Attention-Deficit Disorder: Comparison with
Schizophrenia
Ann Olincy, Randal G. Ross, Josette G. Harris, David A. Young,
Mary Ann McAndrews, Ellen Cawthra, Kara A. McRae, Bernadette Sullivan,
Lawrence E. Adler, and Robert Freedman
Background:
Attention-deficit/hyperactivity
disorder
(ADHD) and schizophrenia are both conceptualized as
disorders of attention. Failure to inhibit the P50 auditory
event– evoked response, extensively studied in
schizophre-nia, could also occur in ADHD patients, if these two
illnesses
have
common
underlying
neurobiological
substrates.
Methods: This study examined the inhibition of the P50
auditory event– evoked potential in 16 unmedicated adults
with ADHD, 16 schizophrenic outpatients, and 16 normal
control subjects. Auditory stimuli were presented in a
paired stimulus, conditioning-testing paradigm.
Results: The amplitude of initial or conditioning P50
response did not differ between the three groups; however,
significant effects of psychiatric diagnosis on the
ampli-tude of the test response and the ratio of the test to the
conditioning response amplitudes were observed.
Schizo-phrenic patients’ P50 ratios and test amplitudes were
higher than both the ADHD and normal groups.
Conclusions: Adults with ADHD do not have the inhibitory
deficit seen in patients with schizophrenia, suggesting that
the mechanism of attentional disturbance in the two illnesses
may be fundamentally different. Biol Psychiatry 2000;47:
969 –977 © 2000 Society of Biological Psychiatry
Key Words: Event-related potentials, attention deficit
disorder, schizophrenia, sensory gating, nicotine, vigilance
Introduction
A
ttention-deficit/hyperactivity disorder (ADHD) is an
early onset disorder of inattention, hyperactivity, and
impulsivity. This disorder was once thought to be
primar-ily a childhood problem; however, approximately 60% of
adolescents with ADHD maintain this status into
adult-hood (Biederman et al 1996; Wender 1995).
Schizophre-nia has also been postulated to be a disorder of attention
(Bleuler 1911). Venables (1964) noted that schizophrenic
patients are unable to control their sensitivity to sensory
stimuli, so that their attention is involuntarily drawn to
many irrelevant elements in their environment, leaving
them unable to maintain concentration on relevant items.
Continuous performance tests (CPT) (Rosvold et al
1956) have been the most widely used measure to examine
vigilance in individuals with schizophrenia (Nuechterlein
1991; Nuechterlein et al 1994) as well as subjects with
ADHD (Koelega 1995). Subjects are instructed to respond
to a predefined target stimulus presented on a computer
monitor, usually a pair of identical stimuli presented in a
row. These tests may help to discern whether these two
illnesses have common underlying neurobiological
sub-strates. A number of response indices are usually recorded,
including the following: proportion of correct responses
(“hits”; where the person responds when the two identical
stimuli are present); number of failures to detect the target
stimulus (omission errors, i.e., missing hits); responses to
a nontarget stimulus (commission errors, i.e., “false
alarms”—responses made to a second stimulus in a “catch
trial,” in which the second stimulus presented was very
similar to the first stimulus, but not identical, or “random
errors”—responses to trials in which the first stimulus is
very different from the second stimulus and is thus filler);
response time for correct detections; the individual’s
ability to distinguish target (signal) from nontarget (noise)
stimuli (perceptual sensitivity, the normal deviate of the
false alarm rate minus the normal deviate of the hit rate, d9
5
z[false alarm]
2
z[hit rate]); and the amount of
perceptual evidence that the person requires to decide that
a stimulus is a target (response criterion, the ratio of the
ordinate of the hit rate to the ordinate of the false alarm
rate,
b 5
[y(hit rate)]/[y(false alarm rate]). Compared with
normal subjects, adults with ADHD were significantly
From the Departments of Psychiatry (AO, RGR, JGH, MAM, KAM, BS, LEA, RF) and Preventive Medicine and Biometrics (DAY), University of Colorado Health Sciences Center, and Denver Veterans Administration Medical Center (AO, RGR, EC, KAM, BS, LEA RF), Denver, Colorado.
Address reprint requests to Ann Olincy, M.D., M.P.H., University of Colorado Health Sciences Center, Department of Psychiatry, 4200 East 9th Avenue, C-268-71, Denver CO 80262.
Received June 21, 1999; revised November 19, 1999; accepted January 7, 2000.
more impaired on an auditory CPT (Seidman et al 1998).
Specifically, ADHD patients make more errors of
omis-sion (missing hits) and late responses (longer response
time to hits), with a slower reaction time to targets,
without increases in commission errors (Holdnack et al
1995; Seidman et al 1998). Patients with schizophrenia
have also consistently shown reduced performances
rela-tive to normal subjects on indices of sustained attention
(Goldstein 1986; Nuechterlein and Dawson 1984).
Schizo-phrenic patients have demonstrated impaired signal/noise
discrimination (sensitivity; they have less hits and more
random errors, and thus are unable to focus on the critical
information in their environment), without altered
re-sponse criterion levels (they do not have increased false
alarms, and thus have little difficulty screening out trials
that were similar but not identical to each other) in several
studies using auditory CPT (Cornblatt et al 1989;
Nuechterlein 1991). Although adult ADHD patients have
not been directly compared to adult schizophrenic patients
on this measure of vigilance, studies of children with
ADHD present contradictory results (Nuechterlein 1983;
O’Doughterty, et al 1984; van Leeuwen 1998). In some
studies, children with ADHD are reported to have a lower
response criterion without a lower perceptual sensitivity
(Nuechterlein 1983) whereas in other studies, children
with ADHD have both reduced response criterion and
perceptual sensitivity (O’Doughterty et al 1984). A
meta-analysis by Losier et al (1996) indicated that children with
ADHD made significantly more errors of commission
(false alarms) and omission (lack of responses to hits) than
normal children. Thus, it is possible that adult
schizo-phrenic patients and adult ADHD patients may be
distin-guished by their levels of impairment in measures of
perceptual sensitivity and response criteria. These
differ-ences in CPT performance may reflect underlying
neuro-biological differences in the two disorders. If
schizo-phrenic patients are unable to discriminate target stimuli
from nontarget noise stimuli, schizophrenia may be a
disorder of inhibition, with patients being flooded with too
many unimportant stimuli, such as the filler stimuli in the
CPT (Venables 1964). In contrast, if subjects with ADHD
need greater perceptual evidence to decide that a stimulus
is a target, and thus are able to distinguish hits from
random errors but not from false alarms, ADHD may be a
disease of understimulation, with patients displaying
at-tention problems if the task requires fine discrimination.
Event-related potentials, also considered measures of
attention (Coull 1998), appear to be abnormal in both
schizophrenic patients and subjects with ADHD. N100,
P300, and mismatch negativity amplitude reductions are
consistent findings in schizophrenic patients (Hirayasu et
al 1998; Javitt et al 1998; Ogura et al 1991; Shelley et al
1991) and children with ADHD (Halliday et al 1983;
Klorman 1991; Loiselle et al 1980; Winsberg et al 1993).
Furthermore, P200 latency range is greater in
schizo-phrenic patients (Ogura et al 1991) and children with
ADHD (Klorman 1991; Winsberg et al 1993) than in
control subjects. Notably, stimulant administration
en-larges these late positive waves in children with ADHD
and shortens the latencies of the P200
(Syrigou-Papavasi-liou et al 1988). Although adult ADHD patients have not
been directly compared to adult schizophrenic patients on
these specific measures, the later components of the
event-related potential do not seem to distinguish
differ-ences between these two groups as previously described in
the CPT.
An earlier component of the event-related potential, the
inhibition of the P50 auditory event– evoked potential, is
related to measures of sustained attention in schizophrenia
(Cullum et al 1993). This early evoked potential has been
extensively studied in schizophrenia (Adler et al 1982,
1990a, 1990b; Baker et al 1987; Boutros et al 1991;
Clementz et al 1997; Erwin et al 1991; Freedman et al
1983; Judd et al 1992). It is not known, however, if this
deficit also occurs in ADHD patients. To test the whether
the underlying pathophysiologic deficit of inhibition
present in schizophrenic patients was also present in
ADHD, this study examined the inhibition of the P50
auditory event– evoked potential in unmedicated adults
diagnosed with ADHD.
Methods and Materials
Subjects
hyperactive or inattentive symptoms of ADHD at the time of assessment; and describing a chronic course of ADHD symp-tomatology from childhood to adulthood. Normal subjects were similarly examined for the diagnosis of ADHD and excluded if they were symptomatic in childhood or adulthood. All other diagnoses in both ADHD and normal subjects were based on the Structured Clinical Interview for DSM-IV—Nonpatient Edition (SCID-IV-NP; First et al 1996) and Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II, Paranoid, Schizo-typal, Schizoid and Borderline personality disorder sections; First et al 1997b). Subjects with current depression, current or past bipolar mood disorder, current anxiety disorders, and current borderline or Cluster A personality disorders were excluded. ADHD and normal subjects were also excluded if they had a family history of psychosis as determined by Family History-Research Diagnostic Criteria (FH-RDC; Endicott et al 1978). Diagnoses for the schizophrenic subjects were made based on the Structured Clinical Interview for DSM-IV-Patient Edition (SCID-IV-P; First et al 1997a). All schizophrenic patients were chronically ill, but were clinically stable outpatients with an average duration of illness of 18 years.
After complete description of the study to the subjects, written informed consent was obtained. Schizophrenic patients were assessed at baseline on their current typical neuroleptic medica-tion. P50 gating in schizophrenic patients is unaffected by typical neuroleptic medication (Adler et al 1982, 1990a, 1990b; Freed-man et al 1983; Ward et al 1996). Patients on atypical neuroleptic medications were excluded, because a previous study found that P50 gating improves in a schizophrenic population on clozapine (Nagamoto et al 1996, 1999). ADHD and normal subjects were not taking any psychotropic medications.
Electrophysiologic Recordings
Subjects were recorded supine, relaxed, and awake with eyes open and fixed on a distant target to decrease drowsiness during the recording. Because nicotine can briefly enhance P50 sensory gating in schizophrenics (Adler et al 1993), subjects were recorded only after abstaining from nicotine for a minimum of 30 min. Electroencephalographic activity was recorded from a gold
disk electrode affixed to the vertex (Cz) and referenced to one ear. Electroencephalogram (EEG) activity was amplified 20,000 times with bandpass filters (250%) between 1 and 300 Hz. Electro-oculogram (EOG) was also recorded between the right superior orbit and lateral canthus. EEG and EOG were collected for 1000 msec, for each paired stimulus presented, by a techni-cian blind to the subjects’ clinical symptoms. Trials were rejected if they contained large muscle artifact or eye blinks as indicated by an EEG or EOG voltage of650mV over the area of the P50. Auditory stimuli were presented in a conditioning testing para-digm with an intrapair interval of 0.5 sec and interstimulus interval of 10 sec. A 0.04 msec duration square wave pulse was amplified in the 20 –12,000 Hz bandwidth and delivered through earphones that produced a 2.5 msec sound with a mean intensity of a 70-dB sound pressure level, as measured at the subject’s ear by a sound meter (Tandy Corporation, Houston; Griffith et al 1995). If the subjects showed a startle response, the intensity was decreased by 5 dB. There were two normal, one schizophrenic, and one ADHD subjects that required decreases in decibel intensity. At least three averaged evoked potential recordings were collected for each patient, each containing 16 trials. Each of the three averages (16 trials) was digitally filtered with a low-pass filter (10 Hz) and a seven point, high-pass filter (300 Hz; A5 0.95; Coppola 1979). Each filter was applied in the forward and reverse positions to increase rolloff and to preserve waveform latency. The three averages were added together to give a grand average (48 trials), which was used for analysis. A previously described computer algorithm (Nagamoto et al 1989, 1991) was used to identify and quantify the P50 wave. The computer selected the most positive peak between 40 and 75 msec after the conditioning stimulus. The test P50 was selected as the most positive waveform at a latency from the test stimulus, which was610 msec of the latency of the conditioning P50 from its stimulus. This criterion represents the 95% confidence limit of the difference between conditioning and test latencies (Naga-moto et al 1989). A blind rater also reviewed tracings; thus any possible P50 occurring up to 15 msec after the latency window in the test response was not overlooked (Clementz et al 1997). The amplitude of each wave was measured relative to the previous Table 1. Demographic Characteristics of the Sample Groups
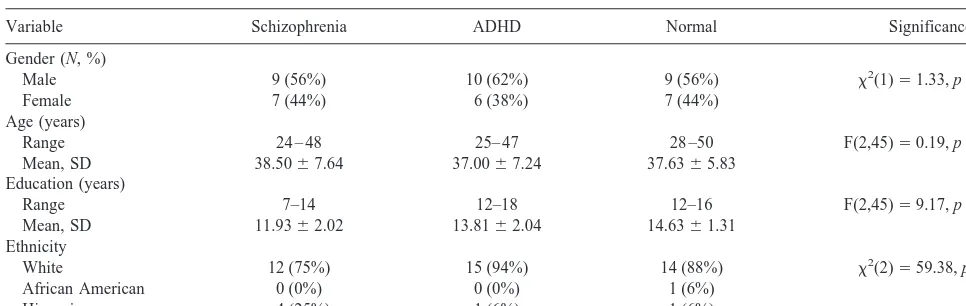
Variable Schizophrenia ADHD Normal Significance
Gender (N, %)
Male 9 (56%) 10 (62%) 9 (56%) x2(1)51.33, p,.25
Female 7 (44%) 6 (38%) 7 (44%)
Age (years)
Range 24 – 48 25– 47 28 –50 F(2,45)50.19, p,.83
Mean, SD 38.5067.64 37.0067.24 37.6365.83
Education (years)
Range 7–14 12–18 12–16 F(2,45)59.17, p,.001
Mean, SD 11.9362.02 13.8162.04 14.6361.31
Ethnicity
White 12 (75%) 15 (94%) 14 (88%) x2(2)559.38, p,.001
African American 0 (0%) 0 (0%) 1 (6%)
Hispanic 4 (25%) 1 (6%) 1 (6%)
negativity. Averages with no discernable conditioning P50 waves or with prominent EOG activity at the same latency as the P50 were excluded from average used for the analysis. Test/condi-tioning ratios were calculated by dividing the test P50 amplitude by the conditioning P50 amplitude. This ratio was multiplied by 100, thus represents a percentage. The 95% confidence interval for the P50 ratio indicates that 5% of normal subjects have ratios greater than 50%, and 5% of schizophrenic patients have P50 ratios less than 40% (Adler et al 1990a, 1990b; Freedman et al 1997; Ward et al 1996).
Statistical Analysis
To determine if the auditory event– evoked potential was affected by diagnosis, overall effects between the three groups were assessed using a Kruskal–Wallis distribution-free analysis of variance. Conditioning amplitude, test amplitude, test/condition-ing ratio, difference (conditiontest/condition-ing amplitude2test amplitude), and conditioning latency were examined as dependent variables, with diagnostic group as the independent variable. In cases where the overallx2
statistic was significant, individualx2
tests were employed for pairwise comparisons. All analyses were imple-mented with the SAS statistical software package (SAS/STAT for Windows, Version 6.12, SAS Institute, Inc., Cary, NC). A one-way analysis of variance (ANOVA) for continuous variables andx2
test of association for categorical variables were used to test for potential differences between groups with respect to demographic variables. Demographic variables for which the groups differed were then tested for their effect on the variables with Pearson correlation coefficients or an ANOVA. To examine the relationship between the severity of ADHD symptoms and the P50 outcome measures, Pearson correlation coefficients were computed for the P50 variables and the number of hyperactive or inattentive symptoms, the Wender patient scores, and the Parent Rating Scale scores.
Results
The Patient’s Wender score for the ADHD group was
(mean) 58.69
6
(SD) 18.45. The Adult ADHD Parent’s
Rating Scale score for the ADHD group was 13.62
6
11.43. All patients had at least six inattentive symptoms in
childhood and in adulthood with a mean of 7.73
6
1.1
symptoms. In addition, six out of 16 patients (38%) had at
least six hyperactive symptoms in childhood and in
adult-hood with a mean of 4.8
6
2.3 symptoms. Two of the
demographic variables were significantly different
be-tween subject groups (Table 1), but neither of them
affected any of the physiologic variables.
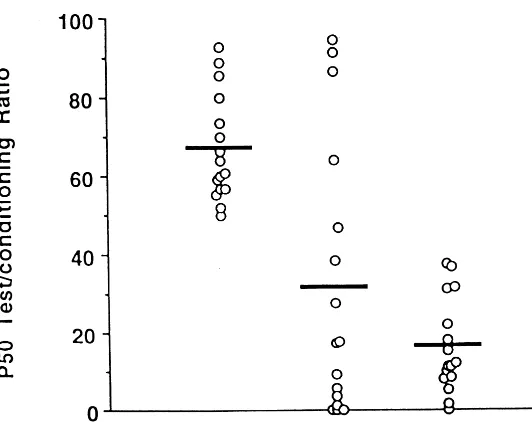
The Kruskal–Wallis showed significant effects of
psy-chiatric diagnosis on the P50 ratio, the P50 difference, and
the P50 test amplitude (Table 2). Significant pairwise
comparisons between groups showed that the
schizo-phrenic group had P50 ratios, P50 differences, and P50
test amplitudes that were higher than both the ADHD and
normal groups (Table 3). The ADHD and normal groups
were not significantly different on P50 ratio or test
amplitude but were significantly different on the P50
difference (Figures 1 and 2). Furthermore, the P50
condi-tioning amplitude or condicondi-tioning latency did not
signifi-cantly differ between groups (Table 2). There were no
significant correlations between any of the symptom
severity markers in the ADHD group and the P50 test
amplitude, P50 difference, or P50 ratio.
Table 2. Means and Standard Deviations of the P50 Parameters Examined
Diagnosis
Conditioning amplitude
Test amplitudeb
Amplitude differencec
(conditioning2test)
Ratioa
(conditioning/test)
Conditioning latency
Schizophrenia (mean6SD)
2.5361.58 1.5360.85 1.0161.04 67.01613.46 61.0065.75
ADHD (mean6SD)
2.0861.21 0.6660.88 1.4261.21 31.46634.77 57.8666.72
Normal (mean6SD)
2.6161.57 0.506.65 2.1061.20 16.22612.10 60.6966.11
Kruskal–Wallis analysis of variance. ADHD, attention-deficit/hyperactivity disorder.
ap,.0001. bp,.001. cp,.014.
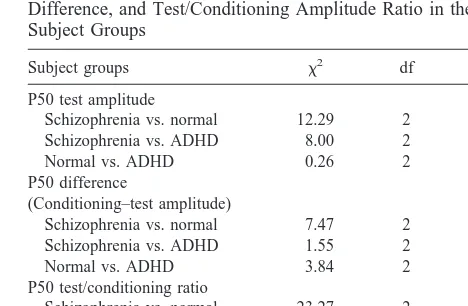
Table 3. P50 Test Amplitude, Conditioning–Test Amplitude Difference, and Test/Conditioning Amplitude Ratio in the Subject Groups
Subject groups x2 df p
P50 test amplitude
Schizophrenia vs. normal 12.29 2 .0005 Schizophrenia vs. ADHD 8.00 2 .005
Normal vs. ADHD 0.26 2 .61
P50 difference
(Conditioning–test amplitude)
Schizophrenia vs. normal 7.47 2 .006 Schizophrenia vs. ADHD 1.55 2 .21
Normal vs. ADHD 3.84 2 .05
P50 test/conditioning ratio
Schizophrenia vs. normal 23.27 2 .0001 Schizophrenia vs. ADHD 7.99 2 .005
Normal vs. ADHD 2.40 2 .62
Discussion
This study replicates previous findings that patients with
schizophrenia have an inability to suppress the P50 wave
of the auditory event– evoked potential in a
conditioning-testing paradigm. The study further demonstrates that most
adults with ADHD do not have this inhibitory deficit.
ADHD subjects generally inhibit the response to repeated
stimuli, although 25% of this group did not suppress the
response to the second stimulus. This rate of
nonsuppres-sion in the ADHD group is higher than the 10% rate
previously observed within groups of normal subjects
(Freedman et al 1994), but the difference is not significant;
however, this lack of significance may be due to the small
number of subjects studied, although this same number of
subjects demonstrated sufficient power to detect a
differ-ence between schizophrenic patients and normal subjects.
The sample has a power of 0.80 to detect a difference
between groups of effect size 1.0.
The P50 deficit is a noninvasive method to elucidate
some of the underlying pathophysiologic deficits in
psy-chiatric disorders. The inhibitory deficit in schizophrenic
patients may be mediated through the nonpyramidal
hip-pocampal interneurons that contain the inhibitory
neuro-transmitter
g-aminobutyric acid (GABA; Freedman et al
1993). Cholinergic synapses activate these interneurons,
which then inhibit the pyramidal response to the second
stimulus (Hershman et al 1995; Miller and Freedman
1995). The receptor type mediating the P50 response is the
low affinity neuronal nicotinic cholinergic receptor, for
which the responsible gene is the
a7-nicotinic receptor
subunit gene (Luntz-Leybman et al 1992). Furthermore,
nicotine, in high doses, transiently normalizes the
abnor-mality in P50 inhibition in schizophrenic patients and in
their relatives (Adler et al 1992, 1993). Schizophrenic
patients are unusually heavy smokers (Olincy et al 1997),
a finding that may signify their attempt to self medicate
this pathophysiologic mechanism.
Nicotinic systems may also play a role in ADHD
(Barkley 1990; Hartsough and Lambert 1987; Lynskey
and Fergusson 1995; Milberger et al 1997a, 1997b).
Children with ADHD are more likely to smoke than other
normal children (Barkley 1990; Hartsough and Lambert
1987). Adults with ADHD also smoke cigarettes at a
higher rate than normal subjects (Borland and Heckman
1976). Forty-two percent of men and 38% of women
diagnosed with ADHD are current smokers, almost twice
as high as the number in an unselected population
(Mil-berger et al 1997b; Pomerleau et al 1995). Nicotine has
been shown to significantly improve clinical ADHD
symptoms as measured by the Clinical Global Impression
scale as well as by measures of attention, vigor, and
arousal in ADHD subjects (Coger et al 1996; Conners et al
1996; Levin et al 1996).
Nicotine has a variety of actions. It can directly
stimu-lates
a7 nicotinic acetylcholine receptors on inhibitory
interneurons, as is hypothesized in schizophrenia. Nicotine
also promotes the release of dopamine by nigrostriatal and
mesolimbic dopaminergic neurons and causes the release
of other neurotransmitters, such as serotonin, through
presynaptic nicotinic receptors (Wonnocott et al 1989). In
addition, the noradrenergic pathways of the locus
coer-uleus, which are involved in regulating attention, may
additionally be affected by nicotine (Levin et al 1996). In
ADHD, dysregulation of norepinephrine release in the
locus coeruleus is suspected to decrease attention and
interfere with subsequent signaling of dopamine-mediated
systems in other areas of the brain (Pliszka et al 1996).
After abrupt withdrawal of nicotine, decreased levels of
dopamine and norepinephrine are believed to exacerbate
the mood and behavioral dysregulation of ADHD patients
(Coger et al 1996). These data provide indirect evidence
that nicotinic pathways may mediate the expression of
ADHD symptoms.
A noradrenergic mechanism can also cause loss of
inhibition in the sensory gating paradigm (L.E. Adler et al,
unpublished data, 2000; Adler et al 1990a). Decreased
inhibition of the P50 response occurs in acute mania, with
the decrease correlated with increased plasma levels of the
noradrenergic metabolite 3-methoxy,
4-hydroxyphenylg-lycol (MHPG; Adler et al 1989). When plasma MHPG
levels return to normal during clinical treatment, the P50
inhibition also normalizes. Decreased inhibition also
oc-curs in normal subjects after administration of the
a2
adrenergic receptor antagonist yohimbine, which increases
presynaptic release of norepinephrine (Adler et al 1994).
McDougle et al (1994) have demonstrated that
noradren-ergic dysregulation occurs in cocaine addicts and persists
into the detoxification period. Abnormalities in the
dopa-mine transporter are also correlated with increased MHPG
levels in cocaine addicts (Bowers et al 1998). An identical
disturbance in the inhibition of the P50 auditory event–
evoked response to repeated stimuli has been found in
recently detoxified cocaine addicts (L.E. Adler et al,
unpublished data, 2000; Boutros 1998; Fein et al 1996).
This P50 deficit corrects in cocaine addicts with nicotine
(L.E. Adler et al, unpublished data, 2000). Thus,
norad-renergic mechanisms that cause a variable P50 deficit may
correct with nicotine, presumably through the interaction
with the high affinity
a4b2 nicotinic cholinergic receptors.
A noradrenergic role in the etiology of ADHD has also
been previously suggested. The urinary concentration of
3,4-dihydroxyphenylglycol (DOPEG), a norepinephrine
metabolite, was found to be significantly lower in boys
with ADHD than in normal control subjects (Hanna et al
1996). Furthermore, children with ADHD have plasma
MHPG levels that vary depending on the presence or
absence of a reading disability, indicating a lack of
homogeneity in noradrenergic function that is associated
with clinical variation (Halperin et al 1997). Additionally,
alterations in noradrenergic function are consistent with
neurobiologic studies that implicate catecholamines in
ADHD by the mechanism of action of stimulants, the class
of drugs that effectively treats many patients with this
disorder. Stimulants block the reuptake of dopamine and
norepinephrine into the presynaptic neuron, and increase
the release of these monoamines into the extraneuronal
space. Additionally, 35% of treatment-seeking cocaine
abusers met DSM-III-R criteria for childhood ADHD
(Caroll and Rounsaville 1993). Thus, a noradrenergic
mechanism may explain the P50 auditory event– evoked
potential variability and the variability often seen in
clinical symptoms of ADHD.
Thus, different aspects of both nicotinic cholinergic
neurotransmission and noradrenergic neurotransmission
are involved in psychotic illness (schizophrenia, mania,
stimulant addiction) and ADHD. For nicotinic cholinergic
neurotransmission, these differences likely reflect
differ-ences in which genes in the family of nicotinic receptor
genes are involved. Although the
a7 nicotinic receptor has
been related to the pathophysiologic deficit in
schizophre-nia, it may not be related to the attentional deficits in
ADHD, which have a different neurophysiologic and
neuropsychological basis. The use of nicotine in ADHD
could reflect other neuronal mechanisms, such as the
release of norepinphrine and dopamine, which is mediated
by presynaptic
a4b2 nicotinic receptors (Wonnocott et al
1989).
Attention is a multifaceted symptom. The abnormality
in P50 inhibition has been related to vigilance, one aspect
of attentional dysfunction (Cullum et al 1993; Erwin et al
1998; Vinogradov et al 1996; Yee et al 1998) and appears
to differentiate schizophrenia from ADHD. The P50
au-ditory event– evoked potential, however, does not
discrim-inate schizophrenia from other psychiatric disorders with
distractibility, such as acute mania and cocaine
with-drawal. Although the P50 abnormality in schizophrenia is
correlated with neuropsychological measures of sustained
attention (Cullum et al 1993), this electrophysiologic
measure is not related to self-report of sensory gating
dysfunction (Jin et al 1998). Thus, the P50 inhibitory
deficit is only a single element in the complex
pathophys-iology of psychosis and attentional abnormalities.
Supported by the National Alliance for Research on Schizophrenia and Depression, the Veterans Administration Medical Research Service, and U.S. Public Health Service Grants Nos. MH4212 and MH38321.
References
Adler LE, Gerhardt GA, Franks R, Baker N, Nagamoto H, Drebing C, et al (1990a): Sensory physiology and cat-echolamines in schizophrenia and mania. Psychiatry Res 31:297–309.
Adler LE, Hoffer L, Nagamoto HT, Waldo MC, Kisley MA, Griffith JM (1994): Yohimbine impairs P50 auditory sensory gating in normal subjects. Neuropsychopharmacology 10: 249 –257.
Adler LE, Hoffer LD, Wiser A, Freedman R (1993): Normaliza-tion of auditory physiology by cigarette smoking in schizo-phrenic patients. Am J Psychiatry 150:1856 –1861.
Adler LE, Hoffer LJ, Griffith J, Waldo M, Freedman R (1992): Normalization of nicotine by deficient auditory sensory gat-ing in the relatives of schizophrenics. Biol Psychiatry 32:607– 616.
Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R (1982): Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating and schizophrenia. Biol Psychiatry 17:639 – 654.
Adler LE, Waldo M, Tatcher A, Cawthra E, Baker N, Freedman R (1990b): Lack of relationship of auditory sensory gating defects to negative symptoms in schizophrenia. Schizophr Res 3:131–138.
American Psychiatric Association (1994): Diagnostic and
Sta-tistical Manual of Mental Disorders, 4th ed. Washington, DC:
American Psychiatric Press.
Baker N, Adler LE, Franks RD, Waldo M, Berry S, Nagamoto H, et al (1987): Neurophysiological assessment of sensory gating in psychiatric inpatients: Comparison between schizophrenia and other diagnoses. Biol Psychiatry 22:603– 617.
Barkley R (1990): Attention Deficit Hyperactivity Disorder: A
Handbook for Diagnosis and Treatment. New York:
Guil-ford.
Biederman J, Faraone S, Milberger S, Guite J, Mick E, Chen L, et al (1996): A prospective four-year follow-up study of attention deficit hyperactivity and related disorders. Arch Gen
Psychiatry 53:437– 446.
Bleuler E (1911): Dementia Praecox or the Group of
Schizo-phrenias. New York: New York International Universities
Press.
Borland BL, Heckman HK (1976): Hyperactive boys and their brothers: A 25-year follow-up study. Arch Gen Psychiatry 33:669 – 675.
Boutros N, Krystal J, Gerlenter J (1998): Sensory gating and cocaine induced paranoia. Biol Psychiatry 43:89S.
Boutros NN, Zouridakis G, Overall J (1991): Replication and extension of P50 findings in schizophrenia. Clin
Electroen-cephalogr 22:40 – 45.
Bowers MB, Malison RT, Seibyl JP, Kosten TR (1998): Plasma homovanillic acid and the dopamine transporter during co-caine withdrawal. Biol Psychiatry 43:278 –281.
Carroll KM, Rounsaville BJ (1993): History and significance of childhood attention deficit disorder in treatment-seeking co-caine abusers. Compr Psychiatry 34:75– 82.
Clementz BA, Geyer MA, Braff DL (1997): P50 suppression among schizophrenia and normal comparison subjects: A methodological analysis. Biol Psychiatry 41:1035–1044. Coger R, Moe KL, Serafetinides EA (1996): Attention deficit
disorder in adults and nicotine dependence: Psychobiological factors in resistance to recover? J Psychoactive Drugs 28: 229 –240.
Coppola R (1979): Isolating low frequency activity in EEG spectrum analysis. Electroencephalogr Clin Neurophysiol 46:224 –226.
Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L (1989): The continuous performance test, identical pairs version (CPT-JP): II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Res 29:65– 86.
Coull JT (1998): Neural correlates of attention and arousal: Insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol 55:343–361. Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A,
Nagamoto HT, et al (1993): Neurophysiological and neuro-psychological evidence for attentional dysfunction in schizo-phrenia. Schizophr Res 10:131–141.
Endicott J, Andreasen N, Spitzer RL (1978): Family
History-Research Diagnostic Criteria, 3rd ed. New York: New York
State Psychiatric Institute, Research Assessment and Training Unit.
Erwin RJ, Mawhinney-Hee M, Gur RC, Gur RE (1991): Midla-tency auditory evoked responses in schizophrenia. Biol
Psy-chiatry 30:430 – 442.
Erwin RJ, Turetsky BJ, Moberg P, Gur RC, Gur RE (1998): P50 abnormalities in schizophrenia: Relationship to clinical and neurophysiological indices of attention. Schizophr Res 33: 157–167.
Fein G, Biggins C, MacKay S (1996): Cocaine abusers have reduced auditory P50 amplitude and suppression compared to both normal controls and alcoholics. Biol Psychiatry 39:955– 965.
First MB, Spitzer RL, Gibbon M, Williams JBW (1996):
Structured Clinical Interview for DSM-IV Axis I Disorders— Non-Patient Edition. New York: New York State Psychiatric
Institute, Biometrics Research Department.
First MB, Spitzer RL, Gibbon M, Williams JBW (1997a):
Structured Clinical Interview for DSM-IV Axis I Disorders— Patient Edition. New York: New York State Psychiatric
Institute, Biometrics Research Department.
First MB, Spitzer RL, Gibbon M, Williams JBW, Benjamin LS (1997b): Structured Clinical Interview for DSM-IV Axis II
Personality Disorders (SCID-II). Washington, DC: American
Psychiatric Press.
Freedman R, Adler LE, Bickford P, Byerley W, Coon H, Cullum MC, et al (1994): Schizophrenia and nicotinic receptors. Harv
Rev Psychiatry 2:179 –92.
Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD (1983): Neurophysiological evidence for a defect in inhibi-tory pathways in schizophrenia: Comparison of medicated and drug-free patients. Biol Psychiatry 18:537–551. Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy
A, Davis A, et al (1997): Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl
Acad Sci U S A 94:587–592.
Freedman R, Wetmore C, Stromberg I, Leonard S, Olson, L (1993): Alpha-bungarotoxin binding to hippocampal inter-neurons: Immunocytochemical characterization and effects on growth factor expression. J Neurosci 13:1965–1975. Goldstein G (1986): The neuropsychology of schizophrenia. In:
Grant I, Adams KM, editors. Neuropsychological Assessment
of Neuropsychiatric Disorders. New York: Oxford University
Press, 147–171.
Goyette CH, Conners CK, Ulrich RF (1978): Normative data on revised Conners Parent and Teacher Rating Scales. J Abnorm
Child Psychol 6:221–236.
Griffith J, Hoffer LD, Adler LE, Zerbe GO, Freedman R (1995): Effects of sound intensity on a midlatency evoked response to repeated auditory stimuli in schizophrenic and normal sub-jects. Psychophysiology 32:460 – 466.
Halliday R, Callaway E, Naylor H (1983): Visual evoked potential changes induced by methylphenidate in hyperactive children dose/response effects. Electroencephalogr Clin
Neu-rophysiol 55:258 –264.
Halperin JM, Newcorn JH, Koda VH, Pick L, McKay KE, Knott P (1997): Noradrenergic mechanisms in children with ADHD with and without reading disabilities: A replication and exten-sion. J Am Acad Child Adolesc Psychiatry 36:1688 –1697. Hanna GL, Ornitz EM, Hariharan M (1996): Urinary
catechol-amine excretion and behavioral differences in ADHD and normal boys. J Child Adolesc Psychopharmacol 6:63–73. Hartsough CS, Lambert NM (1987): Pattern and progression of
drug use among hyperactives and controls: A prospective short-term longitudinal study. J Child Psychol Psychiatry 28:543–553.
Hershman KM, Freeman R, Bickford PC (1995): GABAB antagonists diminish the inhibitory gating of auditory re-sponse in the rat hippocampus. Neurosci Lett 190:133–136. Hirayasu Y, Asato N, Ohta H, Hokama H, Arakaki H, Ogura C
(1998): Abnormalities of auditory event-related potentials in schizophrenia prior to treatment. Biol Psychiatry 43:244 – 253.
Holdnack JA, Moberg PJ, Arnold SE, Gur RC, Gur RE (1995): Speed of processing and verbal learning deficits in adults diagnosed with attention deficit disorder. Neuropsychiatry
Neuropsychol Behav Neurol 8:282–292.
Javitt DC, Grochowski S, Shelley AM, Ritter W (1998): Im-paired mismatch negativity (MMN) generation in schizophre-nia as a function of stimulus deviance, probability, and interstimulus deviant interval. Electroencephalogr Clin
Neu-rophysiol 108:143–153.
Jin Y, Bunney WE Jr, Sandman CA, Patterson JV, Fleming K, Moenter JR, et al (1998): Is P50 supression a measure of sensory gating in schizophrenia? Biol Psychiatry 43:873– 878. Judd LL, McAdams L, Budnick B, Braff DL (1992): Sensory
gating deficits in schizophrenia: New results. Am J Psychiatry 149:488 – 493.
Klorman R (1991): Cognitive event-related potentials in atten-tion deficit disorder. J Learning Disabilities 24:130 –140. Koelega HS (1995): Is the Continuous Performance Task useful
in research with children with ADHD? Comments on a review. J Child Psychol Psychiatry 36:1477–1485.
Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al (1996): Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology 123:55– 63.
Loiselle D, Stamm JS, Maitinsky S, Whipple SC (1980): Evoked potential and behavioral signs of attentive dysfunctions in hyperactive boys. Psychophysiology 17:193–201.
Luntz-Leybman V, Bickford PC, Freedman, R (1992): Cholin-ergic gating of response to auditory stimuli in rat hippocam-pus. Brain Res 587:130 –136.
Lynskey MT, Fergusson DM (1995): Childhood conduct prob-lems, attention deficit behaviors, and adolescent alcohol, tobacco, and illicit drug use. J Abnorm Child Psychol 23:281– 302.
Milberger S, Biederman J, Faraone SV, Chen L, Jones J (1997a): ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc
Psychiatry 36:37– 44.
Milberger S, Biederman J, Faraone SV, Guite J, Tsuang MT (1997b): Pregnancy, delivery and infancy complications and attention deficit hyperactivity disorder: Issues of gene-envi-ronment interaction. Biol Psychiatry 41:65–75.
Miller CL, Freedman R (1995): The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neuroscience 69: 371–381.
Nagamoto HT, Adler LE, Hea RA, Griffith JM, McRae KA, Freedman R (1996): Gating of auditory P50 in schizophren-ics: Unique effects of clozapine. Biol Psychiatry 40:181–188. Nagamoto HT, Adler LE, McRae KA, Huettl P, Cawthra E, Gerhardt G, et al (1999): Auditory P50 in schizophrenics on clozapine: Improved gating parallels clinical improvement and changes in pMHPG. Neuropsychobiology 39:10 –17. Nagamoto HT, Adler LE, Waldo MC, Freedman R (1989): Sensory
gating in schizophrenics and normal controls: Effects of chang-ing stimulation interval. Biol Psychiatry 25:549 –561.
Nagamoto HT, Adler LE, Waldo MC, Griffith J, Freedman R (1991): Gating of auditory response in schizophrenics and normal controls: Effects of recording site and stimulation interval on the P50 wave. Schizophr Res 4:31– 40.
Nuechterlein K, Buchsbaum MS, Dawson ME (1994): Neuro-psychological vulnerability to schizophrenia. In: David AS, Cutting JC, editors. The Neuropsychology of Schizophrenia. Hillsdale, NJ: Lawrence Erlbaum, 53–77.
Nuechterlein KH (1983): Signal detection in vigilance tasks and behavioural attributes among offspring of schizophrenic mothers and among hyperactive children. J Abnorm Psychol 92:4 –28.
Nuechterlein KH (1991): Vigilance in schizophrenia and related disorders. In: Seinhauer SR, Gruzelier JH, Zubin J, editors.
Handbook of Shizophrenia. Vol. 5: Neuropsychology, Psy-chophysiology and Information Processing. New York:
Elsevier Science, 397– 433.
Nuechterlein KH, Dawson ME (1984): Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull 10:160 –203. O’Dougherty M, Nuechterlein KH (1984): Hyperactive and
hypoxic children: Signal detection, sustained attention, and behavior. J Abnorm Psychol 93:178 –191.
Ogura C, Nageishi Y, Matsubayashi M, Omura F, Kishimoto A, Shimokochi M (1991): Abnormalities in event-related poten-tials, N100, P200, P300 and slow wave in schizophrenia. Jpn
J Psychiatry Neurol 45:57– 65.
Olincy A, Young DA, Freedman R (1997): Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry 42:1–5.
Orvaschel H, Puig-Antich J, Chambers WJ, Tabrizi MA, Johnson R (1982): Retrospective assessments of child psychopathol-ogy with the Kiddie-SADS-E. J Am Acad Child Psychiatry 21:392–397.
Pliszka SR, McCracken JT, Maas JW (1996): Catecholamines in attention-deficit hyperactivity disorder: Current perspectives.
J Am Acad Child Adolesc Psychiatry 35:264 –272.
Pomerlau O, Downey K, Stelson F, Pomerlau C (1995): Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse 7:373–378.
Rosvold HE, Mirsky A, Sarason J, Bransome ED Jr, Beck JH (1956): A continuous performance test of brain damage. J
Consult Psychol 20:343–350.
Seidman LJ, Biederman J, Weber W, Hatch M, Faraone SV (1998): Neuropsychological function in adults with atten-tion-deficit hyperactivity disorder. Biol Psychiatry 44: 260 –268.
Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N (1991): Mismatch negativity: An index of preattentive processing deficit in schizophrenia. Biol
Psychi-atry 30:1059 –1067.
Syrigou-Papavasiliou A, Lycaki H, LeWitt PA, Verma NP, Spivak D, Chayasirisobhon S (1988): Dose-response effects of chronic methylphenidate administration on late event-related potentials in attention deficit disorder. Clin
Electro-encephalogr 19:129 –133.
van Leeuwen TH (1998): The continuous performance test revisited with neuroelectric mapping: Impaired orienting in children with attention deficits. Behav Brain Res 94:97– 110.
Venables P (1964): Input dysfunction in schizophrenia. In: Maher BA, editor. Progress in Experimental Personality
Research. Orlando, FL: Academic Press, 1– 47.
Vinogradov S, Solomon S, Ober BA, Biggins CA, Shenaut GK, Fein G (1996): Do semantic priming effects correlate with sensory gating in schizophrenia? Biol Psychiatry 39:821– 824.
Ward MF, Wender PH, Reimherr FW (1993): The Wender Utah Rating Scale: An aid in the retrospective diagnosis of child-hood attention deficit hyperactivity disorder. Am J Psychiatry 150:885– 890.
Ward PB, Hoffer LD, Liebert BJ, Catts SB, O’Donnell M, Adler LE (1996): Replication of a P50 auditory sensory gating deficit in Australian patients with schizophrenia. Psychiatry
Res 64:121–135.
Wender P (1995): Attention-Deficit Hyperactivity Disorder in
Adults. New York: Oxford University Press.
Winsberg BG, Javitt DC, Silipo GS, Doneshka P (1993): Mismatch negativity in hyperactive children: Effects of meth-ylphenidate. Psychopharmacol Bull 29:229 –233.
Wonnacott S, Irons J, Rapier C, Thorne B, Lunt GG (1989): Presynaptic modulation of transmitter release by nicotinic receptors. In: Nordberg A, Fuxe K, Holmstedt B, Sundwall A, editors. Progress in Brain Research. New York: Elsevier, 157–163.