www.elsevier.com/locate/ibmb
Purification and properties of a
β
-glycosidase purified from midgut
cells of Spodoptera frugiperda (Lepidoptera) larvae
Sandro R. Marana, Walter R. Terra, Cle´lia Ferreira
*Departamento de Bioquı´mica, Instituto de Quı´mica, Universidade de Sa˜o Paulo, C.P. 26077, 05513-970 Sa˜o Paulo, Brazil
Received 13 December 1999; received in revised form 3 April 2000; accepted 4 April 2000
Abstract
Two β-glycosidases (BG) (Mr 47,000 and Mr 50,000) were purified from Spodoptera frugiperda (Lepidoptera: Noctuidae) midguts. These two polypeptides associate or dissociate depending on the medium ionic strength. The Mr 47,000 BG probably has two active sites. One of the putative active sites (cellobiase site) hydrolyses p-nitrophenylβ-d-glucoside (NPβGlu) (79% of the total
activity in saturated enzyme), cellobiose, amygdalin and probably also cellotriose, cellotetraose and cellopentaose. The cellobiase site has four subsites for glucose residue binding, as can be deduced from cellodextrin cleavage data. The enzymatic activity in this site is abolished after carbodiimide modification at pH 6.0. Since the inactivation is reduced in the presence of cellobiose, the results suggest the presence of a carboxylate as a catalytic group. The other active site of Mr 47,000 BG (galactosidase site) hydrolyses p-nitrophenylβ-d-galactoside (NPβGal) better than NPβGlu, cleaves glucosylceramide and lactose and is unable to act
on cellobiose, cellodextrins and amygdalin. This active site is not modified by carbodiimide at pH 6.0.
The Mr 47,000 BG N-terminal sequence has high identity to plantβ-glycosidases and to mammalian lactase–phlorizin hydrolase, and contains the QIEGA motif, characteristic of the family of glycosyl hydrolases. The putative physiological role of this enzyme is the digestion of glycolipids (galactosidase site) and di- and oligosaccharides (cellobiase site) derived from hemicelluloses, thus resembling mammalian lactase–phlorizin hydrolase.2000 Elsevier Science Ltd. All rights reserved.
Keywords: Intestinalβ-glycosidase; Substrate specificity; Toxic glycosides; Glycolipid digestion; Insectβ-glucosidase; Spodoptera frugiperda
1. Introduction
β-glycosidases (EC 3.2.1) are exoenzymes, removing monosaccharides from the non-reducing end of di-and/or oligosaccharides. Depending on the monosacch-aride that is removed, theβ-glycosidase is namedβ -glu-cosidase (glucose),β-galactosidase (galactose),β -xylosi-dase (xylose), and so on. Frequently the same β -glycosidase is able to hydrolyse several different monos-accharide residues from glycosides. In this case,β -gluco-sidase is used to name all enzymes, which remove glu-cose efficiently (Terra and Ferreira, 1994).
In insects, β-glycosidases play a role in terminal digestion of cellulose and hemicelluloses, in the cleavage of the carbohydrate moieties of glycoproteins (see Terra and Ferreira, 1994) and may also hydrolyse glycolipids,
* Corresponding author. Fax+55-11-38182186.
E-mail address: [email protected] (C. Ferreira).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 9 0 - 4
as proposed by Marana et al. (1995). However, the pre-cise function of many β-glycosidases is not clear, since some of them only hydrolyse synthetic substrates (Morgan, 1975; Marana et al., 1995).
The insect digestive tract has enzymes hydrolysing a variety ofβ-glycosides. Some insects have three or four digestive β-glycosidases with different substrate speci-ficity. In others we found only one of these enzymes, which is able to hydrolyse as many different β -glycos-ides as the other three or four enzymes together (Ferreira et al., 1998). Among the substrates of insectβ -glycosid-ases there are toxicβ-glycosides, which are produced by plants to avoid insect attack. Some insect species are able to feed on these plants without any harm, whereas others have their growth impaired. These differences in performance may be due to a detoxification mechanism acting after glycoside hydrolysis or may be based on differential β-glycosidase specificity (Ferreira et al., 1997).
even in insects from the same order. To understand the molecular basis and the physiological meaning of those differences, it is necessary to characteriseβ-glycosidases from different insects, describing their specificity, num-ber of active sites and amino acid residues related to catalysis and substrate binding.
In this work, one of the major midgutβ-glycosidases from Spodoptera frugiperda larvae was purified and shown to possess two active sites with different speci-ficities, resembling the mammalian dimeric lactase– phlorizin hydrolase.
2. Materials and methods
2.1. Animals
S. frugiperda (Lepidoptera: Noctuidae) were labora-tory reared according to Parra (1986). The larvae were individually contained in glass vials with a diet based on kidney bean (Phaseolus vulgaris), wheat germ, yeast and agar and were maintained under a natural photoreg-ime (summer, 14L:10D; winter, 10L:14D) at 25°C. Adults were fed a 10% honey solution. Fifth (last) instar larvae of both sexes were used in the experiments.
2.2. Enzyme samples
Larvae were immobilised by placing them on ice, after which they were rinsed in water and blotted with filter paper. Their guts were dissected in cold 125 mM NaCl, and the midgut tissue was pulled apart. Midgut tissue, after being rinsed thoroughly with saline, was homogen-ised in double distilled water, frozen-and-thawed three times and centrifuged at 25,000g for 30 min at 4°C. The resulting supernatant was stored at 220°C until use.
2.3. Gel filtration
Samples were applied to a Superose 12 HR 10/30 col-umn of a FPLC system (Pharmacia-LKB Biotechnology, Sweden) equilibrated and eluted with 20 mM triethanol-amine–HCl buffer pH 7.5, or with this buffer plus 200 mM NaCl. Fractions of 0.4 ml were collected at a flow rate of 0.4 ml/min. The active fractions were pooled and stored at 220°C until use.
2.4. Ion-exchange chromatography
An aliquot of the eluate from the Superose 12 column was applied to a column of Mono Q HR 5/5 (FPLC-system) equilibrated with 20 mM triethanolamine–HCl buffer pH 7.5. The proteins were eluted with 20 ml of a 300–500 mM NaCl gradient. The flow rate was 0.5 ml/min and 0.4 ml fractions were collected. Fractions
21–24 (Q1) and 31–35 (Q2) were pooled and stored at
220°C.
2.5. Hydrophobic chromatography
Sample Q1 or Q2, diluted in 50 mM phosphate buffer pH 7.0, containing 2 M ammonium sulphate, were applied to a column of Alkyl Superose HR 5/5 (FPLC system). The proteins were eluted with 20 ml of 2–0.4 M ammonium sulphate gradient in the same buffer. The flow rate was 0.5 ml/min and 0.4 ml fractions were col-lected. More active fractions from each chromatography were pooled, stored at 220°C and used as an enzyme source.
2.6. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE)
Samples containing approximately 4 µg protein were combined with sample buffer containing 60 mM Tris– HCl buffer pH 6.8, 2.5% (w/v) SDS, 0.36 mM β -mer-captoethanol, 0.5 mM EDTA, 10% (v/v) glycerol and 0.005% (w/v) bromophenol blue. The samples were heated for 5 min at 95°C in a water bath, before being loaded onto a 7.5% (w/v) polyacrylamide gel slab con-taining 0.1% SDS (Laemmli, 1970). The gels were run at a constant voltage of 200 V and stained for protein using a silver stain (Blum et al., 1987). Mr values were calculated according to Shapiro et al. (1967) using the following Mr standards: ovoalbumin (Mr 45,000), bov-ine serum albumin (Mr 66,000), phosphorylase b (Mr 97,400), β-galactosidase (Mr 116,250) and myosin (Mr 200,000).
2.7. Microsequencing of purifiedb-glycosidase
Samples with 100µg of S. frugiperda purifiedβ -gly-cosidase were concentrated in a vacuum desiccator (Heto Lab Equipment, Denmark) and then solubilised in 62.5 mM Tris–HCl buffer pH 6.75, containing 2% (w/v) SDS, 5% (v/v) β-mercaptoethanol, 10% (v/v) glycerol and 0.001% (v/v) bromophenol blue. After a pre-run (10 mA, 30 min), with 0.1 M sodium thioglycolate in the running buffer, the samples and pre-stained standards were loaded and electrophoretically resolved as described above. The resolved peptides in the gel were electroblot-ted onto PVDF (polyvinylidene difluoride) membranes according to Matsudaira (1987). The PVDF membranes were stained for proteins using 0.1% Coomassie Blue R-250 in a 50% (v/v) methanol solution, and destained with a 50% methanol solution. Dried PVDF membranes were the source of peptides for microsequencing.
2.8. Protein determination and hydrolase assays
Protein was determined according to Bradford (1976) using ovoalbumin as a standard.
β-glycosidase activity was determined by measuring the release of p-nitrophenolate (Terra et al., 1979) from NPβGlu (p-nitrophenyl-β-d-glucopiranoside;) and
NPβGal (p-nitrophenyl-β-d-galactopiranoside), reducing
groups (Noelting and Bernfeld, 1948) from laminarin and CMC (carboxymethyl cellulose) or glucose (Dahlqvist, 1968) from different alkylβ-glucosides, cel-lobiose, cellotriose, cellopentaose, gentiobiose, prunasin, amygdalin, phlorizin, lactose, laminaribiose and glucos-ylceramide. In the last case, the solubilization was achi-eved according to Dinur et al. (1984).
All substrates were assayed in 50 mM citrate–sodium phosphate pH 6.0 at 30°C under conditions such that activity was proportional to protein concentration and to time. Controls without enzyme or without substrate were included. One unit of enzyme (U) is defined as the amount that hydrolyses 1 µmol of substrate/min.
2.9. Chemical modification studies
Purifiedβ-glycosidase was incubated at 30°C with 6 mM EDC (1-ethyl-3-(3-dimethylaminopropyl carbodiimide), 40 mM glycine ethyl ester and 100 mM Temed(N,N,N9,N9-tetramethyl-ethylenediamine)/HCl buffer pH 5.2 or 6.0. When the substrate used to follow the inactivation was cellobiose, the incubation with EDC was done with or without 25 mM NPβGal or 14 mM cellobiose. When the substrate was NPβGal, the incu-bation with EDC was done with or without 14 mM cello-biose. Samples were collected at different periods of time and the reaction was stopped by a two-fold dilution with 400 mM citrate–sodium phosphate buffer pH 6.0. When the substrate used to follow the inactivation was cellobiose, after this initial dilution, samples were sub-mitted to two cycles of five-fold dilution followed by concentration in Microcon centrifuge filters YM-10 (Amicon). This procedure is necessary to avoid cello-biose hydrolysis inhibition by NPβGal present in the reaction media.
2.10. Kinetic studies
The effect of substrate concentration on purified β -glycosidase was determined using at least 10 different substrate concentrations. Km and Vm values (mean and SEM) were determined by linear regression using the software Enzfitter (Elsevier, Biosoft).
When the inhibition of the hydrolysis of one substrate (NPβGlu or NPβGal) by another substrate (NPβGlu, NPβGal or cellobiose) was studied, β-glycosidase was incubated with at least five different concentrations of substrate in each of at least five different concentrations
of the substrate used as inhibitor. In these studies, two samples were taken from each reaction medium at the end of incubation. In one sample, p-nitrophenolate was determined to calculate the amount of NPβGlu or NPβGal that was hydrolysed; in the other sample, glu-cose was measured according to Dahlqvist (1968), with the final addition of sulfuric acid to change p-nitrophen-olate into the colourless p-nitrophenol. In the medium with NPβGlu, after allowance for the amount of glucose originating from it, it is possible to calculate the activity on cellobiose or NPβGal. Ki values were determined from replots of slopes of Linewaver–Burk plots against inhibitor concentration (Segel, 1975), using the software Enzfitter (Elsevier, Biosoft).
3. Results
3.1. Purification of a Mr 47,000 b-glycosidase
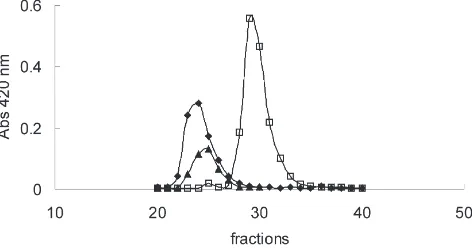
S. frugiperda β-glycosidase activity is almost restric-ted to midgut epithelium and probably locarestric-ted at the cell glycocalix (Ferreira et al., 1994). When a sample of sol-uble midgut cellular fraction was applied to a Superose 12 column at low and high ionic strength, a single β -glycosidase activity is recovered with Mr of 120,000 and 66,000, respectively. The reapplication of the high ionic strength eluate into a low ionic strength chromatography, gave one β-glycosidase activity peak, with a Mr of 125,000, indicating that there are two polypeptides that can associate one with the other, depending on the ionic strength (Fig. 1).
To purify the β-glycosidases, samples from soluble midgut cellular fraction were applied to a Superose col-umn in low and subsequently in high ionic strength. The eluted active fractions of the second run were pooled and then submitted to ion-exchange chromatography, resulting in two partially resolvedβ-glycosidases. Active
Fig. 2. Electrophoresis in SDS-7.5% polyacrylamide gel slab. Lane H, midgut soluble cellular fraction; S1, low and S2 high ionic strength Superose eluates; Q1 and Q2, fractions 21–24 and 31–35 eluted from Mono Q; A1 and A2, more active fractions pooled after elution from hydrophobic chromatography of Q1 and Q2 materials.
fractions of each peak were pooled, trying to avoid cross contamination. Two β-glycosidases were purified to homogeneity submitting these pools to hydrophobic chromatography.
The Mr of theβ-glycosidases obtained by SDS-PAGE are 47,000 and 50,000 (Fig. 2). The recovery and enrich-ment of β-glycosidase activities are shown in Table 1. Taking into account that there are two activities in the initial sample, the recovery of each β-glycosidase is higher than the figures presented in Table 1.
3.2. N-terminal amino acids
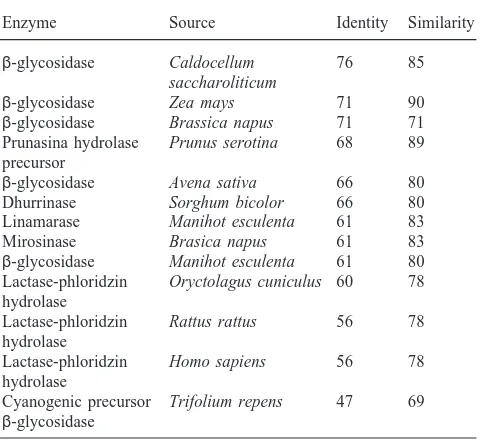
The N-terminal sequencing of the purified Mr 47,000 β-glycosidase resulted in the following sequence: YTKFPNGFTFGVATASHQIEGAWNxxK, where x denotes unidentified amino acid residues. The presence of the motif QIEGA near the amino terminal is a charac-teristic of the family of the glycosyl hydrolases (Rojas et al., 1995).
Table 1
Purification ofβ-glycosidases from S. frugiperda larval midgut
Fraction Specific Yield (%) Purification
activity factor
(mU/mg)
Cellular supernatant 14.6 100 1 Superose (low ionic 47.4 85 3.2 strength) eluate
Superose (high ionic 172 57 11.8 strength) eluate
Mono Q eluate Q1 1441 19 98.7
Mono Q eluate Q2 4500 36 308
Alkyl Superose eluate A1 1041 12 71 Alkyl Superose eluate A2 5770 30 395
The N-terminal sequence of S. frugiperdaβ -glycosid-ase has highest identity with plantβ-glycosidases (Table 2). High identity is also seen with mammalian lactase– phlorizin hydrolase, which is located in the intestinal epithelium and is responsible for lactose and glycosyl-ceramide digestion.
3.3. Specificity of purified Mr 47,000 b-glycosidase
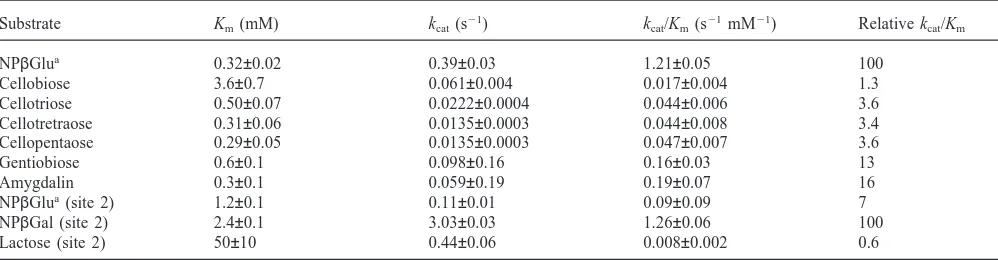
The Mr 47,000β-glycosidase has a broad specificity, hydrolysing aryl-β-glycosides (NPβGlu and NPβGal), di- (cellobiose, gentiobiose and lactose) and oligo-saccharides (cellotriose, cellotetraose and cellopentaose) and the cyanogenic glucoside amygdalin (Table 3). The enzyme is also able to hydrolyse glucosylceramides (0.3 mU/mg). As far as we know, this is the first time that the hydrolysis of glycolipids by an insectβ-glycosidase is described.
The enzyme is unable to hydrolyse the alkylβ -gluco-sides pentyl-, octyl- and decyl-β-d-glucosides (2 mM),
the disaccharide laminariobiose (7 mM), the polysac-charides CMC (0.25% p/v) and laminarin (0.25% p/v), and the plant glucoside phlorizin (1 mM). Prunasin, the cyanogenic glucoside that arises after removing one glu-cosyl residue from amygdalin is poorly hydrolysed by this enzyme, with a Km of more than 50 mM.
Cellobiose is an enzyme substrate but is unable to inhibit NPβGal hydrolysis (Table 4). The simplest expla-nation for this result is that cellobiose and NPβGal are hydrolysed at different sites in theβ-glycosidase (called
Table 2
Percentage of identity and similarity of the N-terminal sequence from
S. frugiperdaβ-glycosidase in relation to otherβ-glycosidasesa
Enzyme Source Identity Similarity
β-glycosidase Caldocellum 76 85
saccharoliticum
β-glycosidase Zea mays 71 90
β-glycosidase Brassica napus 71 71 Prunasina hydrolase Prunus serotina 68 89 precursor
β-glycosidase Avena sativa 66 80 Dhurrinase Sorghum bicolor 66 80 Linamarase Manihot esculenta 61 83
Mirosinase Brasica napus 61 83
β-glycosidase Manihot esculenta 61 80 Lactase-phloridzin Oryctolagus cuniculus 60 78 hydrolase
Lactase-phloridzin Rattus rattus 56 78 hydrolase
Lactase-phloridzin Homo sapiens 56 78 hydrolase
Cyanogenic precursor Trifolium repens 47 69
β-glycosidase
a The amino acid sequence from Mr 47,000β-glycosidase was
Table 3
Substrate specificities of purified Mr 47,000β-glycosidase. Data correspond to site 1 (cellobiase site), except when otherwise specified (site 2, glycosylceramidase site)
Substrate Km(mM) kcat(s21) kcat/Km(s21mM21) Relative kcat/Km
NPβGlua 0.32±0.02 0.39±0.03 1.21±0.05 100
Cellobiose 3.6±0.7 0.061±0.004 0.017±0.004 1.3
Cellotriose 0.50±0.07 0.0222±0.0004 0.044±0.006 3.6
Cellotretraose 0.31±0.06 0.0135±0.0003 0.044±0.008 3.4
Cellopentaose 0.29±0.05 0.0135±0.0003 0.047±0.007 3.6
Gentiobiose 0.6±0.1 0.098±0.16 0.16±0.03 13
Amygdalin 0.3±0.1 0.059±0.19 0.19±0.07 16
NPβGlua(site 2) 1.2±0.1 0.11±0.01 0.09±0.09 7
NPβGal (site 2) 2.4±0.1 3.03±0.03 1.26±0.06 100
Lactose (site 2) 50±10 0.44±0.06 0.008±0.002 0.6
aK
mand Vmfor NPβGlu were calculated from data obtained before and after inactivation of the cellobiase site (details in Section 2). Activity
on lactose is assigned to site 2 based on EDC modification (see text). Figures are means and SEM.
Table 4
Inhibition of Mr 47,000β-glycosidasea
Substrate Inhibitor Ki(mM)
Cellobiose NPβGal 3.4±0.3
NPβGal NPβGlu 1.0±0.2
NPβGal Cellobiose N.I.
NPβGlu NPβGal 6.6±0.3
NPβGlu Cellobiose 1.2±0.1
aSubstrates acting as inhibitors were simple linear competitive
inhibitors. N.I., no inhibition. Figures are means and SEM. Details in Section 2.
cellobiase and galactosidase sites in this study). NPβGlu could be hydrolysed by both active sites, since neither the Ki of NPβGal nor the Ki of cellobiose are equal to their corresponding Km values when NPβGlu is used as substrate (compare Tables 3 and 4). Amygdalin is hydro-lysed only at the cellobiase site, since even a concen-tration of amygdalin as high as 15-fold its Kmwas unable to inhibit the hydrolysis of NPβGal at a concentration equal to one Km.
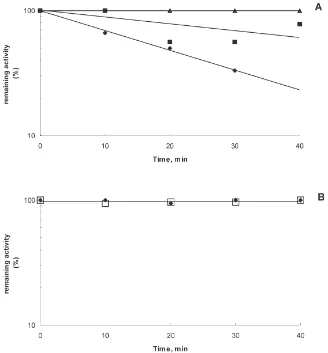
The presence of two different active sites in Mr 47,000 β-glycosidase is also indicated by chemical modification experiments. EDC modifies carboxylates that have been demonstrated to be catalytic groups in many glycosidases (see White and Rose, 1997). The β -glycosidase inactivation by EDC plus glycine ethyl ester follows pseudo first-order kinetics. When the modifi-cation reaction is done at pH 6.0, the activity upon cello-biose decreases, while the activity upon NPβGal remains constant. The cellobiase activity inactivation is protected by the presence of 14 mM cellobiose or 12 mM NPβGal in the reaction media (Fig. 3). The results given above indicate that NPβGal can bind but is not hydrolysed at the cellobiose site.
The activity towards lactose is not affected by EDC modification at pH 6 (not shown). This indicates that
this substrate is hydrolysed at the same site as NPβGal (galactosidase site).
NPβGlu is hydrolysed mainly at the cellobiase site, although the galactoside site also displays some activity on this substrate. The Km and kcatfor NPβGlu were cal-culated in both active sites as followed: after 100% inac-tivation of cellobiose activity by EDC at pH 6.0, the Km and kcat for NPβGlu hydrolysis are, 1.2 mM and 0.11 s21, respectively. Taking into account the activity upon NPβGlu before and after complete modification of the cellobiase site, the kinetic parameters of the cellobiase site in relation to this substrate were estimated as: Km=0.32 mM, kcat=0.39 s21.
4. Discussion
4.1. Specificity of the active sites of Mr 47,000 b-glycosidase
Twoβ-glycosidases were purified from S. frugiperda midguts. These enzymes associate or dissociate depending on the medium ionic strength. The character-isation of the Mr 50,000β-glycosidase is described else-where (Marana et al., 2000). Here only the properties of the Mr 47,000 β-glycosidase will be discussed.
Fig. 3. Inactivation ofβ-glycosidase in 6 mM EDC plus 40 mM glycine ethyl ester (GEE) and 100 mM Temed/HCl buffer pH 6.0. (A) Activity on 14 mM cellobiose, after modification in the presence of EDC-GEE (r) or EDC-GEE plus 14 mM cellobiose (G) or EDC-GEE plus 25 mM NPβGal (j). (B) Activity on 25 mM NPβGal after modification with EDC-GEE (r) or EDC-GEE plus 14 mM cellobiose (h).
When cellobiose is used as an inhibitor of NPβGlu hydrolysis it presents a Ki value different from its Km. This may be explained by the NPβGlu hydrolysis in both active sites of the Mr 47,000β-glycosidase. The finding that activity on NPβGlu remains after 100% inactivation of cellobiase activity by EDC at pH 6.0, shows that the galactosidase active site also has some activity on NPβGlu.
The β-glycosidase active sites may have several monossaccharide residue-binding subsites. The subsite that binds the residue present at the non-reducing end is called21. The other subsites are sequentially called+1, +2, +3, etc. The glycosidic linkage to be broken is the one between the 21 and +1 subsites (Davies et al., 1997).
The Mr 47,000 β-glycosidase cellobiase site accepts only substrates having glucose residues linked by β-1,4 or β-1,6 glucosidic bonds at the 21 and +1 subsites to account for the hydrolysis of cellobiose (glucose β -1,4-glucose), gentiobiose (glucose β-1,6-glucose), and amygdalin (glucoseβ-1,6-glucoseβ-mandelonitrile) and the lack of activity towards NPβGal, lactose (galactose
β-1,4-glucose) and laminaribiose (glucose β -1,3-glucose). It is noteworthing thatβ-1,6 bonds are prefer-entially hydrolysed to β-1,4 glucosidic bonds, whereas they are completely inactive against β-1,3 glucosidic bonds. However, it is not clear why the cellobiase site hydrolyses NPβGlu with such a great efficiency.
Kmvalues decrease from cellobiose up to cellotetraose and cellopentaose. This suggests that the cellobiase site has four subsites that bind glucose residues. The unchanged affinity due to the binding of the fifth glucose residue of cellopentaose and the low kcatobserved with cellotetraose favours the four-subsite hypothesis. Hydrolysis of the non-reducing end glucose residue from cellotetraose results in cellotriose, which is supposed to be the more tightly-bound product (all their residues would be bound) ofβ-glucosidase action. Tightly-bound products are expected to be released slowly from the enzyme, thus decreasing the turnover rate (kcat) of the enzyme.
Neverthe-less, NPβGal is hydrolysed 27 times faster than NPβGlu. Amongst the insect β-glycosidases, only that present in Erinnyis ello midgut has similar properties (Santos and Terra, 1985). Binding of glucose residues at the+1 subs-ite of the galactosidase ssubs-ite is virtually impossible, since it has a very large Km for lactose and its activity is not inhibited by cellobiose and amygdalin. Taking this into account, cellotriose, cellotetraose and cellopentaose are probably hydrolysed by the cellobiase site. In spite of the unfavourable binding, lactose seems to be hydrolysed only in the galactosidase site. Based on the specificity of this site, mainly the preference for hydrophobic moi-eties at the +1 subsite, it is possible that it hydrolyses galactolipids.
The mammalian lactase–phlorizin hydrolase has two active sites, located in two similar peptides (Mr 46,000 and 53,000) of the mature enzyme. One active site is responsible for most disaccharide hydrolysis (cellobiose, laminaribiose, and gentiobiose) and the other hydrolyses the plant glucoside phloridzin, lactose and glucosylcera-mides (Wacker et al., 1992; Freund et al., 1991; Neele et al., 1995).
In the S. frugiperda β-glycosidase studied in this paper, the two active sites are present in a protein that has Mr 47,000. A similar result is found in Abracris fla-volineata midgut β-glycosidase, which has two active sites (one hydrolysing cellobiose and laminaribiose and the other, aryl-β-glucosides) in a Mr 82,000 polypeptide (Marana et al., 1995). It will be interesting to compare the amino acid sequences from the two insectβ -glycos-idases and the mammalian enzyme to understand how the two active sites are placed in a single polypeptide.
4.2. Function of Mr 47,000 b-glycosidase
The function of the cellobiase site of S. frugiperda Mr 47,000 β-glycosidase is the hydrolysis of di- and oligo-saccharides derived from hemicellulose digestion. Hem-icellulose polymers have glucose residues bound by β -1,4,β-1,6, andβ-1,3 glycosidic bonds. Thus, except for the β-1,3 glycosidic bonds, the resulting hemicellulose oligosaccharides are susceptible to further digestion at the cellobiase site. Hemicellulose hydrolysis seems to be widespread among insects, since they have high hem-icellulose digestibility (Terra and Ferreira, 1994).
Gentiobiose (glucose β-1,6 glucose) and amygdalin (glucose β-1,6-glucose β-1-mandelonitrile) are hydro-lysed with the same efficiency (same kcat/Km), whereas prunasin (glucose β-1-mandelonitrile) is poorly hydro-lysed by the Mr 47,000β-glycosidase. These properties ensure the utilisation by the insect of one glucose present in amygdalin. On the other hand, the second glucose and the cyanogenic mandelonitrille are not set free by the enzyme. This result and the finding that the S. frugiperda Mr 50,000 β-glycosidase is almost unable to hydrolyse prunasin (Marana et al., 2000) could explain the fact that
S. frugiperda larvae are able to survive and grow nor-mally feeding on a diet that contains amygdalin (Ferreira et al., 1997).
As mentioned before, the galactosidase site of the S. frugiperda Mr 47,000β-glycosidase may have, as natu-ral substrate glycolipids, mainly galactolipids, as lactose is not found in the diet of a leaf feeder. Digalactosyldig-lycerides (major lipids in photosynthetic tissues; Har-wood, 1980) are hydrolysed by a S. frugiperda midgut α-galactosidase (Grossman and Terra, 2000) resulting in monogalactosyldiglyceride that could be hydrolysed by the Mr 47,000 β-glycosidase.
Acknowledgements
This work was supported by the Brazilian research agencies FAPESP and FINEP (PRONEX Program). We are much indebted to L.Y. Nakabayashi for technical assistance. S.R. Marana is a graduate fellow of FAPESP and C. Ferreira and W.R. Terra are staff members of the Biochemistry Department and research fellows from CNPq.
References
Blum, H., Beier, H., Gross, H.J., 1987. Improved silver staining of plant proteins. RNA and DNA in polyacrylamide gels. Electro-phoresis 8, 93–99.
Bradford, M.M., 1976. A rapid and sensitive method for the quantit-ation of microgram quantities of protein utilising the principle of protein-dye binding. Analyt. Biochem. 72, 248–254.
Dahlqvist, A., 1968. Assay of intestinal disaccharides. Analyt. Biochem. 22, 99–107.
Davies, G.J., Wilson, K.S., Henrisat, B., 1997. Nomenclature for sugar-binding subsites in glycosyl hydrolyses. Biochem. J. 321, 557–559.
Dinur, T., Grabowski, G., Desnick, R.J., Gatt, S., 1984. Synthesis of a fluorescent derivative of glucosilceramide for the sensitive deter-mination of glucocerebrosidase activity. Analyt. Biochem. 13, 223–374.
Ferreira, C., Capella, A.N., Sitnik, R., Terra, W.R., 1994. Digestive enzymes in midgut cells, endo- and ectoperitrophic contents and peritrophic membranes of Spodoptera frugiperda (Lepidoptera) lar-vae. Arch. Insect Biochem. Physiol. 26, 299–313.
Ferreira, C., Parra, J.R.P., Terra, W.R., 1997. The effect of dietary plant glycosides in larval midgutβ-glycosidases from Spodoptera
frugiperda and Diatraea saccharalis. Insect. Biochem. Molec. Biol.
27, 55–59.
Ferreira, C., Torres, B.B., Terra, W.R., 1998. Substrate specificities of midgutβ-glycosidases from insects of different orders. Comp. Biochem. Physiol. 119B, 219–225.
Freund, J.N., Gosse´, F., Raul, F., 1991. Derivatives of plant beta-glu-cans are hydrolysed by intestinal lactase–phlorizin hydrolyse of mammals. Enzyme 45, 71–74.
Grossman, G.A., Terra, W.R., 2000.α-galactosidases from the larval midgut of Tenebrio molitor (coleoptera) and spodoptera frugiperda (Lepidoptera) (submitted).
Comprehensive Treatise. Lipids: Structure and Function, vol. 4. Academic Press, New York, pp. 1–56.
Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685.
Marana, S.R., Jacobs-Lorena, M., Terra, W.R., Ferreira, C., 2000. Amino acid residues involved in substrate binding and catalysis in an insect digestiveβ-glycosidase (submitted).
Marana, S.R., Terra, W.R., Ferreira, C.F., 1995. Midgut β-d -gluco-sidases from Abracris flavolineata (Orthoptera: Acrididae). Physi-cal properties, substrate specificities, and function. Insect. Biochem. Molec. Biol. 25, 835–843.
Matsudaira, P., 1987. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J. Biol. Chem. 262, 10035–10038.
Morgan, M.R., 1975. Relationship between gut cellobiase, lactase, aryl
β-galactosidase activities of Locusta migratoria. Insect Biochem. 5, 609–617.
Neele, A.M., Einerhand, A.W.C., Dekker, J., Bu¨ller, H.A., Freund, J.N., Verhave, M., Grand, R.J., Montgomery, R.K., 1995. Verifi-cation of the lactase site of rat lactase–phlorizin hydrolyse by site-direct mutagenesis. Gastroenterology 109, 1234–1240.
Noelting, G., Bernfeld, P., 1948. Sur les enzymes amylolytiques III. La
β-amylase: dosage d’activite´ et controˆle de l’absence d’α-amylase. Helv. Chim. Acta. 31, 286–290.
Parra, J.R.P., 1986. Criac¸a˜o de insetos para estudos com pato´genos. In: Alves, S.B. (Ed.), Controle de Microbiano de Insetos. Editora Manole, Sa˜o Paulo, pp. 348–373.
Rojas, A., Arola, L.I., Romeu, A., 1995. β-glucosidase families revealed by computer analysis of protein sequences. Biochem. Molec. Biol. Int. 6, 1223–1231.
Santos, C.D., Terra, W.R., 1985. Physical properties, substrate speci-ficities and a probable mechanism for a β-d-glucosidase (cellobiase) from midgut cells of the cassava hornworm (Erinnyis
ello). Biochim. Biophys. Acta. 831, 179–185.
Segel, I.H., 1975. Enzyme Kinetics. Wiley, New York.
Shapiro, A.L., Vin˜uela, E., Maizel, J.V., 1967. Molecular weight esti-mation of polypeptide chains by electrophoresis in SDS-polyacryla-mide gels. Biochem. Biophys. Res. Commun. 28, 815–820. Terra, W.R., Ferreira, C., 1994. Insect digestive enzymes: properties,
compartmentalisation and function. Comp. Biochem. Physiol. 109B, 1–62.
Terra, W.R., Ferreira, C., de Bianchi, A.G., 1979. Distribution of digestive enzymes among the endo- and ectoperitrophic spaces and midgut cells of Rhynchosciara americana and its physiological sig-nificance. J. Insect Physiol. 25, 487–494.
Wacker, H., Keller, P., Salchetto, R., Legler, G., Semenza, G., 1992. Location of the two catalytic sites in intestinal lactase–phlorizin hydrolase. Comparison with sucrase–isomaltase and with other gly-cosidases, the membrane anchor of lactase–phlorizin hydrolase. J. Biol. Chem. 267, 18744–18752.