www.elsevier.com / locate / bres
Research report
Decreased expression of nitric oxide synthase in the colonic myenteric
plexus of aged rats
a ,
*
b a a,bToku Takahashi
, Ammar Qoubaitary , Chung Owyang , John W. Wiley
a
Department of Internal Medicine, The University of Michigan Medical Center, 6520 MSRB I, Box 0682, Ann Arbor, MI 48109, USA
b
VA Medical Centers, Ann Arbor, MI 48109, USA Accepted 15 August 2000
Abstract
Nitric oxide (NO) is a major non-adrenergic, non-cholinergic (NANC) inhibitory neurotransmitter in the gastrointestinal tract. NO released from the myenteric plexus enhances colonic transit and facilitates propulsion of the colonic contents by mediating descending relaxation. Although it has been suggested that colonic transit delays with aging, the mechanism of delayed colonic transit in aging remains unclear. We hypothesized that advanced age is associated with decreased expression of neuronal NO synthase (nNOS) and concomitant reduction in synthesis of NO in the rat colon. We studied nNOS mRNA expression, nNOS-immunohistochemistry, nNOS-immunoblotting and NOS catalytic activity in the mid-colon obtained from young (age 4–8 months) and aged (age 22–28 months) Fisher (F3443BN)F1 rats. Western blot analysis of PGP 9.5, a generic neuronal marker, of the colonic tissues were employed to study whether the total number of neurons of the myenteric plexus is reduced with aging. The number of nNOS-immunoreactive cells and nNOS synthesis in the colonic myenteric plexus were significantly reduced in aged rats. In contrast, expression of PGP 9.5 in colonic tissues was not affected in aged rats. Northern blot analysis demonstrated that the expression of neuronal nNOS mRNA was significantly
3
reduced in the colonic tissues in aged rats. Basal and veratridine-induced release ofL-[ H]citrulline were significantly decreased in colonic
tissues from aged rats, compared to young rats. It is suggested that advanced age is associated with diminished gene expression of nNOS, nNOS synthesis and catalytic activity of NOS. This may explain the mechanism of delayed colonic transit observed in advanced age. 2000 Elsevier Science B.V. All rights reserved.
Theme: Development and regeneration
Topic: Aging process
Keywords: Colonic transit; Fisher (F3443BN)F1 rats; Immunohistochemistry; Western blot; Northern blot
1. Introduction important role in mediating descending relaxation in the rat colon, an essential component of the peristaltic reflex
Nitric oxide (NO) is a major non-adrenergic, non- [13,14].
cholinergic (NANC) inhibitory neurotransmitter candidate In general, gastrointestinal function is relatively well
in the gastrointestinal tract. NO is synthesized from L- preserved with aging [15]. However, colonic transit
ap-arginine by NO synthase (NOS). In the rat and human, pears to slow with aging [21]. Madsen has previously
NOS-immunoreactive neuronal cell bodies and fibers have demonstrated that older subjects (55–74 years) had slower
been detected throughout the entire gastrointestinal tract colonic transit of radiolabeled plastic particles than young
[1,4]. Stimulation of the myenteric plexus releases NO, subjects (21–27 years), while age did not affect gastric
which in turn produces relaxation of the smooth muscle in emptying or small intestinal transit [21]. The mechanism
the gastrointestinal tract [3,4,6,7]. NO appears to play an of delayed colonic transit in aging remains unclear.
Previous studies on aging in animal and human subjects suggest that advanced age is associated with a significant
*Corresponding author. Present address: Department of Surgery,
decline in the number of neurons in the myenteric plexus
DUMC Box 3479, Duke University Medical Center, Durham, NC 27710,
that is most apparent in the colon [11,12,29]. In contrast,
USA. Tel.:11-919-286-0411 (Ext. 6542); fax:11-919-286-1140.
E-mail address: [email protected] (T. Takahashi). Johnson et al. observed no significant loss of neurons in
the small intestine of aged rats, compared to young rats months old, n524) (BN3F344) F1 rats were used in this
using the neuronal marker, PGP 9.5 [18]. In these latter study.
studies, neuronal counts that were corrected for age-associ-ated changes in intestinal length and circumference [18].
2.3. Catalytic activity of NOS A modest decrease in the contribution of nitrergic
innervation to NANC-mediated relaxation was observed
L-Citrulline and NO are produced in a 1:1 ratio from
with advanced age in the rat intestine [30,33]. Few studies
L-arginine by the action of NOS. Production of NO was
have examined the expression of NO synthase (NOS) with 3
measured in colonic tissue preloaded with L-[ H]arginine
advancing age. The number of NADPH diaphorase (a 3
and expressed as amount ofL-[ H]citrulline formed in the
histochemical marker for NOS containing
neurons)-posi-tissue as described by Bredt and Snyder [5]. As previously tive neurons in the small intestine myenteric plexus of rats
described [16], the longitudinal muscles with adherent decreased modestly with age [28]. In contrast, Belai et al.
myenteric plexus (LMMP) preparations were obtained observed a significant increase in NADPH
diaphorase-from the mid-colon in young and aged rats and incubated positive neurons with age in the myenteric plexus of the
in a 1.5 ml organ bath for 30 min at 378C in the presence
proximal colon, but not the ileum of the rat intestine [2].
of cofactors (1 mM NADPH, 10 mM FAD, 10 mM FMN,
The discrepancies in these observations might be explained
and 10 mM tetrahydrobiopterin). LMMP preparations were
by the possibility that NADPH diaphorase is a non-specific 3
further incubated with [ H]arginine (3mCi / ml) for 4 min
marker for NOS containing neurons [35].
at 378C. Immediately following the stimulation of
ver-Therefore, based on available literature it is not clear
atridine (5 mM) for 5 min, the reaction was stopped by
whether advanced age is associated with a decrease in the
flash freezing in liquid nitrogen. The samples were stored
total number of myenteric neurons or preferentially in- 3
at2808C for subsequent measurement of L-[ H]citrulline.
volves a reduction in a subpopulation of myenteric
neu-Muscle tissues were homogenized with a 1-ml straight wall rons. The goals of this study were 2-fold; (1) to examine
grinder. After centrifugation at 3000 rpm for 10 min at whether advanced age is associated with a loss of
myen-48C, the supernatant in 10% TCA was sonicated for 5 min.
teric neurons in the rat colon using the neuronal marker,
After washing with water-saturated ethyl ether, the super-PGP 9.5, and (2) to examine whether advanced age is
natant was applied to a Dowex AG50WX-8 resin column
associated with decreased expression of NOS in the rat 1 3
(Na form) and L-[ H]citrulline was eluted with Hepes
colon.
buffer (pH 5.5) and water, as previously reported [16].
3
L-[ H]Citrulline in the effluent was measured by liquid
3
scintillation spectroscopy, and the production ofL-[
H]cit-2. Materials and methods
rulline in the colonic tissue was expressed as cpm / mg tissue. The protein in the pellet was measured by the 2.1. Materials
Bio-Rad method using BSA.
Aprotinin, diaminobenzidine, NADPH, flavin adenine
dinucleotide (FAD), flavin mononucleotide (FMN), 2.4. NOS immunohistochemistry
leupeptin, Triton X-100, and PMSF were obtained from
Sigma (St. Louis, MO). Tetrahydrobiopterin was obtained LMMP preparations were obtained from the mid-colon.
3
from ICN (Costa Mesa, CA).L-[ H]Arginine was obtained LMMP preparations were pinned and stretched on a
from New England Nuclear (Boston, MA). Dowex silicone-coated dish and fixed overnight in 4%
paraformal-AG50W-X8 was obtained from Bio-Rad Laboratories dehyde. LMMP preparations were washed and rinsed in
(Hercules, CA). Antibody to constitutive nNOS was 0.15 M PBS, and blocked in 0.15 M PBS that contained
obtained from Santa Cruz (Santa Cruz, CA). Antibody to 5% normal goat serum, 0.1% BSA, and 0.1% Tween 20
PGP 9.5 was obtained from UltraClone (Isle of Wight, for 30 min at room temperature. LMMP preparations were
UK). Anti-rabbit IgG and the avidin–biotin labeled kit then incubated with a polyclonal antibody raised in rabbits
(Vectastain ABC kit) were obtained from Vector Lab- against the purified soluble NOS extract from rat
cere-32
oratories (Burlingame, CA). [ P]dCTP and random primer bellum at a dilution of 1:1000 in 0.15 M PBS for 18 h at
labeling system (Rediprime) were obtained from Amer- 48C. After washing in 0.15 M PBS, LMMP preparations
sham (Arlington Heights, IL). TRIzol was obtained from were incubated with biotinylated anti-rabbit IgG for 1 h at
Life Technologies (Gaithersburg, MD). AatII and dithio- room temperature. Horseradish peroxidase staining was
threitol were obtained from Boehringer Mannheim (In- done using the Vectastain ABC kit. Diaminobenzidine and
dianapolis, IN). nickel chloride were used as chromogens.
The number of nNOS–positive cells in 50 ganglia were
2.2. Animals counted in each preparation and the average number of
nNOS–positive cells in one ganglion were determined in
2.5. Western blot analysis of NOS synthesis labeled probes for 16 hrs at 658C. The samples were
washed four times: twice in 23SSC, 0.1% SDS for 5 min
LMMP preparations were obtained from the mid-colon at 658C, and twice in 0.23 SSC, 0.1% SDS for 5 min at
in young and aged rats. Soluble homogenates of these 658C. The samples were autoradiographed with
intensify-samples were prepared in lysis buffer containing 25 mM ing screens at 2808C. Radioactivity of the tissues was
Tris–HCl (pH 7.4) with EGTA (1 mM), dithiothreitol (1 measured by an imaging analyzer. To confirm equivalent
mM), leupeptin (10mg / ml), aprotinin (10mg / ml), PMSF loading of RNA in the various lanes, the membrane was
(1 mM), and Triton X (0.1%). Western blot analysis of washed and rehybridized with a probe against
LMMP preparations was performed, as previously de- glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
scribed [31,32]. Equal amounts of protein (20mg) in each
sample were separated by SDS–PAGE (7.5%, w / w, gel)
and transferred to a nitrocellulose membrane. All pro- 2.8. Statistical analysis
cedures were done in Tris buffer (40 mM, pH 7.55)
containing 0.3 M NaCl and 0.3% Tween 20. The mem- Data were expressed as means6S.E. Statistical analysis
brane was blocked with dried milk (6%, w / v) and incu- was performed using the Student’s t-test or paired t-test.
bated with specific polyclonal nNOS antibody (1:1000 Significance was accepted at the 5% level.
dilution) and a horseradish peroxidase-conjugate of affinity column-purified goat antibody to rabbit IgG. The immune
complexes were detected on photographic film by H O /2 2
luminol chemiluminescence and the bands were measured 3. Results
by an imaging analyzer, as previously described [23,32].
3.1. NOS catalytic activity in mid-colon preparations
2.6. Western blot analysis of PGP 9.5 from young and aged rats
3
It has been demonstrated that antisera raised against Basal L-[ H]citrulline formation was 61506925 cpm /
neuronal cytosolic protein, protein gene product (PGP) 9.5, mg protein of the colonic tissues obtained from young rats.
3
provides excellent staining of the generic neurons in the Veratridine (5mM) significantly increased L-[ H]citrulline
CNS and the myenteric plexus [8]. As previously de- formation to 10 51062590 cpm / mg protein of the colonic
scribed [31,32], possible quantitative changes of the tissues obtained from young rats (Fig. 1; n57, P,0.05, by
generic neurons in the colonic LMMP preparations in aged Student’s t-test).
3
rats was studied by Western blot analysis of PGP 9.5. BasalL-[ H]citrulline release was decreased by 5866%,
Equal amounts of protein (20 mg) in each sample were and veratridine-induced release was reduced by 78612%
separated by SDS–PAGE (15%, w / w, gel) and transferred in preparations from aged animals compared to young
to a nitrocellulose membrane. After blocking with dried animals (Fig. 1; n57, P,0.05, by Student’s t-test).
3
milk (6%, w / v), the membrane was incubated with specific Veratridine-induced L-[ H]citrulline formation did not
in-polyclonal PGP 9.5 antibody (1:10 000 dilution) [8]. creased significantly in aged preparations (Fig. 1; n57,
P50.2). 2.7. Northern blot analysis of NOS mRNA expression
For Northern blot analysis of NOS mRNA, the total RNA was isolated from homogenized colonic LMMP preparations from young and aged rats, as previously described [31,32]. Extraction and preparation of RNA was performed using TRIzol reagent. The amount of RNA was estimated by measuring absorbance at 260 nm. Following
isolation, the total RNA samples (20 mg each) were
electrophoresed on an agarose gel and transferred onto a nylon membrane. As previously demonstrated by Huang et al. [17], we used 2947 base rat neuronal NOS cDNA probe that extends 2.1 kb in the 39direction beyond the first exon (244–3191) of the cloned cDNA. This probe was made from original cDNA (provided by Dr. Solomon H. Snyder)
cut by the restriction endonuclease, AatII. The cDNA 3
Fig. 1. Basal and veratridine-induced L-[ H]citrulline formation in
32
probe (2.9 kb) was labeled with [ P]dCTP (111 TBq / colonic tissues obtained from young and aged rats. Basal and veratridine
3
mmol) by the random primer labeling system. The samples (5mM)-stimulatedL-[ H]citrulline formation were significantly reduced
3.2. NOS immunohistochemistry of the colonic myenteric 3.5. Northern blot analysis of NOS mRNA of the colonic
plexus tissue
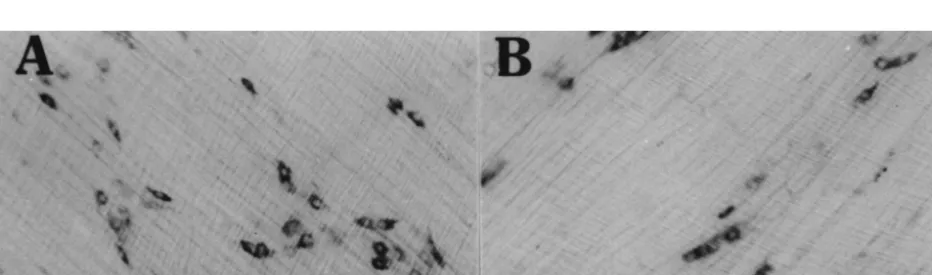
In the young rats, nNOS-immunoreactive neuronal cell We examined the reduced NOS synthesis in the colonic
bodies were found throughout the myenteric plexus of the myenteric plexus in aged rats to determine if it was caused
mid-colon. Non-neuronal tissues (e.g., muscle cells, endo- by down-regulation of nNOS mRNA expression. nNOS
thelium, mucosal cells, and macrophages) were unstained. mRNA expression (9.5 kb) was clearly observed in the
The average number of nNOS-immunoreactive cells of the colonic tissues from young rats. It has been demonstrated
mid-colon was 5.361.2 / ganglion in young rats (n54) that nNOS mRNA expression is abundant in the rat
(Fig. 2A). The average number of nNOS-immunoreactive cerebellum [4,17]. We have previously shown that nNOS
cells was significantly less in the mid-colon of the aged mRNA bands observed in the myenteric plexus
corres-rats (2.360.5 / ganglion, n54, P,0.01, by Student’s t-test) ponded well with the bands observed in the rat cerebellum
(Fig. 2B). [31]. The density of nNOS mRNA of colonic tissues
observed in aged rats was significantly reduced to
35615% of that observed in young rats (n53, P,0.05, by
3.3. Western blot analysis of NOS synthesis paired t-test) (Fig. 4).
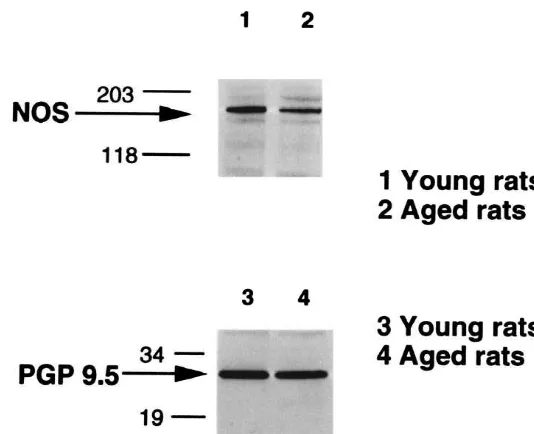
The molecular weight of neuronal NOS (nNOS) and
endothelial NOS (eNOS) has been shown to be 155 and 4. Discussion
130 kDa, respectively [10]. To investigate if changes
occurred in nNOS synthesis in the colonic myenteric We have demonstrated in the Fisher (344313 N)F1 rat
plexus in aged rats, we performed Western blot analysis model that advanced age is associated with decreased
using nNOS antibody. We have previously shown that the nNOS expression and decreased NOS catalytic activity in
nNOS-immunoreactive bands at 155 kDa observed in the the myenteric plexus from the mid-colon.
Semi-quantita-myenteric plexus corresponded well with the bands ob- tive Western blot analysis of PGP 9.5 revealed that
served in the rat cerebellum [31]. The density of NOS- expression of this neuron-specific marker was not
de-immunoreactive bands (155 kDa) obtained from the creased in aged rats. This suggests that advanced age was
colonic tissues in aged rats was significantly reduced to not associated with a significant reduction in the number of
54614% of that in young rats (Fig. 3, n54, P,0.05, by neurons in the rat colon myenteric plexus. Prior studies
paired t-test). examining age-associated changes in gastrointestinal
trans-it indicate that slowing occurs in the colon in humans [21]. Previous reports also suggest that the density of neurons in
3.4. Western blot analysis of PGP 9.5 the myenteric ganglia decreases along the longitudinal axis
of the gastrointestinal tract. The colon demonstrates
rela-In contrast to the nNOS-immunoreactive bands, the tively greater loss of myenteric neurons with advanced age
density of PGP 9.5-immunoreactive bands at 27 kDa of the compared to small intestine and stomach [12,29]. These
colonic tissues observed in the aged rats was not sig- observations lead us to focus our attention on the effects of
nificantly different from that observed in the young rats advanced age on myenteric neurons in the colon. We
(Fig. 3). examined several distinct measures of NOS expression and
Fig. 3. Western blot analysis of nNOS in the tissues obtained from the mid colon in young rats (lane 1) and aged rats (lane 2). The density of nNOS-immunoreactive bands at 155 kDa in the tissues obtained from the aged rats was significantly less, compared to that of young rats. Western blot analysis of PGP 9.5 in the tissues obtained from the mid colon of young rats (lane 3) and aged rats (lane 4). The density of PGP 9.5-immunoreactive bands at 27 kDa was not significantly different between the tissues obtained from the young and aged rats.
NOS catalytic activity because previous studies indicate ‘dilution’ effect was to perform semi-quantitative
immuno-blotting analysis using preparations of the mid-colon from that indirect assessment of NOS expression using NADPH
young and aged rats. diaphorase-positive neurons as a marker for NOS
con-Our results are in general agreement with Johnson et al. taining neurons can generate non-specific results [35].
[18] that the total number of PGP 9.5 immunoreactive The accurate quantification of age-associated changes in
neurons are preserved in advanced age. PGP 9.5 is a neuron numbers in the gastrointestinal tract is a
compli-well-established generic marker for enteric neurons [8,19], cated endeavor. For example, because of the large number
although it has been suggested that PGP 9.5 may fail to of myenteric neurons distributed along the longitudinal
stain up to 20% of myenteric neurons [8]. axis of the gut, it is realistically possible to survey only a
NOS can be classified into two types: constitutive and limited number of areas of the gut wall to estimate the
inducible. The constitutive form of NOS is calcium- and number of neurons. The intestines of the rat undergo
3
calmodulin-dependent. L-[ H]Citrulline formation was
age-associated increase in length and circumference [18].
21
A consequence of this increase in surface area is to completely abolished in Ca -free medium, which
sug-substantially decrease direct counts of neurons in enteric gests that the constitutive type of NOS was dominantly
3
preparations from aged animals compared to their young expressed in the colonic tissues. However,L-[ H]citrulline
counterparts. Our approach to address this age-associated formation does not distinguish between NO generated from
nNOS in the nerves and eNOS that is expressed in smooth nNOS synthesis and catalytic activity of NOS. These
muscle cells [34] and endothelium [10,22]. Therefore, we observations provide a rationale to examine the
physiologi-performed nNOS immunohistochemistry and Western blot cal consequences of decreased nNOS expression on
analysis in colonic tissues using a specific nNOS antibody colonic transit.
that has no cross-reactivity with eNOS [27]. Our results indicate that the number of neurons expressing nNOS in
the colonic myenteric plexus was significantly reduced in Acknowledgements
aged rats. The impaired nNOS synthesis in the colonic
myenteric plexus in aged rats was further demonstrated by This study was supported by VA Merit (JWW) and
Western blot analysis. The density of a 155-kDa NOS University of Michigan Geriatrics Center Awards (TT and
species, i.e., nNOS, was significantly reduced in the JWW). The authors wish to thank Dr. Solomon H. Snyder
colonic tissues from aged rats. We favor the interpretation (Johns Hopkins University School of Medicine, Baltimore,
that advanced age is associated with a significant reduction MD) for providing the NOS cDNA. The authors are also
in nNOS expression and not a loss of neurons based on our indebted to Marcus Campbell, Gary Blum, Luke Saski,
observation that the number of colonic myenteric neurons Chun Xiao Hsu, Kazumi Nakamura, Koji Nakao, and
does not decrease with advanced age. Jennifer Thomas for their technical assistance.
The impaired nNOS expression observed in the aged colonic myenteric plexus could result from transcriptional,
translational, or post-translational events. To examine if References
the gene transcription of nNOS in the colonic myenteric
plexus was impaired in aged rats, we performed Northern [1] Y. Aimi, H. Kimura, T. Kinoshita, Y. Minami, M. Fujimura, S.
Vincent, Histochemical localization of nitric oxide synthase in rat
blot analysis using cDNA specific for nNOS. The size of
enteric nervous system, Neuroscience 53 (1993) 553–560.
nNOS mRNA and eNOS mRNA have been demonstrated
[2] A. Belai, C. Cooper, G. Burnsrock, Effect of age on
NADPH-to be 9.5 and 4.5 kb, respectively [10]. We observed that diaphorase-containing myenteric neurones of rat ileum and proximal
nNOS mRNA expression at 9.5 kb was significantly colon, Cell Tissue Res. 279 (1995) 379–383.
reduced in the colonic tissues obtained from aged rats. This [3] G.E. Boeckxstaens, P.A. Pelckmans, J.J. Bogers, H. Bult, J.G. De Man, L. Oosterbosch, A.G. Herman, Y.M. Van Maercke, Release of
suggests that the impaired nNOS synthesis observed in the
nitric oxide upon stimulation of nonadrenergic noncholinergic
aged rat colon is in the result of impaired gene
transcrip-nerves in the rat gastric fundus, J. Pharmacol. Exp. Ther. 256 (1991)
tion of nNOS. We believe that this is the first demonstra- 441–447.
tion supporting impaired gene transcription of nNOS in the [4] D.S. Bredt, P.M. Hwang, S.H. Snyder, Localization of nitric oxide
colonic myenteric plexus of aged rats. We conclude that synthase indicating a neural role for nitric oxide, Nature 347 (1990)
768–770.
impaired nNOS expression observed in the preparations
[5] D.S. Bredt, S.H. Snyder, Nitric oxide mediates glutamate-linked
obtained from aged rats was responsible for the diminished
enhancement of cGMP levels in the cerebellum, Proc. Natl. Acad.
catalytic activity of the NO pathway in the rat colon. Sci. USA 86 (1989) 9030–9033.
The mechanism responsible for the impaired nNOS [6] H. Bult, G.E. Boeckxstaens, P.A. Pelckmans, F.H. Jordaens, Y.M.
mRNA expression in the colonic myenteric plexus of aged Van Maercke, A.G. Herman, Nitric oxide as an inhibitory
non-adrenergic non-cholinergic neurotransmitter, Nature 345 (1990)
rats remains to be investigated. We have previously shown
346–347.
that truncal vagotomy significantly reduced nNOS mRNA
[7] M. D’Amato, D. Curro, P. Montuschi, Evidence for dual components
expression in the gastric myenteric plexus in rats and that in the non-adrenergic non-cholinergic relaxation in the rat gastric
nicotinic receptor mediated-stimulation of nNOS mRNA fundus: role of endogenous nitric oxide and vasoactive intestinal
expression in cultured gastric myenteric plexus was sig- polypeptide, J. Auton. Nerv. Syst. 37 (1992) 175–186.
[8] E.Y. Eaker, J.E. Sallustio, The distribution of novel intermediate
nificantly reduced by blocking the protein kinase C (PKC)
filament proteins defines subpopulations of myenteric neurons in rat
pathway [24]. These observations suggest that vagal
intestine, Gastroenterology 107 (1994) 666–674.
21
cholinergic innervation acting through the Ca –PKC [9] M.M. Facchinetti, A.R. de Boland, Effect of ageing on the
expres-pathways may play an important role in regulating the sion of protein kinase C and its activation by 1,25(OH) -vitamin D2 3
transcription of nNOS mRNA in the gastric myenteric in rat skeletal muscle, Cell Signal 11 (1999) 39–44.
[10] U. Forstermann, J.S. Pollock, W.R. Tracey, M. Nakane, Isoforms of
plexus. It is noteworthy that advanced age is associated
nitric-oxide synthase: purification and regulation, Methods Enzymol.
with decreased release of acetylcholine in the rat colon
233 (1994) 258–264.
21
[26]. Moreover, abnormalities of the intracellular Ca – [11] G. Gabella, Fall in the number of myenteric neurons in aging guinea
PKC pathways have been reported in aged animals pigs, Gastroenterology 96 (1989) 1487–1493.
[9,20,25]. Therefore, it is possible that impaired choliner- [12] O.A. Gomes, R.R. de Souza, E.A. Liberti, A preliminary
inves-21 tigation of the effects of aging on the nerve cell number in the
gic tone and / or intracellular Ca –PKC pathways may
myenteric ganglia of the human colon, Gerontology 43 (1997)
contribute to the reduction of nNOS mRNA expression in
210–217.
the colonic myenteric plexus of aged rats. [13] J.R. Grider, Interplay of VIP and nitric oxide in regulation of the
Our present study demonstrates that advanced age is descending relaxation phase of peristalsis, Am. J. Physiol. 264
[14] J.R. Grider, K.S. Murthy, J.G. Jin, G.M. Makhlouf, Stimulation of [26] D. Roberts, D. Gelperin, J. Wiley, Evidence for age-associated nitric oxide from muscle cells by VIP: prejunctional enhancement of reduction in acetylcholine release and smooth muscle response in VIP release, Am. J. Physiol. 262 (1992) G774–G778. the rat colon, Am. J. Physiol. 267 (1994) G515–G522.
[15] K. Hall, J. Wiley, Age-associated changes in gastrointestinal func- [27] M.J. Saffrey, C.J. Hassall, C.H. Hoyle, A. Belai, J. Moss, H.H. tion, in: J.P. Hazzard W.R Blass, W.H. Ettinger, J.B. Hatter, J.P. Ous Schmidt, U. Forstermann, F. Murad, G. Burnstock, Colocalization of Pander (Eds.), Principles of Geriatric Medicine and Gerontology, 4th nitric oxide synthase and NADPH-diaphorase in cultured myenteric Edition, McGraw-Hill, New York, NY, 1999, pp. 835–842. neurones, Neuroreport 3 (1992) 333–336.
[16] K. Hosoda, T. Takahashi, M.A. Fujino, C. Owyang, Inhibitory [28] R. Santer, Survival of the population of NADPH-diaphorase stained effects of nitric oxide donors on nitric oxide synthesis in rat gastric myenteric neurons in the small intestine of aged rats, J. Auton. Nerv. myenteric plexus, J. Pharmacol. Exp. Ther. 286 (1998) 1222–1230. Syst. 49 (1994) 115–121.
[17] P.L. Huang, T.M. Dawson, D.S. Bredt, S.H. Snyder, M.C. Fishman, [29] R.M. Santer, D.M. Baker, Enteric neuron numbers and sizes in Targeted disruption of the neuronal nitric oxide synthase gene, Cell Auerbach’s plexus in the small and large intestine of adult and aged 75 (1993) 1273–1286. rats, J. Auton. Nerv. Syst. 25 (1988) 59–67.
[18] R.J. Johnson, M. Schemann, R.M. Santer, T. Cowen, The effects of [30] G.J. Smits, R.A. Lefebvre, Influence of age on cholinergic and age on the overall population and on sub-populations of myenteric inhibitory nonadrenergic noncholinergic responses in the rat ileum, neurons in the rat small intestine, J. Anat. 192 (1998) 479–488. Eur. J. Pharmacol. 303 (1996) 79–86.
[19] H.J. Krammer, S.T. Karahan, E. Rumpel, M. Klinger, W. Kuhnel, [31] T. Takahashi, K. Nakamura, H. Itoh, A.A. Sima, C. Owyang, Immunohistochemical visualization of the enteric nervous system Impaired expression of nitric oxide synthase in the gastric myenteric using antibodies against protein gene product (PGP) 9.5, Anat. Anz. plexus of spontaneously diabetic rats, Gastroenterology 113 (1997)
175 (1993) 321–325. 1535–1544.
[20] T. Kurumatani, J. Fastbom, W.L. Bonkale, N. Bogdanovic, B. [32] T. Takahashi, C. Owyang, Regional differences in the nitrergic Winblad, T.G. Ohm, R.F. Cowburn, Loss of inositol 1,4,5-tris- innervation between the proximal and the distal colon in rats, phosphate receptor sites and decreased PKC levels correlate with Gastroenterology 115 (1998) 1504–1512.
staging of Alzheimer’s disease neurofibrillary pathology, Brain Res. [33] T. Takeuchi, S. Niioka, M. Yamaji, Y. Okishio, T. Ishii, H. Nishio, 796 (1998) 209–221. K. Takatsuji, F. Hata, Decrease in participation of nitric oxide in [21] J.L. Madsen, Effects of gender, age, and body mass index on nonadrenergic, noncholinergic relaxation of rat intestine with age,
gastrointestinal transit times, Dig. Dis. Sci. 37 (1992) 1548–1553. Jpn. J. Pharmacol. 78 (1998) 293–302.
[22] M.A. Marletta, Nitric oxide synthase structure and mechanism, J. [34] B. Teng, K.S. Murthy, J.F. Kuemmerle, J.R. Grider, K. Sase, T. Biol. Chem. 268 (1993) 12231–12234. Michel, G.M. Makhlouf, Expression of endothelial nitric oxide [23] Y. Mizuta, T. Takahashi, C. Owyang, Nitrergic regulation of colonic synthase in human and rabbit gastrointestinal smooth muscle cells,
transit in rats, Am. J. Physiol. 277 (1999) G275–279. Am. J. Physiol. 275 (1998) G342–351.
[24] K. Nakamura, T. Takahashi, M. Taniuchi, C.X. Hsu, C. Owyang, [35] W. Tracey, M. Nakane, J. Pollock, U. Forstermann, Nitric oxide Nicotinic receptor mediates nitric oxide synthase expression in the synthases in neuronal cells, macrophages and endothelium are rat gastric myenteric plexus, J. Clin. Invest. 101 (1998) 1479–1489. NADPH diaphorases, but represent only a fraction of total cellular [25] J.J. Proust, C.R. Filburn, S.A. Harrison, M.A. Buchholz, A.A. NADPH diaphorase activity, Biochem. Biophys. Res. Commun. 195
Nordin, Age-related defect in signal transduction during lectin (1993) 1035–1040. activation of murine T lymphocytes, J. Immunol. 139 (1987) 1472–
![Fig. 1. Basal and veratridine-induced3in aged rats (*L-[ H]citrulline formation incolonic tissues obtained from young and aged rats](https://thumb-ap.123doks.com/thumbv2/123dok/3138958.1382690/3.612.312.548.533.683/basal-veratridine-induced-citrulline-formation-incolonic-tissues-obtained.webp)