Industrial Crops and Products 12 (2000) 53 – 56
Chemical transformation of 1,8-cineole:
synthesis of seudenone, an insect pheromone
Armando J.D. Silvestre *, Mo´nica Va´lega, Jose´ A.S. Cavaleiro
Department of Chemistry,Uni6ersity of A6eiro,3810A6eiro,Portugal
Accepted 2 December 1999
Abstract
A simple method to transform the easily available 1,8-cineole (1) into the more valuable compound seudenone, 3-methyl-2-cyclohexenone (4), a sex pheromone ofDendroctonus pseudotsugaeHopkins, is described. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Seudenone; 3-Methyl-2-cyclohexenone; Sex pheromone; 1,8-Cineole; Chemical transformation; Eucalyptus globulus www.elsevier.com/locate/indcrop
1. Introduction
Eucalyptus globulus Labill. occupies 18.3% of the Portuguese forest area; it is a very important source of raw materials for the pulp industries, which have a relevant impact in the economies of several countries, Portugal being one of them (CELPA, 1996). However, the economical value of the E. globulus trees can be increased if new applications are found for the essential oil-rich parts of the plant (essentially leaves and small branches), which are normally left behind on the fields by the pulp producers.
E. globulus essential oil is mainly composed of 1,8-cineole (1) (Silvestre et al., 1994, 1997a) but, despite its natural abundance, no major or
signifi-cant chemical transformations of 1 into valuable products are known (Silvestre and Cavaleiro, 1996).
Following our interest in the valorisation of E. globulus essential oil (Silvestre et al., 1994, 1997a) and in the chemical transformation of 1,8-cineole (1) (Cavaleiro et al., 1996; Silvestre et al., 1996, 1997b), we now report a simple way to prepare seudenone (4) in high yields, starting from easily available and cheap 1,8-cineole (1).
Seudenone (4), 3-methyl-2-cyclohexenone, is an insect pheromone, which has been isolated from the indguts of the male of Douglas-fir beetle (Dendroctonus pseudotsugae Hopkins), an insect well known as a very destructive pest for the Douglas-fir trees (Kinzer et al., 1971; Pitman and Vite, 1974). Several methods are reported for the synthesis of seudenone (4) starting from formalde-hyde and ethyl acetoacetate (Rabe and Pollock, 1912; Natelson and Gottfried, 1939; Robinson * Corresponding author. Tel.: +351-234-370711; fax: +
351-234-370084.
E-mail address:[email protected] (A.J.D. Silvestre)
A.J.D.Sil6estre et al./Industrial Crops and Products12 (2000) 53 – 56 54
and Evans, 1985), from d-ketocarboxylic acids and derivatives through Wittig reactions (Best-mann et al., 1985, 1993), and also from copper-catalysed addition of methyl manganese chloride to cyclohexanone (Cahiez and Alami, 1989). The method now reported uses a natural and abun-dant monoterpene, and it is highly efficient in its overall yield.
2. Materials and methods
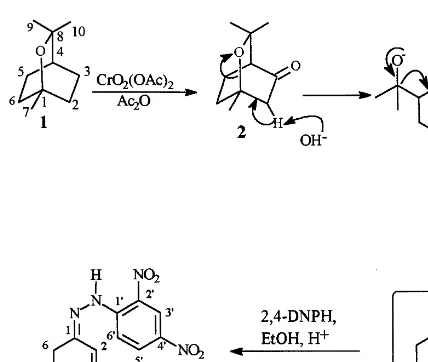
3-Keto-1,8-cineole (2) can be obtained by oxi-dation of 1 with hydrogen peroxide (Cavaleiro et al., 1996) or with chromyl acetate (Boggiato et al., 1987). In the present work, we prepared com-pound 2 according to Boggiato et al. with 65% yield.
Seudenone 4 was obtained according to the following procedure. 3-Keto-1,8-cineole (1.0 g) was added to a stirred solution of KOH (1.5 g) in ethanol (100 ml) under a nitrogen atmosphere, at room temperature. After 80 min, the mixture was poured into water, extracted with dichloro-methane and dried over anhydrous sodium sul-phate; after concentration on the rotary evaporator, the compound was further purified by thin-layer chromatography on silica gel, eluting with chloroform. After removing the solvent, seu-denone ) was obtained as a light-sensitive dark-yellow liquid (637 mg, 97% yield): boiling point, 199 – 200°C. Mass spectrometry/electron ionisa-tion (MS/EI):m/z(%), 110 (40), 82 (100), 67 (10), 54 (33), 39 (38).
The identification of compound 4 was further confirmed through its conversion into the corre-sponding 2,4-dinitrophenylhydrazone (5) by treat-ment of 4 with 2,4-dinitrophenylhydrazine in ethanol. The spectroscopic results obtained for the characterisations of hydrazone 5 are the fol-lowing: melting point, 177.5 – 178.5°C.1
H nuclear magnetic resonance (NMR): (300.13 MHz, CDCl3)d: 1.93 – 1.99 (m, 5H, H-5, 3-CH3), 2.23 (t,
The oxidation of 1,8-cineole (1) and the conver-sion of compound 2 into seudenone 4 were fol-lowed by gas chromatography-mass spectrometry (GC-MS) using a Hewlett Packard chro-matograph 5890 equipped with a mass selective detector MDS series 2 and using a DB-5 capillary column (30 m×0.25 mm i.d.; 0.25 mm film thick-ness). The chromatographic conditions were as follows: initial temperature, 100°C; temperature rate, 5°C min−1; final temperature, 200°C; injec-tor temperature, 200°C; detecinjec-tor temperature, 210°C.
3. Results and discussion
When the ketone 2, obtained as already men-tioned, was treated with KOH in ethanol, seude-none 4was obtained as the only reaction product in very high yields (97%). This transformation occurs through the formation under basic condi-tions of an anion in the position 2 of ketone 2; this anion can rearrange to form the alcoxide 3
which, under basic conditions, can easily undergo a retro-Aldol condensation reaction to form the ketone 4 in a selective way (Fig. 1).
Seudenone 4 was identified by GC-MS, com-paring its mass spectrum with literature data (Kinzer et al., 1971) and with the equipment mass spectral library. The structure of4 was also confi-rmed, after treatment with 2,4-dinitrophenylhy-drazine, through the structural characterisation of the corresponding 2,4-dinitrophenylhydrazone (5) by NMR and MS. The multiplet at d 1.93 – 1.99
liter-A.J.D.Sil6estre et al./Industrial Crops and Products12 (2000) 53 – 56 55
Fig. 1. Chemical synthesis of seudenone4from 1,8-cineole (1).
References
Bestmann, H.J., Schade, G., Lu¨tke, H., Mo¨nius, T., 1985. Synthese von cyclischen verbindungen aus triphenyl[(phenylimino)ethyliden]phosphoran und oxocar-bonsa¨uren — eine neue anellierungsmethodik. Chem. Ber. 118, 2640.
Bestmann, H.J., Pichl, R., Zimmermann, R., 1993. Synthese vona,b-ungesa¨ttigten cycloalkanonen aus bis[(1-acylalkyli-den)-triphenylphosphoranen] — eine methode zur u¨ber-fu¨hrung von carbonsa¨ure-anydriden in carbocyclische und heterocyclische verbindungen. Chem. Ber. 126, 725. Boggiato, M.V., Heluani, C.S., Fenik, I.J.S., Catala´n, C.A.N.,
1987. Regiospecific functionalization of the monoterpene ether 1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane (1,8-cine-ole). Synthesis of the useful bridgedg-lactone ether 1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-35-olide. J. Org.
Chem. 52, 1505.
Breitmeier, E., Voelter, W., 1990. Carbon-13 NMR Spec-troscopy. High Resolution Methods and Applications in Organic Chemistry and Biochemistry. VCH, New York. Cahiez, G., Alami, M., 1989. Organomanganese (II) reagents
XVI: copper-catalysed 1,4-addition of organomanganese chlorides to conjugated enones. Tetrahedron Lett. 30, 3541.
Cavaleiro, J.A.S., Nascimento, G.M.S.F., Neves, M.G.P.M.S., Pinto, M.T., Silvestre, A.J.D., Vicente, M.G.H., 1996. Oxidation of natural compounds using Mn(III) porphyrin complexes. Tetrahedon Lett. 37, 1893.
CELPA, 1996. Annual Statistics of the Portuguese Paper Industry. Associac¸a˜o da Indu´stria Papeleira, Lisbon. Kinzer, G.W., Fentiman, A.F., Foltz, R.Z., Rudinsky, J.A.,
1971. Bark beetle atractants: 3-methyl-2-cyclohexen-1-one isolated from Dendroctonus pseudotsugae. J. Econ. Ento-mol. 64, 970.
Natelson, S., Gottfried, S.P., 1939. Synthesis of derivatives of symmetrical diphenylethane related to materials occurring naturally. II 3,4-Dihydro-5-methyl-3%-methoxydibenzyl, a compound related to oestrone in structure. J. Am. Chem. Soc. 61, 1001.
Pitman, G.B., Vite, J.P., 1974. Biosynthesis of methylcyclo-hexenone by male douglas-fir beetle. Environ. Entomol. 3, 886.
Rabe, P., Pollock, E., 1912. U8der das vermeintliche auftreten von isomerie bei 3-methyl-cyclohexeh-(2)-on(1). Ber. 45, 2926.
Robinson, P.L., Evans, S.A., 1985. Reactions of di-ethoxyphenylphosphorane with diasteriomeric 3-methylcy-clohexane-1,2-diols. Control of regioselectivity by methyl substitution during cyclodehydration and rearrangement of 1,2-diols. J. Org. Chem. 50, 3860.
Silvestre, A.J.D., Cavaleiro, J.A.S., 1996. 1,8-Cineole: applica-tions, metabolism and chemical transformations. Rev. Farm. (Argentina) 2 – 3, 49 – 57.
Silvestre, A.J.D., Cavaleiro, J.A.S., Delmond, B., Filliatre, C., Bourgeois, G., 1994. The essential oils ofEucalyptus glob -ulusLabill. from Portugal. Flavour Fragr. J. 9, 51 – 53. ature data (Torri and Azzaro, 1974; Breitmeier
and Voelter, 1990).
4. Conclusions
This transformation can be considered as an alternative way to prepare the expensive seude-none 4, since 1,8-cineole (1) is an easily available and low-cost raw material, and, at the same time, this might represent an important contribution to the valorisation of essential oil-rich parts of E. globulus trees. This alternative can be even more promising considering that scaling up the process would not result in a yield loss (assays up to 5 g scale have been shown to be as successful as that described in Section 2); in addition, taking into account the high yield obtained and the boiling point of seudenone4(199 – 200°C), purification of the reaction mixture will be successfully attained by distillation.
Acknowledgements
A.J.D.Sil6estre et al./Industrial Crops and Products12 (2000) 53 – 56 56
Silvestre, A.J.D., Cavaleiro, J.A.S., Silva, A.M.S., Delmond, B., Filliatre, C., 1996. Regioselective synthesis of some derivatives of cineolic acid: confirmation by NMR and MS. Heterocycl. Commun. 2, 371.
Silvestre, A.J.D., Cavaleiro, J.A.S., Delmond, B., Filliatre, C., Bourgeois, G., 1997a. Analysis of the variation of the essential oil composition of Eucalyptus globulus Labill. from Portugal using multivariate statistical analysis. Ind. Crops Prod. 6, 27.
Silvestre, A.J.D., Cavaleiro, J.A.S., Silva, A.M.S., Delmond, B., Filliatre, C. 1997. Chemical transformation of 1,8-cineole. Synthesis of N-phenylimides from cineolic acid. J. Chem. Res. (S), 228; J. Chem. Res. (M), 1516.
Torri, J., Azzaro, M., 1974. Re´sonance magne´tique nucle´are du 13C. Effets des substituants me´thyle´s sur les
de´place-ments chimiques du13C dans une se´rie de
2-cyclohexe`ne-1-ones. Bull. Soc. Chim. Fr., 1633.