www.elsevier.com / locate / bres
Research report
Potassium-induced enhancement of persistent inward current in
hippocampal neurons in isolation and in tissue slices
1
*
¨
G.G. Somjen , M. Muller
Department of Cell Biology, Box 3709, Duke University Medical Center, Durham, NC 27710, USA Received 19 May 2000; accepted 12 September 2000
Abstract
Previous work suggested a role for the voltage-dependent persistent sodium current, INa,P, in the generation of seizures and spreading
1
depression (SD). Ordinarily, INa,P is small in hippocampal neurons. We investigated the effect of raising external K concentration,
1
[K ] , on whole-cell persistent inward current in freshly isolated hippocampal CA1 pyramidal neurons. Io Na,P was identified by
1
TTX-sensitivity and dependence on external Na concentration. When none of the ion channels were blocked, INa,P was not usually
1 1 1
detectable, probably because competing K current masked it, but after raising [K ] Io Na,Pappeared, while K currents diminished. With
1 1
K channels blocked, INa,Pcould usually be evoked in control solution and raising [K ] caused its reversible increase in most cells. Theo
21
increase did not depend on external calcium [Ca ] . In CA1 pyramidal neurons in hippocampal slices a TTX-sensitive persistent inwardo
1
current was always recorded and when [K ] was raised, it was reversibly enhanced. Strong depolarization evoked irregular currento
1
fluctuations, which were also augmented in high [K ] . The findings support a role of potassium-mediated positive feedback in theo
generation of seizures and spreading depression. 2000 Elsevier Science B.V. All rights reserved.
Theme: Excitable membranes and synaptic transmission
Topic: Sodium channels
Keywords: Persistent sodium current; Sodium channel; External potassium; Intracellular calcium; Seizure; Spreading depression
1. Introduction component of the extracellular potential shift during ictal
seizures revealed a long-lasting prominent current sink 1
Accumulation of K ions in cerebral interstitial spaces limited to cell body layers, for which the source was has been blamed for the eruption of spreading depression distributed over the dendritic fields [45]. By contrast,
1
(SD) by Grafstein [17] and for the generation of seizure voltage shifts and [K ] increase associated with SD starto
discharges by Green [18] and by Fertziger and Ranck [12]. in the dendrite layers [24] where intrinsic optical signals Gloor et al. [15,16] identified a sustained extracellular are also maximal [1,34] and CSD shows powerful inward negative potential shift during epileptiform seizures that currents [45]. In spite of the obvious differences between was limited to the cell body layers in dentate gyrus and seizures and SD, there are also important similarities. Both, hippocampus. We have confirmed this observation [40] and seizures and SD can be triggered by similar insults, and
1
demonstrated that the accumulation of K ions was also both are inhibited by similar physical or pharmacological maximal in the interstitium of cell body layers [41]. interventions, such as cooling, hypertonicity, acidosis and Current source density (CSD) analysis of the sustained certain depressant drugs [4,32]. An event that begins with an epileptiform discharge can sometimes terminate in SD [39] and a seizure that starts in a focus can then spread
*Corresponding author. Tel.: 11-919-681-8404; fax: 11-919-684- over a large area with a velocity resembling that of SD
5481. [4,44].
E-mail address: g.somjen@cellbio.duke.edu (G.G. Somjen).
1 From these and similar observations it has been clear ¨
Present address: Zentrum fur Physiologie und Pathophysiologie,
Ab-that both tonic seizures and spreading depression require
¨ teilung Neuro- und Sinnesphysiologie Humboldtallee 23, D-37073
Gott-ingen, Germany. inward currents that inactivate very slowly or not at all.
G.G. Somjen, M. Muller / Brain Research 885 (2000) 102 –110 103
The nature of these currents was, however not known. A microscope objective with patch pipettes; tight seal was voltage-dependent persistent sodium current was demon- established, and the whole-cell recording condition created strated, amongst others, in hippocampal neurons [13,26] by suction. To record Na as well as K currents the pipettes (reviewed by Crill [7] and by Taylor [43]). It is not known were filled with a solution containing (in mmol / l): KF
1
whether INa,P flows through distinct classes of Na chan- 129, NaCl 4, EGTA 10, CaCl 0.5, MgCl 2, Hepes 10,2 2
nels, or whether it represents failure of inactivation of the Na ATP 4, pH 7.1 or 7.3, tip resistance 2.5–4.5 M2 V. To
1 1
‘conventional’, fast, transient Na current. Recently the block K currents, KF was substituted by 109 mM CsF weight of evidence seemed to be shifting toward the latter and 20 mM TEA-Cl.
interpretation [2,31]. An Axopatch 1D amplifier in voltage clamp configura-The physiological role attributed to INa,P is the regula- tion and the pClamp-6 (Axon Instruments) suite of pro-tion of the ‘resting’ excitability of neurons. Its usual grams was used to record whole-cell currents. Pipette and conductance is too weak to explain the depolarization seen cell capacitances were compensated in the customary in such severely pathological conditions as seizures or SD. manner. Series resistance was compensated to 70%. The
INa,P greatly increases, however, under pathological con- holding potential was 270 mV pipette voltage. Current– 1
ditions. In heart and skeletal muscle a persistent Na voltage (I –V ) curves were recorded usually at one, some-1
current is enhanced by elevated [K ]o [9] and hypoxia times at 2-min intervals. Two different protocols were causes an increase in heart muscle [5,28] as well as in used: either eight sweeps, each beginning with a pre-pulse neurons [19]. In computer-based simulations we recently of 100 ms to290 mV to remove inactivation, followed by
1
demonstrated that Na -mediated, voltage-dependent, slow- 200-ms depolarizing steps at 2-s intervals in 15-mV ly inactivating inward current can generate an SD-like increments, taking the pipette voltage from 270 to 135 depolarized state [29]. We have therefore asked whether mV; or 12 sweeps of a 200-ms hyperpolarizing pre-pulse
1
elevation of [K ] would also cause an increase in Io Na,P in followed by 400-ms depolarizing steps in 10-mV incre-hippocampal neurons. This indeed turned out to be the ments, taking the pipette from 270 to 140 mV.
case. Some of the findings have been reported in an The current records were read with Clampfit (Axon abstract [38]. Instruments) software. After subtraction of linear leak and holding currents, the data were further processed with the Excel (Microsoft) program. Junction potentials were
calcu-2. Methods lated with the JPCalc program [3].
2.1. Isolation of neurons
2.3. Fluorescence imaging
Hippocampal CA1 pyramidal cells were isolated
accord-The non-permeant forms of the fluorescent calcium ing to the method of Kay and Wong [30]. Briefly: rats of
indicator dyes fluo-3 (10 mM) and fura-red (30 mM) 60–120 g body weight were decapitated under ether
(Molecular Probes) were added to the pipette solution [22]. anesthesia. Brains were removed and 500-mm thick slices
The 488 nm excitation light was used and emission was were cut from hippocampus. The CA1 region was cut into
recorded at 520 nm (fluo-3) as well as 640 nm (fura-red) smaller pieces and these tissue fragments were digested for
with COMOS (Biorad) software. Fluorescence intensities 75 min. The digestion medium contained (in mmol / l):
were recorded from two intersecting elongated rectangular NaCl 125, KCl 5, CaCl2 1, MgCl2 2, D-glucose 25,
areas of interest at 10- or 20-s intervals; images were [2-hydroxyethyl]piperazine-[2-ethanesulfonic acid]
recorded at 60-s intervals. Background-corrected fluores-(Hepes) 10, pH 7.0, with trypsin 0.75 mg / ml, at room
cence ratios (fluo-3 / fura-red) were computed subsequently temperature. After digestion the tissue pieces were washed
using Excel (Microsoft) software. Fluo-3 fluorescence and then incubated in trypsin-free oxygenated medium at
increases while fura-red fluorescence decreases with rising room temperature. Tissue fragments were dispersed by 21
[Ca ] .i
trituration with a graded series of fire polished Pasteur pipettes.
2.4. Hippocampal slices 2.2. Recording of voltage-dependent currents
Tissue slices of 400 mm thickness were prepared as Cell suspensions were placed in a chamber of about 0.7 described above, and placed in an ‘Oslo’ style interface ml capacity on the stage of a Zeiss Axioskop and main- chamber in flowing artificial cerebrospinal fluid (ACSF) of tained in flowing Hepes-buffered medium of the following the following composition (in mmol / l): 130 NaCl, 3.5 composition: (in mmol / l:) NaCl 130, KCl 3.5, CaCl 1.2,2 KCl, 1.25 NaH PO , 24 NaHCO , 1.2 CaCl , 1.2 MgSO ,2 4 3 2 4
MgCl2 1.0, glucose 25, Hepes 10, pH 7.3 or 7.35, at 10 glucose, pH 7.4, saturated with 95% O , 5% CO ,2 2
1 1 1 1
Slices were left undisturbed for 90 min before recording slowly inactivating inward current was detectable in all but began. two of a population of 42 isolated CA1 hippocampal neurons. The mean amplitude (normalized to cell capaci-2.5. Whole-cell recording in tissue slices tance) was 27.166.5 pA / pF (mean6st.d.), with a range from 0 to 224.3 pA / pF in cells with average capacitance
1
Patch pipettes were pulled from thick-walled glass tubes of 10.762.2 pF. In 10 trials the bath K concentration was and filled with the following solution (in mmol / l): 120 raised from 3 to 20 mM; the mean persistent inward K-gluconate, 8 KCl, 10 Hepes, 11 EGTA, 0.5 CaCl , 22 current in this sample in control solution was 24.860.9 MgCl , 4 ATP-Na , pH 7.35, osmolarity 290, pipette tip2 2 pA / pF (mean6S.E.M.) and it increased to 28.461.6 pA / resistance 3–5 MV. Cells in CA1 stratum pyramidale were pF (P,0.03 by paired t-test). Fig. 1 illustrates an example found by ‘blind’ search. Seal resistances of $0.5 GV, of the reversible enhancement of the persistent inward more usually $1.0 GVwere accepted. Pipette capacitance current in one of these cells. In this case the maximal was compensated. Whole-cell recording condition was persistent inward current increased during elevation of
1
established by gentle suction. Cell capacitance and series [K ]o by a factor of almost 4 (Fig. 1A,C) while the resistance were not compensated. Holding potential was calculated maximal conductance doubled (Fig. 1D). The
265 mV. Series of test pulses were delivered at 90-s sample currents of Fig. 1B show an increase not only of intervals. Each protocol consisted of a pre-pulse of 400 ms the slowly inactivating inward current, but also of an to 290 mV followed by a series of either eight or 10 inward tail current that follows repolarization. As long as depolarizing steps of 600 ms in 10-mV increments, either the persistent inward current was small, tail currents were from 270 to 0, or from270 to 120 mV. Data processing usually small or absent in recordings made with CsF was similar to that for isolated cells. pipettes. Marked tail currents were seen whenever the Each cell was examined first for 15 min in normal persistent inward current grew large, but there was no
1
solution, then for 15 min in elevated K (10 mmol / l) simple correlation between the amplitudes of the two. solution. If the seal held, then either recovery was ob- In the absence of channel blocking agents, when KF-served during washing with normal ACSF for 15–30 min, filled pipettes were used, the persistent current was a
1 1
or the cell was exposed to high K solution with tet- mixture mainly composed of the delayed rectifier K rodotoxin (TTX) 1.0 mM added. Slices were exposed to current, IK,DR [36] and INa,P. As long as the cells were
1
high K only once. bathed in normal solution, a persistent inward current was 1 detected in only two out of 11 cells. When the K 2.6. Statistics concentration in the bath was raised, such an inward
current appeared in nine of the 11 cells. Its amplitude was Except when otherwise noted, numerical data are given 21.1 pA / pF (n53) at 10 mM, 23.6 pA / pF (n57) at 20
1
as the mean6S.E.M. Significance was calculated by paired mM and216.9 pA / pF (n53) at 40 mM [K ] . Apparent-o
two-tailed t-test. ly in control solution the powerful IK,DRmasked the weak 1
INa,P, but as [K ] increased, Io Na,Pstrengthened sufficiently to compete. Fig. 2B illustrates the emergence of INa,P in
1
3. Results high [K ] solution.o
1
The transient Na current, INa,T, usually increased in the 3.1. The effect of elevated potassium concentration, first few minutes of recording from a cell, and then its
1
[K ] , on isolated neuronso amplitude drifted slowly over time, either increasing or 1
decreasing. Raising [K ] did not alter this course. In Fig.o
Whole-cell currents were evoked by depolarizing volt- 2 the I –V curves and the normalized conductances of INa,T
age steps in patch-clamped neurons freshly isolated from and INa,P of one cell are compared. CA1 region of rat hippocampus. The recording pipettes
were filled either with a KF-based solution that does not 3.2. Elevated potassium concentration and fluorescence impede any of the known major ion currents, or a solution
1
with CsF as the major electrolyte and 20 mM TEA , to Laser-induced fluorescence in cells filled with calcium 1
block most K -mediated outward currents. The holding indicator dyes causes a powerful, slowly reversing poten-potential was 270 mV. Pipette and cell capacitances were tiation of INa,P [37,38]. To see whether the effects of
1
neutralized and series resistance was 70% compensated. elevated [K ] and of fluorescence interact, dye-filled cellso
Linear leak and holding current were subtracted off line. were intermittently exposed to laser light while voltage-To construct current–voltage (I –V ) curves, cells were dependent currents were also tested, first in normal
solu-1
stimulated by series of depolarizing steps, preceded by tion and then in elevated [K ] . Currents were recordedo
G.G. Somjen, M. Muller / Brain Research 885 (2000) 102 –110 105
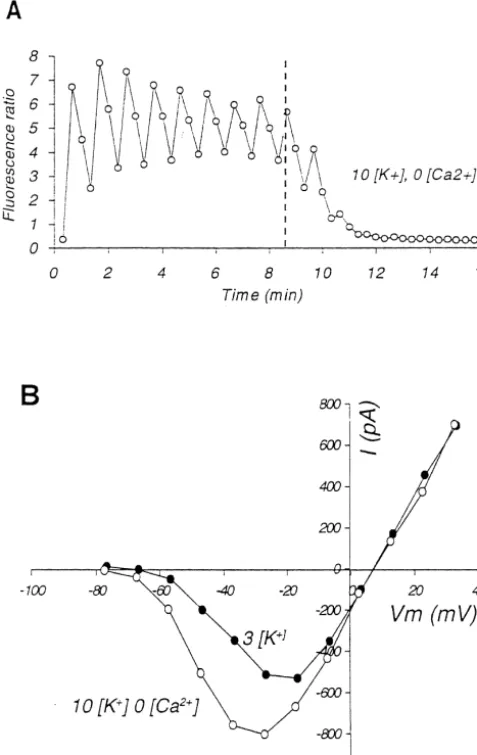
Fig. 1. Reversible enhancement of persistent inward sodium current, INa,P in an isolated neuron during exposure to elevated external potassium
1 1
concentration. (A–D) Data from the same cell, recorded with a pipette filled with a solution based on CsF, containing 20 mM TEA , to block most K currents. No fluorescent dyes were used. Current amplitudes for (A), (C) and (D) were measured as the average during the last 15 ms of depolarization. (A)
1
The maximal amplitude of the persistent inward current recorded before, during and after exposure to 20 mM [K ] in the bath. The broken vertical lines indicate change of bath solution. The horizontal bar indicates intermittent illumination by laser but, in the absence of dyes, the illumination had no effect on
INa,P. (B) Sample recordings of currents evoked by voltage step to210 mV pipette potential, corresponding to membrane potential (V ) ofm 216.7 mV after
1 1
correction for junction potential. Superimposed recordings obtained before raising [K ] , 8 min after switching to high Ko solution, and after 20 min of washing with normal solution. Sampling rate 2000 Hz, filtered off-line at 100 Hz. (C) Current–voltage (I –V ) curves of the slowly inactivating current
1 1
obtained just before switching to high K solution, after 8 min of exposure to 20 mM [K ] , after 8 min of intermittent laser illumination while exposed too 1
high [K ] , and after 20 min washing with normal ACSF. (D) Whole-cell conductance in nanoSiemens as function of V , calculated by dividing theo m currents shown in part (C) by the driving potential, defined as the difference between V and the reversal potential of the current.m
21
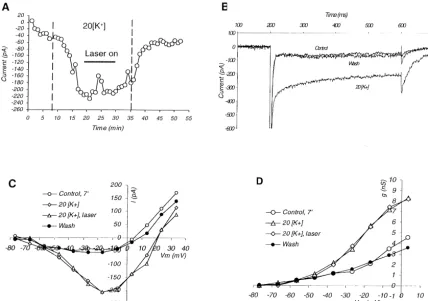
currents recorded at one min intervals from 10 such dye- of influx of Ca through voltage-dependent channels. These increases dissipated slowly, creating a ‘sawtooth’ filled cells. The rapid increase of the inward current after
1
pattern when the fluorescence ratio was plotted against high-K solution was introduced into the bath, strongly
1
time (Fig. 4A). The amplitude of these voltage-dependent suggests that raising [K ]o accelerated the laser-induced
21 1
Ca responses gradually decreased with repetition, proba-increase. Of the 10 cells three were lost during the high K
1
bly due to rundown. Raising [K ] caused a small increase treatment. When the seven remaining cells were washed in o
in the baseline fluorescence ratio in most cells, indicating normal solution, the inward current decreased for a short
21
an increase in ‘resting’ [Ca ] . The magnitude of the period, but in five of the cells it then resumed to increase i
enhancement of I and of the change in fluorescence under the influence of the continuing laser pulses. Na,P
ratio baseline were, however, not correlated. Laser illumination of cells that were not filled with dye
The effect of calcium on I was further tested in three had no effect on the persistent inward current, regardless of Na,P
21 1
1
cells by removing Ca from the bath while raising [K ]. whether the bath K concentration was normal, or elevated
21 21
(Fig. 1). Lack of [Ca ]o was compensated by raising Mg concentration from 1.0 to 2.2 mM. Fig. 4B,C illustrates 1
3.3. K -induced augmentation of INa,P is not calcium one such trial, recorded with a CsF-filled pipette. As
21
dependent expected, removing Ca suppressed the ‘sawtooth’ oscil-lations and depressed the baseline fluorescence (Fig. 4A).
21
1 1
N-methyl-D-glucamine (NMDG ) for 50 mM Na in the
1
bath, in the presence of 20 mM [K ] in four fluorescento
cells, depressed INa,P from 2117639 to 238610 pA / pF 1 (P,0.04). TTX-sensitivity, reversal potential, and Na dependence confirmed that this is a slowly inactivating sodium current, INa,P.
3.5. Neurons in tissue slices
Since isolated neurons are truncated and are in an 1 abnormal environment, we asked whether raising [K ]o
would also enhance INa,P in intact neurons in their natural habitat. To answer this question, whole-cell recordings were made from pyramidal cells in CA1 region of rat hippocampal tissue slices. The slices were maintained in an interface tissue slice chamber perfused with bicar-bonate-buffered artificial cerebrospinal fluid (ACSF) at 368C. Glucose in the bath was 10 mM, instead of the 25 mM used for isolated cells. The pipettes were filled with K-gluconate-based solution and no ion channel blocking drugs were used, except at the end of some trials when 1 1
mM TTX was added to the bath to verify the Na dependence of the inward currents.
The voltage of cells in situ cannot perfectly be con-trolled by the whole-cell patch clamp method, because the membrane potential of the axon and of the dendritic tree cannot be controlled by feedback applied to the cell body. Nonetheless, with this technique changes in the relative magnitude of the voltage-induced currents can reliably be assessed. Since the membrane potential was presumably not uniform over the entire cell surface, in this section we report pipette voltages without correction for series resist-ance or junction potential, because this was the only reliably known voltage. The capacitance of the cells in tissue was not neutralized, but this does not introduce a serious error in the measurement of currents that change
Fig. 2. Comparing the persistent and the transient sodium currents. From only slowly. The holding potential was265 mV; to obtain one cell, recorded by KF filled micropipette. (A) I –V curves of the
I –V curves the pipette was first stepped to 290 mV for 400
maximal amplitude of the fast transient inward current, INa,T, in control
1 ms and then a series of 600-ms depolarizing pulses were solution and in 20 mM bath [K ]. (B) I –V curves of the persistent current
applied in 10-mV increments, either from 270 to 0 mV or
measured during the last 15 ms of depolarization from the same traces as
those used for (A). In the absence of channel blocking drugs the persistent from 270 to 120 mV. Depolarization evoked trains of
current is a mixture consisting mainly of INa,P and IK,DR. (C) Voltage current spikes presumably drawn by action potentials fired dependence of the normalized conductances of INa,T and of the mixed in the axon, outside the voltage clamped region, as well as
1
persistent current in elevated [K ] . Conductance was calculated byo
slower transient inward current surges probably reflecting
dividing current by driving voltage, normalized to the maximal amplitude.
calcium action potentials generated in the dendritic tree [8,27]. Persistent currents were measured within segments 1
ion. During the same bath change, [K ] was raised to 10o of traces toward the end of the depolarizing voltage steps mM. INa,Pwas augmented, demonstrating the independence where firing ceased, or else between spikes.
1 21
of the high [K ] effect from Cao influx (Fig. 4B). After subtracting linear ‘leak’ and holding current off line, a slowly inactivating inward current was evident in
1 1
3.4. TTX and low [Na ] suppress high K -augmentedo recordings from all observed cells in normal solution (Figs.
persistent inward current 5 and 6) (also noted in an earlier study, see Ref. [27]). I –V curves were recorded at 90-s intervals over a period of Exposing cells to 0.5 mM tetrodotoxin (TTX) sup- 15–16.5 min in control solution, followed by a similar
1
pressed the laser- and high [K ] -enhanced persistento length of time during perfusion with solution containing 10 1
G.G. Somjen, M. Muller / Brain Research 885 (2000) 102 –110 107
1
Fig. 3. Raising [K ] hastens fluorescence-induced potentiation of Io Na,P. The maximal persistent inward currents measured in 10 fluorescent isolated cells 1
before, during and after raising bath [K ] to 20 mM. All recordings were made with pipettes filled with KF-based solution, with the fluorescent dyes fluo-3 and fura red added. During the entire period, the cells were illuminated at 10- or 20-s intervals by 1-s pulses of laser light. The initial control period varied
1
from 5 to 14 min, exposure to 20 mM [K ] from 6 to 14 min.o
interstitial fluid of the slice and the bath in an interface 2509 pA and after washing for 15–20 min with normal chamber is usually completed in about 30 min [6,10]. Even solution it decreased to 2344 pA (Fig. 5).
1 1
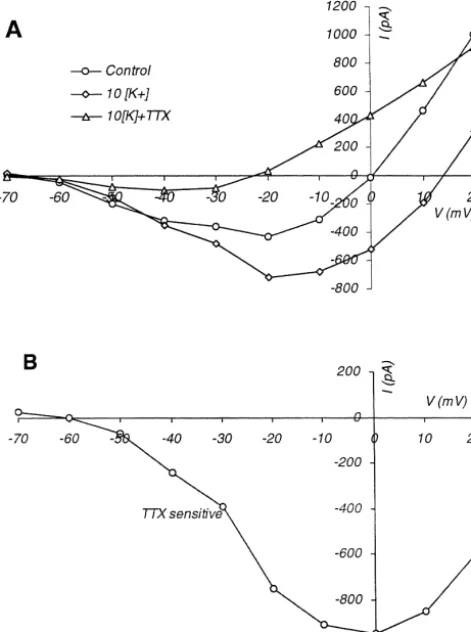
though tissue [K ] probably did not reach the bath level,o At the end of high [K ] treatment 1 mM tetrodotoxin persistent inward currents were enhanced in all trials (Figs. (TTX) was added to the bath in four trials. Persistent 5 and 6). The maximal amplitude and the reversal potential inward currents were substantially reduced by TTX, even of the current shifted to a more depolarized voltage range. though the loss of the seal between pipette and cell
1
When bath [K ] was raised, the maximal persistent current membrane 10–15 min after the start of TTX administration increased from 2325643 pA (mean6S.E.M.; n516) to precluded recording the full effect of the drug. Fig. 6A
2445658 pA at 230 mV pipette voltage, while at 220 shows average I –V curves from all four TTX-treated cells. mV it increased from 2262654 pA to 2577661 pA. The The depression by TTX affected the persistent inward difference was significant at both voltages (P,0.02 and currents over the entire voltage range. The curve of Fig.
P,0.0001, respectively, by two-tailed paired t-test). An 6B was constructed by subtracting the composite I –V curve 1
even more striking change occurred at a pipette potential obtained in the presence of TTX and high [K ] from that 1
of 0 mV, at which level in normal solution the current flow taken in high [K ] without TTX, and it represents the 1
was outward,13596174 pA, but in 10 mM [K ] it turned TTX-sensitive part of the persistent inward current. Ex-inward, to 23356117 pA (P,0.0001). trapolation indicates that the TTX-sensitive current
re-1
With bath [K ] elevated, ‘spontaneous’ fluctuations of verses close to 142 mV, similarly to the TTX-sensitive the holding current were noticed in several of the cells. INa,P of isolated neurons [37,38]).
Strongly depolarized potentials evoked irregular current fluctuations even in normal solution, which could be
described as voltage-dependent noise. The depolarization- 4. Discussion
1 induced fluctuations became more marked when [K ] was
1 raised (Fig. 5A). These irregular currents, which need not The data show that elevation of extracellular K
con-1
be related to INa,P, added to the persistent current am- centration, [K ] , causes a reversible increase of persistento
plitude, which was measured as an average over 30–50-ms voltage-gated inward current in hippocampal CA1 pyrami-segments of trace. This addition was most marked at the dal cells. In isolated cells this current was shown to depend
1
more depolarized voltages (Fig. 6). on external Na concentration, while both in neurons in Recovery during washing with normal solution follow- slices and in isolation it was suppressed by TTX,
identify-1
ing high [K ] exposure was recorded in four cells. In this ing it as persistent sodium current, INa,P.
sub-group the control recording of the mean persistent Our goal was to investigate how this current might inward current was, at 230 mV depolarization, 2408 pA, behave under clinically relevant conditions, and for this
1
1 Fig. 5. Enhancement of persistent inward current by elevated [K ] in ao pyramidal neuron in a hippocampal tissue slice. (A) Sample whole-cell currents evoked by depolarizing steps to pipette voltage of230 mV, in
1
normal ACSF, 16 min after raising [K ] in the bath to 10 mM, and after 15 min of washing with normal ACSF. (B) I –V curves of the same cell as in (A). The current was measured during the last 50 ms of the Fig. 4. External calcium is not required for the enhancement of INa,P. (A) depolarizing voltage step.
Ratio of fluorescences of fluo-3 / fura red recorded at 20-s intervals from 21 an isolated neuron. The ‘sawtooth’ pattern is created by Ca influx
21 Patch clamp recordings made from isolated cells and through voltage-gated Ca currents due to depolarizing voltage steps
from cells in tissue slices each have their limitations,
applied at 1-min intervals. At the broken vertical line the bath solution 1
was changed to one containing 10 mM K , and no added calcium. (B) theoretical and practical, and the two methods complement
I –V curves of the slowly inactivating currents of the same cell as in (A), one another. The isolated neurons had lost most of their
1 21
obtained just before and 4 min after switching to high K , low Ca
dendritic tree and were kept at room temperature in a bath
solution. The recording pipette contained CsF-based solution.
buffered by Hepes. Voltage clamping neurons in slices was less perfect than in isolated cells, but the conditions were channel blocking drugs. When all ion channels were more nearly normal, because cells retained morphological available for activation, INa,P was detectable only in a integrity, they were at a temperature that is normal for rats, minority of the isolated cells in normal solution. When in CO / bicarbonate-buffered solution. In addition, the2
1
[K ] was raised, Io Na,P made its appearance even in the recording pipettes were filled with K-gluconate-based cells where its presence was not evident before. It seems solution, instead of the more alien KF or CsF. The reliable that in isolated neurons in control solution INa,P was augmentation of the persistent inward current in the cells
1
usually hidden by the strong outward K currents. Its in slices thus validates the electrophysiologically more 1
enhancement in elevated [K ]o was, in part, due to perfect data obtained from isolated cells. 1
weakening of the outward K currents resulting from the In neurons in slices a persistent inward current was
1 1
positive shift of the K equilibrium potential. That this is always detected even though K currents were not sup-not the whole explanation is apparent from the fact that pressed. The reason could be the presence of persistent
1
elevated [K ] did enhance Io Na,P in the majority of cells inward current in the dendrites that are lost during the 1
even when K currents were pharmacologically sup- dissociation of isolated cells [33]. In many cells the high 1
G.G. Somjen, M. Muller / Brain Research 885 (2000) 102 –110 109
1
During epileptiform ictal events [K ] rises in hippocam-o
pal formation and neocortex to 8–12 mM, and during spreading depression (SD) several times higher [21,23,41]. It is likely that during seizures, hypoxia and SD the enhancement of the persistent inward currents is even more marked than it was in these trials.
1
Elevation of [K ] caused less of an increase in Io Na,P
than did intracellular fluorescence of isolated neurons 1
[37,38], but the effect of high [K ] was comparable too
that seen in similar isolated hippocampal neurons deprived ¨
of oxygen, as reported by Hammarstrom and Gage [19]. In intact brains, as also in brain tissue slices, hypoxia causes
1
[K ]o to increase, and this increase begins soon after withdrawal of oxygen [20,35]. Simultaneous decrease of
1
oxygen tension and increase of [K ] probably reinforceo
1 each other‘s effect on INa,P. It may be, that elevated [K ] ,o
hypoxia, and intracellular fluorescence modulate the per-sistent sodium current through a common intermediate step or steps. Regardless of mechanism, enhancement of persis-tent inward current probably plays an important part in the generation of tonic seizures, SD, and SD-like hypoxic depolarization. In computer-based simulation either INa,Por an NMDA receptor-controlled current could generate SD-like depolarization. When both currents were available, as they are in live brains, the threshold was lower and the onset faster [29].
Fig. 6. I –V curves averaged from four cells, demonstrating TTX
sen-sitivity of persistent inward current. (A) I –V curves of the persistent Acknowledgements component of currents. Points are the averages from four cells, in normal
1 1
ACSF, in elevated bath [K ], and in elevated bath [K ] with 1.0mM
Supported by NIH grant NS 18670.
TTX added. (B) The TTX-sensitive component of the persistent current, calculated from the data of (A), by subtracting data obtained in the
1 1
presence of TTX and high [K ] from those in high [K ] without TTX.
References
voltages, in the range from 0 to 120 mV (Fig. 6). It must
[1] P.G. Aitken, G.C. Tombaugh, D.A. Turner, G.G. Somjen, Similar
be remembered, however, that these positive pipette
volt-propagation of SD and hypoxic SD-like depolarization in rat
ages are translated to more moderate depolarization at
hippocampus recorded optically and electrically, J. Neurophysiol. 80
some distance from the cell soma. The disproportionate (1998) 1514–1521.
growth of the current evoked by the most positive pipette [2] C. Alzheimer, P.C. Schwindt, W.E. Crill, Modal gating of Na1
channels as a mechanism of persistent Na1 current in pyramidal
voltage may thus signal the increasing contribution by the
neurons from rat and cat sensorimotor cortex, J. Neurosci. 13 (1993)
more distal dendrites. The irregular current fluctuations
660–673.
was also most conspicuously boosted at strongly
depolar-[3] P.H. Barry, JPCalc, a software package for calculating liquid
ized pipette voltage. Conceivably these fluctuations repre- junction potential corrections in patch-clamp, intracellular, epithelial sent dendritic NMDA receptor-controlled currents. Voltage and bilayer measurements and for correcting junction potential
21
dependence mediated by Mg is a well-documented measurements, J. Neurosci. Methods 51 (1994) 107–116.
1 [4] J. Bures, O. Buresova, J. Krivanek, in: The Mechanism andˇ ˇ ´ ˇ ´ feature of NMDA-controlled currents [25]. High [K ]o
˜
Applications of Leao’s Spreading Depression of
Electroencephalog-induces irregular firing in afferent neurons and thus release
raphic Activity, Academia, Prague, 1974.
glutamate at synaptic sites. Such firing is suppressed by [5] E. Carmeliet, Cardiac ionic currents and acute ischemia: from 1
TTX, but elevated [K ] is also expected to cause non-o channels to arrhythmias, Physiol. Rev. 79 (1999) 917–1017.
synaptic glutamate release [11,14,42] that is not affected [6] S.R. Chebabo, M.A. Hester, J. Jing, P.G. Aitken, G.G. Somjen, Interstitial space, electrical resistance and ion concentrations during
by TTX. These conjectures, although plausible, require
hypotonia of hippocampal slices of rats, J. Physiol. 487 (1995)
further study.
685–697.
1
After only 15–16 min of raising bath [K ], the con- [7] W.E. Crill, Persistent sodium current in mammalian central neurons, 1
centration of K in interstitial fluid of the tissue slice is Annu. Rev. Physiol. 58 (1996) 349–362. ´
hippocampal cells during spreading depression (SD) and SD-like [28] Y.-K. Ju, D.A. Saint, P.W. Gage, Hypoxia increases persistent hypoxic depolarization, Brain Res. 632 (1993) 195–208. sodium current in rat ventricular myocytes, J. Physiol. 497 (1999) [9] L. Dei Cas, M. Metra, C.V. Leier, Electrolyte disturbances in chronic 337–347.
heart failure: metabolic and clinical aspects, Clin. Cardiol. 18 (1995) [29] H. Kager, W.J. Wadman, G.G. Somjen, Simulated seizure discharges
370–376. and spreading depression-like depolarization in a neuron model
[10] R. Dingledine, G.G. Somjen, Calcium dependence of synaptic incorporating interstitial space and ion concentration changes, transmission in the hippocampal slice, Brain Res. 207 (1981) 218– J.Neurophysiol. 84 (2000) 495–512.
222. [30] A.R. Kay, R.K.S. Wong, Isolation of neurons suitable for patch
[11] J. Drejer, H. Benveniste, N.H. Diemer, A. Schousboe, Cellular clamping from adult mammalian central nervous system, J. Neuro-origin of ischemia-induced glutamate release from brain tissue in sci. Methods 16 (1986) 227–238.
vivo and in vitro, J. Neurochem. 45 (1985) 145–151. [31] J.Y. Ma, W.A. Catterall, T. Scheuer, Persistent sodium currents [12] A.P. Fertziger, J.B. Ranck, Potassium accumulation in interstitial through brain sodium channels induced by G protein betagamma
space during epileptiform seizures, Exp. Neurol. 26 (1970) 571– subunits, Neuron 19 (1997) 443–452.
585. [32] W.H. Marshall, Spreading cortical depression of Leao, Physiol. Rev. [13] C.R. French, P. Sah, K.J. Buckett, P.W. Gage, A voltage-dependent 39 (1959) 239–279.
persistent sodium current in mammalian hippocampal neurons, J. [33] T. Mittmann, S.M. Linton, P. Schwindt, W. Crill, Evidence for Gen. Physiol. 95 (1990) 1139–1157. persistent Na1current in apical dendrites of rat neocortical neurons [14] D.G. Fujikawa, J.S. Kim, A.H. Daniels, A.F. Alcaraz, T.B. Sohn, In from imaging of Na1-sensitive dye, J. Neurophysiol. 78 (1997)
vivo elevation of extracellular potassium in the rat amygdala 1188–1192. ¨
increases extracellular glutamate and aspartate and damages neu- [34] M. Muller, G.G. Somjen, Intrinsic optical signals in rat hippocampal rons, Neuroscience 74 (1996) 695–706. slices during hypoxia-induced spreading depression-like deplariza-[15] P. Gloor, L. Sperti, C.L. Vera, A consideration of feedback mecha- tion, J. Neurophysiol. 82 (1999) 1818–1831.
1 1
¨
nisms in the genesis and maintenance of hippocampal seizure [35] M. Muller, G.G. Somjen, Na and K concentrations, extra- and activity, Epilepsia 5 (1964) 213–238. intracellular voltages and the effect of TTX in hypoxic rat hip-[16] P. Gloor, C.L. Vera, L. Sperti, S.N. Ray, Investigation on the pocampal slices, J. Neurophysiol. 83 (2000) 735–745.
mechanism of epileptic discharge in the hippocampus, Epilepsia 2 [36] R.E. Numann, W.J. Wadman, R.K.S. Wong, Outward currents of (1961) 42–62. single hippocampal cells obtained from the adult guinea pig, J. [17] B. Grafstein, Mechanism of spreading cortical depression, J. Neuro- Physiol. 393 (1987) 331–353.
physiol. 19 (1956) 154–171. [37] G.G. Somjen, Enhancement of persistent sodium current by internal [18] J.D. Green, The hippocampus, Physiol. Rev. 44 (1964) 561–608. fluorescence in isolated hippocampal neurons, Brain Res. 885
¨
[19] A.K.M. Hammarstrom, P.W. Gage, Inhibition of oxidative metabo- (2000)
1 lism increases persistent sodium current in rat CA1 hippocampal [38] G.G. Somjen, Intracellular fluorescence and elevated [K ] poten-o neurons, J. Physiol. 510 (1998) 735–741. tiate persistent sodium current in isolated hippocampal neurons, [20] A.J. Hansen, Effects of anoxia on ion distribution in the brain, FASEB J. 14 (2000b) A107, (Abstract).
Physiol. Rev. 65 (1985) 101–148. [39] G.G. Somjen, P.G. Aitken, The ionic and metabolic responses [21] A.J. Hansen, T. Zeuthen, Extracellular ion concentrations during associated with neuronal depression of Leao’s type in cerebral
ˆ spreading depression and ischemia in the rat brain cortex, Acta cortex and in hippocampal formation, An. Acad. Bras. Cien. 56 Physiol. Scand. 113 (1981) 437–445. (1984) 495–504.
[22] R.P. Haugland, in: Handbook of Fluorescent Probes and Research [40] G.G. Somjen, P.G. Aitken, J.L. Giacchino, J.O. McNamara, Sus-Chemicals, Molecular Probes, Eugene, OH, 1996. tained potential shifts and paroxysmal discharges in hippocampal [23] U. Heinemann, H.D. Lux, M.J. Gutnick, Extracellular free calcium formation, J. Neurophysiol. 53 (1985) 1079–1097.
and potassium during paroxysmal activity in the cerebral cortex of [41] G.G. Somjen, J.L. Giacchino, Potassium and calcium concentrations the cat, Exp. Brain Res. 27 (1977) 237–243. in interstitial fluid of hippocampal formation during paroxysmal [24] O. Herreras, G.G. Somjen, Analysis of potential shifts associated responses, J. Neurophysiol. 53 (1985) 1098–1108.
with recurrent spreading depression and prolonged unstable SD [42] M. Szatkowski, B. Barbour, D. Attwell, Non-vesicular release of induced by microdialysis of elevated K1 in hippocampus of glutamate from glial cells by reversed electrogenic glutamate uptake, anesthetized rats, Brain Res. 610 (1993) 283–294. Nature 348 (1990) 443–446.
1
[25] B. Hille, in: Ionic Channels of Excitable Membranes, Sinauer [43] C.P. Taylor, Na currents that fail to inactivate, Trends Neurosci. 16 Associates, Sunderland, MA, 1992. (1993) 455–460.
[26] J.R. Hotson, D.A. Prince, P.A. Schwartzkroin, Anomalous inward [44] A. Van Harreveld, J.S. Stamm, Spreading cortical convulsions and rectification in hippocampal neurons, J. Neurophysiol. 42 (1979) depressions, J. Neurophysiol. 16 (1953) 352–366.
889–895. [45] W.J. Wadman, A.J.A. Juta, W. Kamphuis, G.G. Somjen, Current
![Fig. 3. Raising [K ] hastens fluorescence-induced potentiation of1 I. The maximal persistent inward currents measured in 10 fluorescent isolated cellsbefore, during and after raising bath [K ] to 20 mM](https://thumb-ap.123doks.com/thumbv2/123dok/3138456.1382589/6.612.138.483.63.282/raising-uorescence-potentiation-persistent-currents-uorescent-isolated-cellsbefore.webp)