The Cytotoxicity of Mekai (
Albertisia papuana
Becc.) Root
Extract on Breast Cancer Cell Lines T47D and Vero Cell
Lines
Elizabeth B.E. Kristiani
1, a), Laurentius H. Nugroho
2, b), Soekarti Moeljopawiro
2, c),
Sitarina Widyarini
3,d)1)Faculty of Biology, Universitas Kristen Satya Wacana, Salatiga, Indonesia 2) Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, Indonesia 3) Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia
a)[email protected] b) [email protected] c) [email protected]
Abstract. Mekai (Albertisia papuana Becc.) root is usually used for treatment of various diseases especially cancer by
Dayak people, Kalimantan. The objective of this study was to evaluate the cytotoxic activities of A. papuana Becc. root extract and its fractions on breast cancer cell lines (T47D) and normal cell lines (Vero). The plant roots were macerated using ethanol. The MTT assay was applied to determine the cytotoxic activity of ethanol extract on T47D and Vero cell lines. Moreover, SPSS (Probit analysis) was used for calculating IC50 extract. The ethanol root extract was fractionated
using vacuum liquid chromatography. The same method was used for determined the cytotoxic activities and of IC50 of
the fractions. The result showed that the ethanol extract less toxic on T47D cell lines less toxic than ethyl acetate : ethanol (2:3 v/v) fractions that have IC50 21.3 and 9.1 µg/ml respectively. The IC50 values of ethanol extract and ethyl
acetate : ethanol (2:3 v/v) fraction on Vero cell lines were 233.0 µg/ml (SI = 11) and 42.5 µg/ml (SI = 5), respectively. Both of those two solutions were toxic to breast cancer cell lines T47D but not to Vero cell lines.
INTRODUCTION
Medicinal herbs were usually used as traditional health care since thousand years ago, including for cancer treatment. However, most of them have not been scientific studied for proving their pharmacology properties [1]. Lately, many researchers found that certain compounds in plants were showed anticancer activity, so that it could be the potential source to obtain the new alternative cancer drug [2, 3, 4; 5].
Albertisia papuana Becc., known as Mekai, is one of thetraditional medicinal plants in Sumatra and Kalimantan, Indonesia [6].The Dayak people in Kalimantan usually use the root of A. papuana Becc. for cancer treatment by boiling them. According to researchers, some plants from Albertisia genus showed many pharmacological activities, such as antiplasmodium and cytotoxic activities on breast cancer, blood cancer, and kidney of methanol extract of A. delagoensis [7], antibacterial, antifungal, antiplasmoidum, and cytotoxic activities of A. vilosa extract [8], antitumor activity ethanol extract of A. laurifolia [9], and ethanol extract of A. papuana on HeLa cell lines [10].
Based on those reports, A. papuana Becc. has a potential source for discovering of anticancer drug. Therefore the objective of this study was to evaluate the cytotoxic activity and selectivity of ethanol root extracts of A. papuana Becc. and its active fraction on breast cancer cell lines, T47D.
MATERIAL AND METHODS
Materials of experiment
Albertisia papuana Becc. was collected from Dayak, East Kalimantan, Indonesia at April 2014. They were authenticated by botanist at Herbarium Bogoriense, Biology Research Center, Bogor, Indonesia. The voucher specimen was kept in Primary Biology Laboratory, Faculty of Biology, Universitas Kristen Satya Wacana, Salatiga, Indonesia. The breast cancer cell lines T47D and Vero normal cell lines were obtained from Parasitology Laboratory, Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia.
Preparation of ethanol extract of
A. papuana
Becc. root
The ethanol extract of A. papuana Becc. root was prepared using maceration methods [11] with a slight modification. The roots were cleaned, cut into small piece, and air-dried for at least a week then dried in an oven at 40ᵒC for 5 hours. The dried root was ground. Ethanol were added to the root powder (4:1 (v/w) and macerated for 4 x 24 hours. The extract was filtrated and dry by rotary evaporator (Rotavapor R-114 Buchi) under vacuum (Eyela A-1000S) at 40ᵒC.
Fractionation of ethanol extract of
A. papuana
Becc. root
The fractionation process was done using vacuum liquid chromatography (VLC) [12]. Silica gel GF254 was used as stationary phase. The mobile phase was consisted of hexane, chloroform, ethyl acetate, and ethanol in different composition (Table 1). Each fraction was concentrated using rotary evaporator (Rotavapor R-114 Buchi) under vacuum (Eyela A-1000S) at 40ᵒC. The profile of each fraction was identified using Thin Layer Chromatography (TLC). The similar profiles of TLC chromatogram were combined into a new fraction for cytotoxic activity assay.
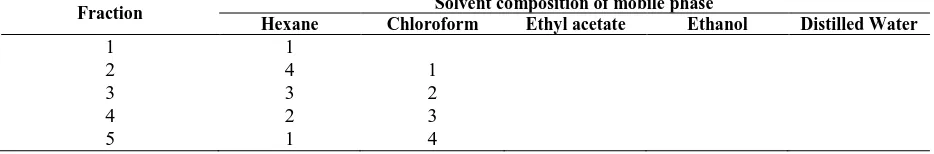
TABLE 1. The composition of mobile phase for fractionation of ethanol root extract of A. papuana Becc. Fraction Solvent composition of mobile phase
Hexane Chloroform Ethyl acetate Ethanol Distilled Water
1 1
2 4 1
3 3 2
4 2 3
6 1
TLC was used to identify the profile of component in fraction. Silica gel 60 F254 precoated plate (Merck, Darmstadt, Germany) was used as stationary phase and mixture of hexane : chloroform : ethyl acetate : ethanol (1:1:1:1, v/v/v/v) as mobile phase. An aliquot 1.5 μl samples were spotted to the plate and then developed in a developing chamber. The chromatogram was visualized using ultraviolet light at 312 nm and then photographed using digital camera (Nikon COOLPIX S3500 20.1 Megapixel).
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
The cytotoxicity of fraction and doxorubicin on T47D cell lines and Vero cell were identified using MTT assay [3] with slight modification. An aliquot of 100 µl cell suspensions (± 1 x 104 T47D cells) were loaded into each well of 96-well plate and incubated at 37°C in a 5% CO2 incubator (Heraeus) for 24 hours. The treatment in
various concentrations (fractions 0-200 μg/ml and doxorubicin 0-100 μg/ml) were added and then incubated at the same condition for 24 hours. Each test concentration was performed three replicated. At the end of treatment, the medium were removed and MTT (Sigma) was added to the plate. The plate was incubated in the dark for 3 to 4 hours. The reaction was stopped with SDS (Sigma) and then incubated overnight at room temperature. The absorbance of each well was measured by ELISA reader (SLT 240 ATC) at 595 nm. The IC50 values (mean ± SD)
were calculated using SPSS 16.0 for Windows® (Probit analysis). The selectivity index (SI) of extract and potential fraction was calculated. SI is the ratio between IC50 value of a matter (extract/fraction/pure compound) on normal
cancer cell and cancer cell [13, 14].
RESULT
Cytotoxicity activity of ethanol extract of
A. papuana
Becc. root
The cytotoxic activity of ethanol extract of A. papuana Becc. root on T47D cell lines was determined using MTT method. The result showed that this extract was a potent cytotoxic with IC50 value of 21.3 µg/ml. For further
purification, this extract was fractionated with gradient polarity of solvent (Table 1).
Fractionation of ethanol root extract of
A. papuana
becc.
FIGURE 1. Chromatogram profiles of fractions resulted from VLC of ethanol root extract of A. papuana Becc. using some types
of mobile phase composition that visualized using ultraviolet light at 312 nm. Spot number 1-17 are number of fractions according to Table 1. Code F1.1-F1.9 are fractions resulted from combination of TLC spot (Table 2).
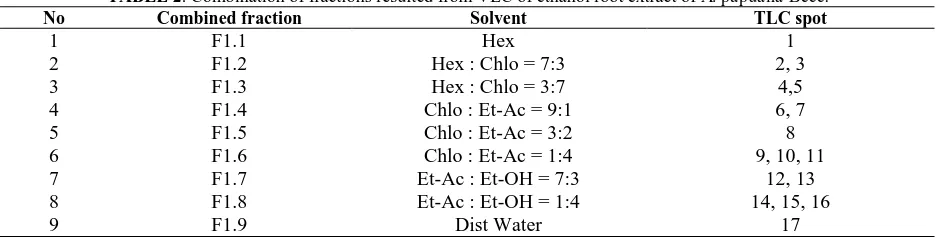
TABLE 2. Combination of fractions resulted from VLC of ethanol root extract of A. papuana Becc.
No Combined fraction Solvent TLC spot
1 F1.1 Hex 1
2 F1.2 Hex : Chlo = 7:3 2, 3
3 F1.3 Hex : Chlo = 3:7 4,5
4 F1.4 Chlo : Et-Ac = 9:1 6, 7 5 F1.5 Chlo : Et-Ac = 3:2 8 6 F1.6 Chlo : Et-Ac = 1:4 9, 10, 11 7 F1.7 Et-Ac : Et-OH = 7:3 12, 13 8 F1.8 Et-Ac : Et-OH = 1:4 14, 15, 16
9 F1.9 Dist Water 17
Hex = hexane; Chlo = chloroform; Et-Ac = ethyl acetate; Et-OH = ethanol; Dist Water = distilled water
Cytotoxicity activity of fraction on T47D
Nine fractions had certain cytotoxic activity on T47D cell lines (Fig. 2). F1.1 and F1.9 were had no cytotoxic activity on T47D cell lines. F1.2 until F1.6 was showed cytotoxic activities with IC50 value of 119.9;
140.7; 144.8; 67.7; 66.7 µg/ml, respectively but less than cytotoxic activity of EE with IC50 value of 21.3 µg/ml).
Two extracts were had cytotoxic activity more than EE that were F1.7 with IC50 value of 4.7 µg/ml and F1.8 with
IC50 value of 9.1 µg/ml). Those activities were no significantly different with cytotoxic activity of doxorubicin on
FIGURE 2. The viability of T47D cell lines on fractions resulted from VLC of ethanol root extract of A. papuana Becc. F1.1 =
fraction of hexane; F1.2 = fraction of hexane : chloroform (7:3); F1.3 = fraction of hexane : chloroform (7:3); F1.4 = fraction of chloroform : ethyl acetate (9:1); F1.5 = fraction of chloroform : ethyl acetate (3:2); F1.6 = fraction of chloroform : ethyl acetate (1:4); F1.7 = fraction of ethyl acetate : ethanol (7:3); F1.8 = fraction of ethyl acetate : ethanol (1:4); F1.9 = fraction ofdistilled
water.
TABLE 3. IC50 value (mean ± SD) of fractions resulted from VLC of ethanol root extract of A. papuana Becc.
No. Fraction IC50 (µg/ml)
1 F1.1 ND 2 F1.2 119.9 ± 37.5 BC 3 F1.3 140.7 ± 12.1 C 4 F1.4 144.8 ± 32.8 C 5 F1.5 67.7 ± 4.3 B 6 F1.6 66.7 ± 11.9 B 7 F1.7 4.7 ± 3.0 A 8 F1.8 9.1 ± 3.6 A 9 F1.9 > 1000 D 10 Doxorubicin 8.1 ± 3.6 A
The same superscript showed no significant difference in the concentration of the fraction/sample between the concentration
F1.1 = fraction of hexane; F1.2 = fraction of hexane : chloroform (7:3); F1.3 = fraction of hexane : chloroform (7:3); F1.4 = fraction of chloroform : ethyl acetate (9:1); F1.5 = fraction of chloroform : ethyl acetate (3:2); F1.6 = fraction of chloroform : ethyl acetate (1:4); F1.7 = fraction of ethyl acetate : ethanol (7:3); F1.8 = fraction of ethyl acetate : ethanol (1:4); F1.9 = fraction of distilled water.
The fractions with cytotoxicity less than ethanol root extract that were F1.7 and F1.8 were combined into one fraction that was ethyl acetate : ethanol (2:3) fraction (EE23F) with the IC50 value of 9.1µg/ml. The cytotoxicity
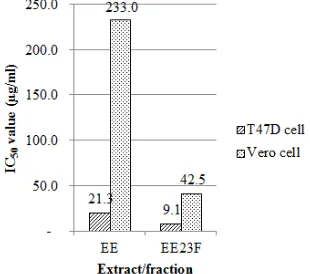
FIGURE 3. Comparison of IC50 value between T47D cell and Vero cell on ethanol extract (EE) and ethyl acetate : ethanol (2:3)
fraction (EE23F) of A. papuana Becc.
The selectivity index of extract was calculated by dividing the IC50 value on normal human cell lines Vero
and breast cancer cell lines T47D (Fig. 3 and Table 4). The selectivity index of EE was higher than EE23F with the SI value of 11 and 5, respectively.
TABLE 4. Selectivity index of ethanol extract (EE) and ethyl acetate : ethanol (2:3) fraction (EE23F) of A. papuana Becc. root Extract/Fraction IC50 value (µg/ml) Selectivity Index
Vero T47D
EE 233,0 21,3 11 EE23F 42,5 9,1 5
Discussion
Various studies on plants extract cytotoxicity on cancer cell lines were done to obtain anticancer agent. Mekai (Albertisia papuana Becc.) is one of the endogenous plants in Dayak, East Kalimantan. The Dayak people usually use Mekai for cancer treatment by boiling the root of plant using water. In this study, we used ethanol to extract root of plant in order to know the cytotoxic activity of this extract. In traditional treatment water is usually use as a solvent, while in modern medicine use the varying polarities of organic solvent to obtain the bioactive compounds in the matter [15].
MTT assay was an in vitro cytotoxicity screening which was used to calculate the amount of cells growth inhibition caused by an anticancer agent [3]. In this study, IC50 of ethanol root extract of A. papuana Becc. on T47D
cell lines was 21.3 µg/ml. According to The National Cancer Institute (NCI) USA, the upper limit of the crude extract which is qualified for further purification with IC50 values of 30 µg/ml [16], so the ethanol root extract was
promising for further purification.
The steps were consisted of fractionated the active crude extract using chromatography procedure followed by bioassay of each fraction was known as Bioassay-Guided Isolation [17]. The cycle of them could be repeated until obtained pure compound with the desired activities. Vacuum Liquid Chromatography (VLC) is an efficient, quick, and cheap method to separate compounds into its fraction component [12]. The separation sequentially of ethanol root extract of A. papuana Becc. using solvent in different polarity would extract compounds according to the polarity of solvent [15]. The compounds extracted using mixture of chloroform with ethyl acetate and ethyl acetate with ethanol (F1.5 – F1.8) could separate well by eluting of the mixture of hexane : chloroform : ethyl acetate : ethanol (1:1:1:1, v/v/v/v) on TLC method (Fig. 1).
The fraction of which fractionated using the mixture of ethyl acetate and ethanol (F1.7 and F1.8) had cytotoxic activities with IC50 value of 4.7 µg/ml and 9.1 µg/ml, respectively less than ethanol root extract (21.3
extract flavonoid, terpene and tannin [19]. According to this study, the bioactive compounds of both extracts were flavonoid and tannin.
Selectivity index (SI) is use to know selectivity a matter toward a cancer cell and normal cell. The greater the SI, the more selective drug is. An anticancer drug was called had general selectivity if the SI less than 2.0 [13]. Therefore it could be concluded that both the ethanol root extract (SI = 11) and ethyl acetate : ethanol (2:3) fraction (SI = 5) were toxic to breast cancer cell lines T47D but not to normal cell lines Vero. The ethyl acetate : ethanol (2:3) fraction was promising for further purification to obtain the anticancer bioactive.
ACKNOWLEDGMENTS
The authors were appreciated to Ministry of Education and Culture of Indonesia for providing BPPS scholarship in Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, Indonesia.
REFERENCES
1. C. Kirana, G. P. Jones, I. R. Record, G. H. McIntosh, J Nat Med 61, 131–137 (2007).
2. H. Almehdar, H. M. Abdallah, A-M. M. Osman, E. A..Abdel-Sattar, J Nat Med 66, 406–412 (2012) 3. S. Arullappan, S. Muhamad, Z. Zakaria. Tropical Journal of Pharmaceutical Research 12, 743-746 (2013). 4. E. Prakash and D.K.Gupta, Universal Journal of Plant Science 1, 113-117 (2013).
5. R. Akter, J. Shaikh, I. Uddin, D. Grice, E. Tiralongo, J Nat Med 68, 246–252 (2014).
6. H. Lusiana,T. T. Irawadi, H. Suprapto, “Uji antiplasmodium senyawa alkaloid dari Albertisia papuana Becc.”
in Seminar Nasional Kimia Terapan Indonesia (2013), pp. 75-78. 7. H. De Wet, G. Fouche, F. R. Van Herden, AJOL. 8, 3332-3335 (2009).
8. M. L. Lohombo-Ekombo, P. N. Okusa, O. Penge, C. Kabongo, M. I. Choundary, O. E. Kasende, Journal Ethnopharmacology 93, 331-335 (2004).
9. X. Zh, W. Yun, Z. Pei-Ling, M. Jian-Min, H. Ping, Acta Botanica Sinica 27, 630-634 (1985).
10. C.K. Angerhofer, H. Guinaudeau, V. Wongpanich, J. M. Pezzuto, G. A. CordellJ. Nat. Prod 62, 59-66 (1999). 11. H. Rahman, V. B. S. Ghosh, G. Pant, G. Sibi. American Journal of Life Sciences 2, 7-10 (2014).
12. J. C. Coll and B. F. Bowden, Journal of Natural Product 49, 934-936 (1986).
13. R. B. Basida, S. F. darling-Reed, P. JOSEPH, S. J. S. Cooperwood, L. M. Latinwo, C. B. Goodman. Anticancer Res. 29, 2993–2996 (2009).
14. M. A. Musa, V. L. D. Badisa, L. M. Latinwo, C. Waryoba, N. Ugochukwu, Anticancer Reserach 30, 4613-4618 (2010).
15. S. S. Handa, S. P. S. Khanuja, G. Longo, D. D. Rakesh, D.D. Extraction Technologies for Medicinal and Aromatic Plants. (United Nations Industrial Development Organization and the International Centre for Science and High Technology, Trieste, Italy, 2008), pp. 21-25.
16. V. Kuete, H. K. Wabo, K. O. Eyong, M. T. Feussi, B. Wiench, B. Krusche, P. Tane, G. N. Folefoc, T. Efferth. PLoS ONE 6, 1-7 (2011).
17. A. M. Rimando, M. Olofsdotter, F. E. Dayan, and S. O. Duke, Agronomy Journal, 93, 16–20 (2001). 18. M. M. Cowan, Clinical Microbiology Review 12, 564–582 (1999).