FINAL REPORT
INDONESIA TORAY SCIENCE FOUNDATION
RESEARCH GRANT 13THIdentification of Microorganisms
Producing New
-lactamase Inhibitor From Indonesia
By :
Sri Agung Fitri Kusuma Valentina Yurina
PADJADJARAN UNIVERSITY BANDUNG
1
Identification of New
-lactamase Inhibitor
Producing Microorganisms From Indonesia
Sri Agung Fitri Kusuma1 and Valentina Yurina2
Abstract
Despite of increasing numbers of Escherichia coli resistant to combination of ampicillin and clavulanic acid stated in recent reports, discovery of new -lactamase inhibitor become s important. Our previous experiment showed that some microorganisms isol ated from Indonesia have potency to prod uce inhibitor against clinical isolate -lactamase producing E. coli. Objectives of this research were to determine the dose dependent effect of extracellular products of ten β-lactamase inhibitor producing bacteria from Indonesia and to identify the species of the producers. β-lactamase inhibitor activity assay was conducted by agar diffusion method using clin ical isolate E. coli producing -lactamase. Our results showed that extracellular product of nine bacteria under study had -lactamase inhibitor activity. These bacteria also have antibiotic activity against both resistant and sensitive E. coli strains. The dose-dependent effect assay of one extracellular product showed more potent antibiotic activity than betalactamase inhibiting activity. Bacterial identification with phenotypic approach was done by colony and cell morphology observation as well as biochemical assays; and with genotypic approach was done by 16S rDNA-based method. Species identification of nine beta-lactamase inhibitor producing bacteria were Streptomyces brasiliensis, Staphylococcus equorum, Salmonella typhi, Pseudomonas fluorescens, P . putida, Enterobacter hormaechei, Aerom onas popoffii, Enterobacter sp. and Serratia marcescens. While the species of antibiotic producer was Alcaligenes faecalis.
Key words: beta-lactamase inhibitor, ampicillin resistant Escherichia coli, Indonesia.
Introduction
Escherichia coli is a Gram negative bacterium and represents normal inhabitant of human intestines. Although E. coli has been considered to be human normal flora, it can cause a wide variety of intestinal and extra intestinal infection, such as diarrhea, urinary tract infection, septicemia and neonatal meningitis (Jawetz et al., 1995).
An example of drug of choice for E. coli infection is ampicillin. However, many strains of E. coli were reported to be resistant to ampicillin; consequently it is not widely used anymore. -lactamase represents the main mechanism of bacterial resistance to -lactam antibiotics by hydrolyzing -lactam ring to form an inactive derivative of -lactam substance. To overcome microbial resistance to -lactam, combinations of hydrolyzable -lactam antibiotics and a -lactamase inhibitor are successfully used. Clavulanic acid, Sulbactam and Tazobactam are examples of potent -lactamase inhibitor for many -lactamase enzymes. However, about 62 % of E. coli isolates were resistant to the combination (Vanjak et al., 1995).
Production of -lactamase is very important for -lactam resistance in human pathogen including E. coli. One study reported that -lactamase was found in more than 90 % of urinary pathogens with mostly (94.8 %) of E. coli (Orret and Shurland, 1996). Therefore, this research is focused on an E. coli isolate from urine sample of cystitis patient obtained from one of hospital in Bandung and was isolated in previous study (Kusuma, 2005, unpublished data). In previous study, the existence of ampicillin resistance gene (bla gene) was detected by Polymerase Chain Reaction (PCR) using bla specific primer (Bonar and Kusuma, 2005).
Our preliminary data showed that extracellular product of some marine and soil bacteria exhibit potency to contain -lactamase inhibitor that inactivates -lactamase produced by clinical isolate of ampicillin resistant Escherichia coli. Since the extracellular products of some marine and soil microorganisms have ability to kill ampicillin-resistant strain producing -lactamase, the compound of microbial products may contain new -lactamase inhibitor. The discovery of new -lactamase will give a significant impact in the development of drug. This research will also to enhance the exploration of microbial biodiversity because the source of -lactamase inhibitor candidates are collected from Indonesian soil and marine. The objectives of this current research were to study the dose-dependent inhibition of extracellular products from microorganisms with potential -lactamase inhibitory activity and to identify
1
Faculty of Pharmacy, Padjadjaran University, Bandung, Indonesia
2
the species of ten -lactamase inhibitor producing microorganisms by phenotypic and genotypic approach.
Material and Methods
Materials
Nutrient Broth, Nutrient Agar, Primer 16S rRNA, Taq Polymerase, dNTP, MgCl2, Agarosa, Ethidium bromide, Trisma base, EDTA, DNA Marker, Ampicillin pro injection, Gentian violet, Potassium iodide, iodine, Fuchsin acid, Lugol, Emersi oil, Xylene, Citrate simmon, Urea indole, MR-VP broth, Kovac’s reagen, Methyl red indicator, Barrit reagen, Glucose broth, Lactose broth, Mannose broth, Maltose broth, Sucrose broth, Phenol red indicator, Sodium chloride, Sodium dihydrophosphate, Potassium chloride, and Potassium dihydrophosphate.
Methods
1. Production of extracellular products of marine and soil microorganisms
Six marine and four soil microorganisms previously shown to produce β-lactamase inhibitor were cultured in 30 mL of Nutrient Broth at room temperature for 18 hours. Supernatants were obtained by centrifugation at 4 oC and cell pellets were removed. The
supernatants were lyophilized and dissolved in 500 µL of sterile phosphate buffer saline (PBS). These concentrated extracellular products were tested for growth inhibition of an -lactamase producing ampicillin resistant E. coli in ampicillin containing agar. This E. coli strain had been confirmed to contain bla gene (gene encoding for β-lactamase) and produced
β-lactamase using colorimetric assay. The concentration of supernatants was varied i.e.20, 40, 60, 80 and 100 % to examine dose-dependent activity.
2. Identification of Marine and Soil Microorganisms 2.1Phenotypic Identification
Ten microorganisms were identified by observing colony and cell morphology, biochemical assays and sugar utilizing activity. Shape and color of the colonies were observed visually, while cell morphologies were observed by microscope using Gram staining method. Biochemical assays were conducted by citrate acid, motile indole urea, red methyl-Voges Proskauer (MR-VP), VP, and sugar fermentation assay.
2.2 Genotypic Identification
3
The PCR products were isolated from agarose gel and purified using GFX column. The purified PCR products were then analyzed with an automatic sequencer. Then, nucleotide sequences were analyzed using Basic Local Alignment Search Tools (BLAST) program (http//www.ncbi.nih.nlm.gov) to find the highest homology/identity values with sequences in Gene Bank. Final identification of the ten microorganisms was determined by combining phenotypic and genotypic data.
Result and Discussion
Dose-Dependent Activity
Dose dependent activity assay was conducted to determine the effect of -lactamase inhibitor activity for inactivating -lactamase produced by clinical isolate of ampicillin resistant Escherichia coli. The inhibition zone from one of inhibitor producing bacteria i.e CL3 was shown in Fig. 1. While other bacteria gave similar result as CL3 bacteria.
Figure1. Inhibition Zones of CL3 Bacteria at Various Concentrations a. Ampicillin resistant E. coli; b. Ampicillin sensitive E. coli
Increasing concentrations of nine tested extracellular products gave progressive greater effects on the diameter of inhibition zone of resistant E. coli, while the inhibition zone diameters of sensitive E. coli was significantly smaller. An example, the effect of CL3 extracellular product concentration to inhibition zone diameter was shown in Fig. 2. These data indicated that the extracellular products contain -lactamase inhibiting activity. Besides, it also contains antibiotics activity because the inhibition zones were formed for both of sensitive and resistant E. coli. The discovery of antibiotics substances in extracellular product could gave a new expectation to overcome E. coli resistant infection.
Figure 2. The Effect of CL3 Extracellular Product Concentration to Inhibition Zone Diameter
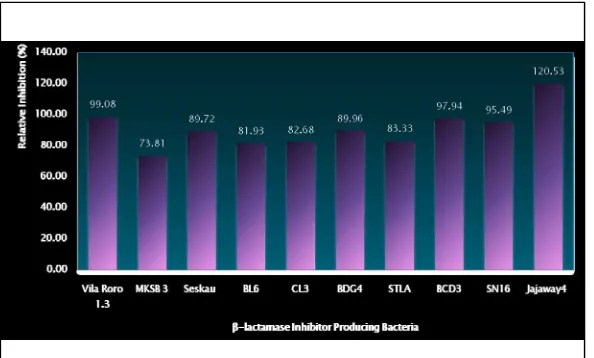
The relative inhibition of undiluted extracellular product to ampicillin sensitive and resistant E. coli was shown in Fig. 3. Relative inhibition (%) was measured by comparing the diameter of inhibition zone of undiluted extracellular products given by ampicillin sensitive to that of resistant E. coli X 100%.
Figure 3. Inhibition of Undiluted Extracellular Product of Tested Bacteria
From figure 3, we can see that among all tested bacteria, the extracellular product of jajaway 4 gave very different result from the other. Inhibition zone in sensitive E. coli from this sample was bigger than in the resistant one. We hypothesized that the extracellular product of sample jajaway 4 have more potent antibiotic activity than betalactamase inhibiting activity.
Identification of Marine and Soil Microorganisms Using Phenotypic Identification
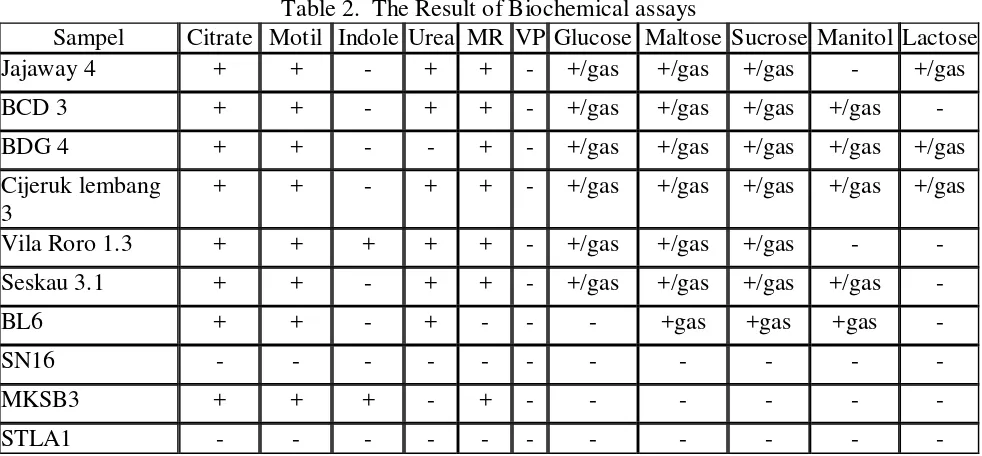
The identification data of ten tested bacteria using phenotypic approach by colony and cell morphology observation and biochemical assays shown in Table 1-2.
Table 1. The Morphology of Ten Betalactamase Inhibitory Producing Bacteria
No. Sample name Shape Gram
Grouping Colony color
1. Jajaway 4 Rod _ Yellowish white
2. BDG4 Rod _ Yellowish white,
tranparant
3. Cijeruk lembang3 Rod _ Yellowish white,
tranparant
4. BCD3 Rod _ Tranparant white
5. SLTA1 Coccus + Tranparant white
6. SN16 Coccus + White,opaque,
7. Vila Roro 1.3 Rod - White, opaque
8. BL6 Rod _ yellow
9. Seskau 3.1 Coccus _ Yellowish White
5
Table 2. The Result of Biochemical assays
Sampel Citrate Motil Indole Urea MR VP Glucose Maltose Sucrose Manitol Lactose
Jajaway 4 + + - + + - +/gas +/gas +/gas - +/gas
BCD 3 + + - + + - +/gas +/gas +/gas +/gas -
BDG 4 + + - - + - +/gas +/gas +/gas +/gas +/gas
Cijeruk lembang 3
+ + - + + - +/gas +/gas +/gas +/gas +/gas
Vila Roro 1.3 + + + + + - +/gas +/gas +/gas - -
Seskau 3.1 + + - + + - +/gas +/gas +/gas +/gas -
BL6 + + - + - - - +gas +gas +gas -
SN16 - - - -
MKSB3 + + + - + - - - -
STLA1 - - - -
Based on morphology and biochemical assays, bacteria can be identified at genus level only. The identity of tested bacteria is present in Table 3.
Table 3. Bacteria Identity using phenotypic Analysis
No. Sample Genus
1 Jajaway 4 Alcaligenes
2 BCD3 Pseudomonas
3 BDG4 Salmonella
4 Cijeruk lembang 3 Enterobacter
5 Vila Roro 1.3 Serratia
6 Seskau 3.1 Aeromonas
7 BL6 Pseudomonas
8 SN16 Stapylococus
9 MKSB3 Enterobacter
10 STLA1 Staphylococcus
Identification of Marine and Soil Microorganisms Using Genotypic Identification
Figure 4. PCR Product of 16S rDNA of marine and soil microorganisms under study 1 = BDG4; 2 = Vila Roro 1.3; 3 = SN16; 4 = Jajaway4; 5, 6, 16 = 100 bps DNA marker 7= Seskau 3.1; 8 = STLA; 9 = negative control; 10= positive control, 11 = BL6; 12 =CL3,13= BCD3; 14 = Seskau; 15 = MKSB3
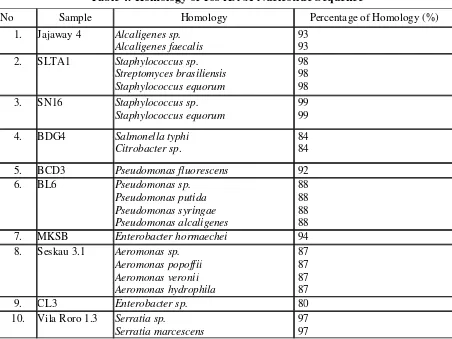
Nucleotide sequence analysis using forward and reverse specific for 16S rDNA gene is shown in Table 4.
Table 4. Homology of 16s rDNA Nucleotide Sequence
No Sample Homology Percentage of Homology (%)
1. Jajaway 4 Alcaligenes sp. Alcaligenes faecalis
93 93 2. SLTA1 Staphylococcus sp.
Streptomyces brasiliensis Staphylococcus equorum
98 98 98 3. SN16 Staphylococcus sp.
Staphylococcus equorum
5. BCD3 Pseudomonas fluorescens 92
7
Identification of bacteria using genotypic and phenotypic analysis gives a suitable result which is lead to the correct identity of each bacterium.
Conclusions
This research strongly showed that the extracellular product of nine bacteria under study contain -lactamase inhibitor activity and in less extend contain activity to kill both resistant and sensitive E. coli strains. One bacteria showed more potent antibiotic activity than betalactamase inhibiting activity. The substances found in this research have good potency as antibiotics to treat ampicillin resistant E. coli. The species of ten tested bacteria were Alcaligenes faecalis, Streptomyces brasiliensis, Staphylococcus equorum, Salmonella typhi, Pseudomonas fluorescens, Pseudomonas putida, Enterobacter hormaechei, Aeromonas popoffii, Enterobacter sp. and Serratia marcescens. From our knowledge, betalactamase inhibitor substances previously available are obtained from published betalactamase inhibitor producing microorganisms.
Acknowledgment
This work was financially supported by Indonesia Toray Science Foundation (ITSF).
References
Jawetz E., J. L. Melnick, E. A. Adelberg, G. F. Brooks, J. S. Butel, L. N. Ornston, 1995, Mikrobiologi Kedokteran, ed. 20, University of California, San Francisco.
Johnson J. R., M. A. Kuskowsky, T. T. O’Bryan, R. Colodner, and R. Raz, 2005, Virulence Genotype an Phylogenetic Origin in Relation to Antibiotic Resistence Profile among Escherichia coli Urine Sample Isolates from Israeli Woman with Acute Uncomplicated Cystitis, Antimicrob. Agents Chemother., 49(1), 26-31.
Karlowsky J. A., L. J. Kelly, C. Thornsberry, M. E. Jones, and D. F. Sahm, 2002, Trends in Antimicrobial Resistance among Urinary Tract Infection Isolates of Escherichia coli from Female Outpatient in the United States, Antimicrob. Agents Chemother., 46(8), 2540-2545. Madigan M.T. et al., 1997. Biology of Microorganisms, Eighth Edition. New Jersey, Prentice Hall International.
Manges A. R., J. R. Johnson, B. Foxman, T. T. O’Bryan, K. E. Fullerton, and L. W. Riley, 2001, Widespread Distribution of Urinary Tract Infections Caused by A Multridrug Resistance Escherichia coli Clonal Group, N. Engl. J. Med., 345(14), 1007-1009.
Oliver A., M. Perez-Vazquez, M. Martinez-Ferrer, F. Baquero, L. de Rafael, and R. Canton, 1999, Ampicillin-Sulbactam and Amoxicillin-Clavulanate Susceptibility Testing on Escherichia coli of Isolates with Different Beta-Lactam Resistance Phenotypes, Antimicrob Agents Chemother., 43, 862-867.
Saves I., O. Burletschiltz, P. Swaren, F. Lefevre, J. M. Masson, J. C. Prome, J. P. Samama, 1995, The Asparagine to Aspartic Acid Substitution at Position-276 of TEM-35 and TEM-36 is Involved in the Betalactamase Resistance to Clavulanic Acid, J. Bio. Chem., 270(31). Teale C. J., 2005, Detection and Characterisation of Betalactamase Resistance in Gram Negatif Bacteria of Veterinary Significance, UK National Guidelines for Laboratories, 102, 1-5.
9
CONSUMABLE LIST
NO ITEM COST (Rp)
1. Small Equipment 5.660.000
2. Consumables 28.340.000
3. Other expenditures 1.900.000
TOTAL 35.900.000
1. Cost of Small Equipment
No. Small equipment Amount Cost per item (Rp) Cost (Rp)
1. Micropipette 1000 uL 1 2.500.000 2.500.000
2. Micropipette 10-100 uL 1 2.500.000 2.500.000
24. tissue 10 rolls 2.000 20.000 25. Spiritus 10 bottles 8000 80.000 26. Alkohol 70% 3 Liter 15.000 45.000 27. Aquadest 10 Liter 1000 10.000
28. Cotton 500 g 40 20.000
29. Object glass 2 boxes 30.000 60.000 30. Gentian violet 150 mL 800 120.000 31. Potassium iodide 200 g 3.875 775.000 32. iodine 1 pack 330.000 330.000 33. Fuchsin acid 250 mL 1.000 250.000 34. Lugol 250 mL 1.000 250.000 35. Emersi oil 100 mL 1.000 100.000 36. Xylene 200 mL 1.200 240.000 37. Citrate simmon 1 boxes 350.000 350.000 38. Urea indole 1 boxes 400.000 400.000 39. MR-VP broth 100 g 2.500 250.000 40. Kovac’s reagen 50 mL 5.100 255.000 41. Methyl red indicator 25 g 29.600 740.000 42. Barrit reagen 50 mL 5.600 280.000 43. Glucose broth 1 box 400.000 400.000 44. Lactose broth 1 box 400.000 400.000 45. Mannose broth 100 g 3.000 300.000 46. Maltose broth 100 g 3.000 300.000 47. Sucrose broth 100 g 3.000 300.000 48. Phenol red indicator 5 g 116.000 580.000 49. Sodium chloride 125 gr 2.000 250.000 50. Para film paper 1 roll 300.000 300.000 51. Sodium dihydrophosphate 50 g 1.500 75.000 52. Potassium chloride 50 g 1.500 75.000 53. Potassium
dihydrophosphate
50 g 2.300 115.000
TOTAL 28.340.000
3. Other expenditures
Other expenditures Cost (Rp)
Administration 1.100.000
Data Documentation 800.000