R.F. Hellon, Noxious thermal input from the rat tail: tefano, Physiological characterization of Taxol-induced large-fiber modulation by descending inhibitory influences, Pain 4 (1978) sensory neuropathy in the rat, Ann. Neurol. 43 (1998) 46–55. 231–242.
[13] F. Coudore, N. Authier, D. Guillaume, A. Beal, E. Duroux, J. Fialip, [28] E. Nogales, S.G. Wolf, I.A. Khan, R.F. Luduena, K.H. Downing, High-performance liquid chromatographic determination of paclitax- Structure of tubulin at 6.5 A and location of the taxol-binding site el in rat serum: application to a toxicokinetic study, J. Chromatogr. [see comments], Nature 375 (1995) 424–427.
B Biomed. Sci. Appl. 721 (1999) 317–320. [29] L.O. Randall, J.J. Selitto, A method for measurement of analgesic [14] C. Courteix, A. Eschalier, J. Lavarenne, Streptozocin-induced activity on inflamed tissue, Arch. Int. Pharmacodyn. 61 (1957)
diabetic rats: behavioural evidence for a model of chronic pain, Pain 409–419.
53 (1993) 81–88. [30] E.K. Rowinsky, V. Chaudhry, D.R. Cornblath, R.C. Donehower, [15] M. De Brabander, G. Geuens, R. Nuydens, R. Willebrords, J. De Neurotoxicity of Taxol, J, Natl. Cancer Inst. Monogr. 15 (1993)
Mey, Taxol induces the assembly of free microtubules in living cells 107–115.
and blocks the organizing capacity of the centrosomes and kineto- [31] M. Roytta, C.S. Raine, Taxol-induced neuropathy: chronic effects of chores, Proc. Natl. Acad. Sci. USA 78 (1981) 5608–5612. local injection, J. Neurocytol. 15 (1986) 483–496.
[16] K.L. Donaldson, G.L. Goolsby, A.F. Wahl, Cytotoxicity of the [32] Z. Sahenk, R. Barohn, P. New, J.R. Mendell, Taxol neuropathy. anticancer agents cisplatin and taxol during cell proliferation and the Electrodiagnostic and sural nerve biopsy findings, Arch. Neurol. 51 cell cycle, Int. J. Cancer 57 (1994) 847–855. (1994) 726–729.
[17] R. Dubner, Three decades of pain research and its control, J. Dent. [33] H.C. Shin, S.J. Oh, S.C. Jung, Y.R. Choi, C.K. Won, J.W. Leem, Res. 76 (1997) 730–733. Activity-dependent conduction latency changes in A beta fibres of [18] F.P. Hamers, C. Pette, J.P. Neijt, W.H. Gispen, The ACTH-(4–9) neuropathic rats, NeuroReport 8 (1997) 2813–2816.
analog, ORG 2766, prevents taxol-induced neuropathy in rats, Eur. [34] V.S. Vuorinen, M. Roytta, Taxol-induced neuropathy after nerve J. Pharmacol. 233 (1993) 177–178. crush: long-term effects on Schwann and endoneurial cells, Acta [19] K. Hargreaves, R. Dubner, F. Brown, C. Flores, J. Joris, A new and Neuropathol. (Berl) 79 (1990) 653–662.
sensitive method for measuring thermal nociception in cutaneous [35] P.D. Wall, The gate control theory of pain mechanisms. A re-hyperalgesia, Pain 32 (1988) 77–88. examination and re-statement, Brain 101 (1978) 1–18.
[20] N.R. Holland, A. Stocks, P. Hauer, D.R. Cornblath, J.W. Griffin, J.C. [36] P.H. Wiernik, E.L. Schwartz, A. Einzig, J.J. Strauman, R.B. Lipton, McArthur, Intraepidermal nerve fibre density in patients with painful J.P. Dutcher, Phase I trial of taxol given as a 24-hour infusion every sensory neuropathy, Neurology 48 (1997) 708–711. 21 days: responses observed in metastatic melanoma, J. Clin. Oncol. [21] S.H. Kim, J.M. Chung, An experimental model for peripheral 5 (1987) 1232–1239.
neuropathy produced by segmental spinal nerve ligation in the rat, [37] A.J. Windebank, M.D. Blexrud, P.C. de Groen, Potential neuro-Pain 50 (1992) 355–363. toxicity of the solvent vehicle for cyclosporine, J. Pharmacol. Exp. [22] G. Lauria, N. Holland, P. Hauer, D.R. Cornblath, J.W. Griffin, J.C. Ther. 268 (1994) 1051–1056.
McArthur, Epidermal innervation: changes with aging, topographic [38] J.H. Wokke, G.W. van Dijk, Sensory neuropathies including painful location, and in sensory neuropathy, J. Neurol. Sci. 164 (1999) and toxic neuropathies, J. Neurol. 244 (1997) 209–221.
172–178. [39] D.R. Woolfolk, S.G. Holtzman, Rat strain differences in the potentia-[23] R.B. Lipton, S.C. Apfel, J.P. Dutcher, R. Rosenberg, J. Kaplan, A. tion of morphine-induced analgesia by stress, Pharmacol. Biochem.
Berger, A.I. Einzig, P. Wiernik, H.H. Schaumburg, Taxol produces a Behav. 51 (1995) 699–703.
predominantly sensory neuropathy, Neurology 39 (1989) 368–373. [40] M. Zimmermann, Ethical guidelines for investigations of experimen-[24] R. Melzack, P.D. Wall, Pain mechanisms: a new theory, Science 150 tal pain in conscious animals [editorial], Pain 16 (1983) 109–110.