www.elsevier.com / locate / bres
Short communication
Effects of microinjection of OFQ into PAG on spinal dorsal horn
WDR neurons in rats
*
Zhi-Lan Yang, Yu-Qiu Zhang, Gen-Cheng Wu
State Key Laboratory of Medical Neurobiology, Department of Neurobiology, Shanghai Medical University, 138 Yi Xue Yuan Road,
Shanghai200032, PR China Accepted 10 October 2000
Abstract
The aim of the present study was to examine the effect of microinjection of orphanin FQ (OFQ) into periaqueductal gray (PAG) on sensory processing in the wide dynamic range (WDR) neurons of the spinal dorsal horn and to explore the effect of OFQ on a descending system of pain modulation. The results show that microinjection of OFQ into ipsilateral PAG significantly facilitated C-fibre evoked response and post-discharge of spinal dorsal horn WDR neurons. This is consistent with our previous results obtained in behavioral studies. It suggests that the supraspinal effect of OFQ on pain may partly be mediated by PAG neurons. 2001 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Opioids: anatomy, physiology, and behavior
Keywords: OFQ; PAG; WDR neurons of the spinal dorsal horn; C-fiber evoked response; Post-discharge; Pain modulation
The newly discovered neuropeptide orphanin FQ these sites suggests its involvement in nociceptive process-(OFQ), also called nociceptin, has been reported to be the ing. The present study was designed to explore the effect endogenous ligand of the opioid-like orphan receptor of OFQ microinjected into PAG on sensory processing in (ORL1) [5,8]. In spite of its structural homology with the the wide dynamic range (WDR) neurons of the spinal classic opioid peptides, OFQ does not show appreciable dorsal horn, and whether the OFQ-induced supraspinal binding tom,dandk receptors. Some behavioural studies effect resulted from a descending system of pain modula-have shown that supraspinal OFQ induces hyperalgesia tion.
[9], whereas spinal OFQ tends to produce analgesia [13]. Sprague-Dawley rats (250–320 g) were supplied by the Electrophysiological studies in vivo demonstrated that Experimental Animal Center, Shanghai Medical Universi-spinal OFQ inhibits the C-fibre evoked wind-up and post- ty. Treatment of the animals conformed to the guidelines discharge of the dorsal horn neurons to noxious stimulation of the International Association for the Study of Pain [16]. [10]. In situ hybridization and immunohistochemistry Rats were anesthetized with urethane (1.0 g / kg, i.p.). showed that the ORL1 receptor was widely distributed in After cannulations of trachea and left jugular vein, the rat’s the central neural system, especially in periaqueductal gray head was fixed in a stereotaxic frame (Narishige SN-3, (PAG), dorsal raphe nucleus and spinal cord dorsal horn Japan). A laminectomy of vertebra L was performed to1
[1]. The high level expression of the ORL1 receptor in expose the lumbar enlargement of the spinal cord. The vertebral column was fixed in the frame by clamps and the wound was covered by a pool of warm paraffin oil. The guide cannule (0.5 mm outer diameter) was inserted
*Corresponding author. Tel.:186-21-64041900, extn. 2397; fax:1
86-vertically into the ventral PAG (vPAG: P 7.6 mm, R 0.6
21-64174579.
E-mail address: [email protected] (G.-C. Wu). mm, H 5.0 mm). Drug microinjection was made through
an injection cannula (0.26 mm outer diameter) that extend- responses which was more than 20% of the control level. ed 1 mm beyond the tip of the guide cannula. All data are expressed as mean6S.E.M. Statistical analysis When electrical stimulation of receptive fields was was performed using one-way analysis of variance performed, the animals were paralyzed with gallamine (ANOVA) and Student’s t-test. Statistical significance was
21
thiethiodide (flaxedil, 40–50 mg kg , i.v., supplemental concluded at P,0.05.
21 21
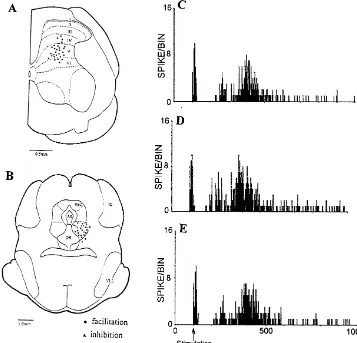
8–10 mg kg h ) and artificially ventilated. The rectal A total of 39 WDR neurons (responding to both non-temperature, electrocardiogram and CO of terminal blood2 noxious and noxious stimuli in a graded manner [3]) were were monitored continuously and maintained within phys- recorded on 28 rats (1–2 units per rat, 1–2 times injection iological limits throughout the experiment. per unit). The recording electrode was located at depths of A single unit extracellular recording was obtained from 550–1200 mm below the dorsal surface of the cord, the lumber dorsal horn neurons with glass micro-pipettes corresponding to laminae IV–VI of the dorsal horn [7] filled with 0.5 mol / l sodium acetate containing 2% pon- (Fig. 1A). The microinjection sites are shown in Fig. 1B. tamine sky blue (impedance 5–15 MV at 1000 Hz) [15]. Note that there are three injection sites located beyond Briefly, the receptive fields of the dorsal horn neurons were vPAG. One out of three was inhibited and two out of three stimulated electrically with a pair of steel needle electrodes were not influenced, therefore they are not included in the applied to the skin. A single electrical pulse was delivered curve.
to the receptive field at an interval of 3 s. The intensity of The NS injected into PAG had no effect on the re-the stimuli (0.5 ms, 20–30 mA) was about three times re-the sponses of WDR neurons, with the mean A -fibre andb
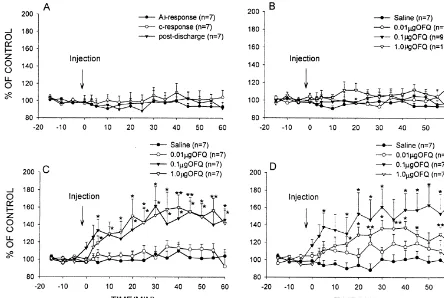
C-fibre threshold. A series of 10 stimuli were applied in C-fibre evoked response and post-discharge being, respec-each trial and the recorded responses were displayed using tively, 96.2163.62, 100.9666.77 and 96.8566.57% (con-a post-stimulus histogr(con-am. The numbers of spikes in the trol %, respectively) throughout the 60 min observation evoked responses were calculated by the computer data period (Fig. 2A). There were no statistically significant collection system (SMUP). These evoked responses were differences compared with before administration.
separated and quantified on the basis of threshold and OFQ injected into PAG (0.01, 0.1 and 1.0mg) had no latency into A -fibre (0–20 ms), A -fibre (20–90 ms),b d effect on A -fibre evoked responses at three differentb
C-fibre (90–300 ms) and post-discharge (300–800 ms) doses (P.0.05) (Fig. 1C–E, Fig. 2B).
[10]. The effect of 0.01 mg OFQ on the C-fibre evoked
Heptadecapeptide OFQ were synthesized and purified by response was not significant (P.0.05, KW-ANOVA, n5
the Shanghai Institute of Biochemistry, Chinese Academy 7), whereas injection of 0.1 and 1.0mg OFQ significantly of Science. The peptide was dissolved in sterilized normal increased the C-fibre evoked responses in most of the saline (NS). Since OFQ is rich in alkaline amino acids, the WDR neurons (7 / 9 and 7 / 10, respectively). The maximal solution, as well as NS, was added with arrowhead double- effect was usually observed within 30–40 min after headed proteinase inhibitor (1 g / l, a product of the administration (Fig. 1C–E, Fig. 2C). The C-fibre evoked Shanghai Institute of Biochemistry), which has been response changes elicited by both 0.1 and 1.0 mg OFQ reported to be able to inhibit trypsin, chymotrypsin and were statistically significant compared with before ad-kallikrein to prevent proteolysis after injection [14]. ministration (P,0.01, KW-ANOVA, n57). The effects After a stable control response, NS or OFQ (0.01, 0.1 obtained from 1.0 mg OFQ were greater than those and 1.0 mg, respectively) in a volume of 0.1 ml was obtained from 0.1mg OFQ.
administered into the vPAG, and spinal neuronal responses The post-discharge was not influenced after injecting were observed for 60–100 min. At the end of the experi- 0.01 mg OFQ (P.0.05, KW-ANOVA, n57), whereas ments, the microinjection site was marked by injecting 0.1 injection of 0.1 and 1.0 mg OFQ significantly facilitated
ml pontamine sky blue. The injected location was de- post-discharge in most of the WDR neurons (7 / 9 and termined in histological section on the basis of the atlas of 7 / 10, respectively). The peak effect of the post-discharge Paxinos and Watson [7]. The lamina location of each usually occurred at 30–40 min after administration (Fig. neuron recorded was obtained from readings of the micro- 1C–E, Fig. 2D). The effects elicited by 0.1 and 1.0 mg manipulator. OFQ were statistically significant compared with before The effects of OFQ administered into PAG on A -fibreb administration (P,0.001, KW-ANOVA, n57). The ef-and C-fibre evoked response ef-and post-discharge of WDR fects obtained from 0.1mg OFQ were greater than those neurons were indicated quantitatively as a percentages of obtained from 1.0mg OFQ.
Fig. 1. Histological reconstructions of the right lateral L4 spinal cord recording sites (A) and OFQ microinjected into ipsilateral ventral PAG sites (B). (C–E) Examples showing histograms of 0.1mg OFQ microinjected into ventral PAG on the A -fibre and C-fibre response and post-discharge of WDRb
neurons evoked by cutaneous electrical stimulation (10 stimuli). (C) Control; (D) 40 min after OFQ injection; (E) 60 min after OFQ injection. Abbreviations: PAG, periaqueductal gray; Aq, aqueduct; DR, dorsal raphe nucleus; IC, inferior colliculus; VLL, ventral nucleus of the lateral lemniscus.
C-fibre evoked response and post-discharge of a WDR system which, when activated by some stimulation, neuron facilitated by microinjection of 0.1 mg OFQ into produces a powerful inhibition of excitation of dorsal horn vPAG. neurons [4]. Morgan’s study showed that microinjection of This is the first report demonstrating by electrophysio- OFQ into PAG inhibited the antinociceptive effect of both logical techniques that microinjection of OFQ into PAG morphine and kainic acid by different mechanisms [6]. modulates the spinal nociceptive sensory processing in Opioids may inhibit some inhibitory interneurons in PAG, vivo. Our results show that microinjection of OFQ into thus disinhibiting PAG output neurons, whereas kainic acid PAG significantly facilitates C-fibre response and post- may directly excite PAG output neurons. Since OFQ discharge of WDR neurons evoked by noxious subcuta- inhibits nearly all PAG neurons, the only way that OFQ neous electrical stimulation in a dose-dependent manner. modulates the effects of exogenous opioid is to reverse the This is consistent with previous behavioral studies report- effects of opioids on neurons downstream from opioid ing that supraspinal OFQ induces hyperalgesia [6,12]. sensitive neurons. Our study reveals that OFQ microinject-PAG is known to play a pivotal role in the modulation of ed into PAG significantly facilitated the C-fiber evoked nociception. Anatomical and pharmacological studies have response and post-discharge of spinal dorsal horn neurons. established that ventral PAG output neurons project to the This is consistent with Morgan’s study.
Fig. 2. Effects of NS and OFQ (at three different doses) microinjected into PAG on dorsal horn WDR neurons in rats. (A) Effects of injecting NS on A -fibre and C-fibre evoked response and post-discharge. (B) Effects of injecting OFQ on A -fibre evoked response. (C) Effects of injecting OFQ onb b
C-fibre evoked response. (D) Effects of injecting OFQ on post-discharge. Data are expressed as mean6S.E.M. *P,0.05 and **P,0.01 indicate significant differences compared with the NS.
cyclase [5,8], to increase inwardly rectifying potassium stimulation. This suggests selective actions of OFQ on conductance and inhibit calcium channel currents [11]. We noxious evoked activity. Our present result demonstrates believe that in the effect of microinjection of OFQ into that the effect of supraspinal OFQ may be partly mediated PAG facilitating C-fibre response and post-discharge of by PAG neurons.
WDR neurons evoked by noxious subcutaneous electrical stimulation, it is likely that OFQ acts on the ORL-1
receptor on opioidergic neurons to inhibit opioid release, Acknowledgements facilitating the excitation of dorsal horn neurons. The
co-existence on neurons ofb-endophin, leu-enkephalin and This study was supported by grants from the National dynorphin with ORL1 mRNA in PAG has been observed Natural Science Foundation of China (39970925) and the (unreported data). It is also likely that OFQ acts directly on Research Fund for the Doctoral Program of Higher Educa-the ORL-1 receptor on PAG output neurons that project to tion (9835).
the RVM, so as to inhibit the activities of the descending inhibition system. Further experiments are still required for elucidation of the mechanism of microinjection of OFQ
References
into PAG enhancing the nociceptive responses of spinal dorsal horn neurons.
[1] B. Anton, J. Fein, T. To, X. Li, L. Silberstein, C.J. Evans,
The effect of 0.1 mg OFQ was greater than that of 1.0 Immunohistochemical localization of ORL-1 in the central nervous
mg OFQ on post-discharge, perhaps some opioid receptors system of the rat, J. Comp. Neurol. 368 (1996) 229–251.
(for example, the m receptor) are activated besides the [2] V. Chapman, A. Diaz, A.H. Dickenson, Distinct inhibitory effects of spinal endomorphin-1 and endokorphin-2 on evoked dorsal horn
ORL1 receptor when 1.0 mg OFQ is administered. We
neuronal responses in the rat, Br. J. Pharmacol. 122 (1997) 1527–
know that the activities ofm receptors can inhibit C-fibre
1539.
evoked responses of dorsal horn neurons [2]. There was no [3] H.O. Handwerker, A. Iggo, M. Zimmerman, Segmental and sup-clear influence of microinjection of OFQ into PAG on raspinal actions on dorsal horn neurons responding to noxious and
[4] S.L. Jones, G.F. Gebhart, Spinal pathways mediating tonic, [11] C.W. Vaughan, S.L. Ingram, M.J. Christie, Actions of the ORL1 coeruleospinal, and raphe-spinal descending inhibition in the rat, J. receptor ligand nociceptin on membrane properties of rat Neurophysiol. 58 (1987) 138–159. periaqueductal gray neurons in vitro, J. Neurosci. 17 (1997) 996– [5] J.C. Meunier, C. Mollereau, L. Toll, C. Suaudeau, C. Moisand, P. 1003.
Alvinerie, J.L. Butour, J.C. Guillemot, P. Ferrara, B. Monsarrat, H. [12] H. Wang, C.B. Zhu, X.D. Cao, G.C. Wu, Effect of orphanin FQ on Mazargull, G. Vassart, M. Parmentier, J. Costentin, Isolation and acupuncture analgesia and noxious stimulation in the periaqueductal structure of the endogenous agonist of opioid receptor-like ORL1 gray, Acta Physiol. Sin. 50 (1998) 263–267.
receptor, Nature 377 (1995) 532–535. [13] T. Yamamoto, N. Nozaki-Taguchi, S. Kimura, Analgesia effect of [6] M.M. Morgan, J.E. Grisel, C.S. Robbins, D.K. Grandy, Antinocicep- intrathecally administered nociceptin, an opioid receptor-like re-tion mediated by the periaqueductal gray is attenuated by orphanin ceptor agonist, in the rat formalin test, Neuroscience 81 (1997) FQ, Neuroreport 8 (1997) 3431–3434. 249–254.
[7] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, [14] H.L. Yang, R.S. Luo, L.X. Wamg, D.X. Zhu, C.W. Chi, Primary 2nd Edition, Academic Press, Sydney, 1986. structure and disulfide bridge location of arrowhead double-headed [8] R.K. Reinscheid, H.P. Nothacker, A. Bourson, A. Ardati, R.A. proteinase inhibitors, J. Biochem. 11 (1992) 537–545.
Henningsen, J.R. Bunzow, D.K. Grandy, H. Langen, F.J. Monsma, [15] Y.Q. Zhang, J.S. Tang, B. Yuan, Inhibitory effects of electrical O. Civelli, Orphanin FQ: a neuropeptide that activates an opioidlike stimulation of thalamic nucleus submediums on the nociceptive G protein-coupled receptor, Science 270 (1995) 792–794. responses of spinal dorsal horn neurons in the rat, Brain Res. 737 [9] G.C. Rossi, L. Leventhal, E. Bolan, G.K. Pasternak, Pharmaco- (1996) 16–24.
logical characterization of orphanin FQ / nociceptin and its frag- [16] M. Zimmermann, Ethical guidelines for investigations of experimen-ments, J. Pharmacol. Exp. Ther. 282 (1997) 858–865. tal pain in conscious animals, Pain 16 (1983) 109–110.