www.elsevier.nlrlocateraqua-online
Optimization of gonad growth by manipulation of
temperature and photoperiod in cultivated sea
ž
/
urchins, Paracentrotus li
Õ
idus Lamarck
ž
Echinodermata
/
Christine Spirlet
a,b,), Philippe Grosjean
a, Michel Jangoux
a,b,ca
Laboratoire de Biologie Marine CP 160r15, UniÕersite Libre de Bruxelles, 50 A´ Õ. F.D. RooseÕelt, B-1050
Brussels, Belgium
b
Centre regional d’etudes cotieres, Uni´ ´ ˆ ` Õersite de Caen, Station marine, B.P. 49,´
F-14530 Luc-sur-Mer, France
c
Laboratories de Biologie Marine, UniÕersite de Mons-Hainaut, 19 rue Maistriau, B-7000 Mons, Belgium´
Accepted 20 October 1999
Abstract
A starvation and then feeding method was developed to produce about 100% marketable sea urchins, Paracentrotus liÕidus, in 3 1r2 months. This method is needed because the reproduction cycle is desynchronized in the conditions imposed during the somatic growth stage in land-based closed systems. The major advantages of starving the animals are resetting the reproductive cycle
Ž .
to the spent stage gonads almost devoid of sexual cells and stressing the individuals so that they mobilize and restore the nutritive phagocytes, filling them with nutrients. Batches of sea urchins starved 2 months beforehand were fed ad libitum for 45 days with enriched food under eight
Ž . Ž
combinations of four temperatures 128C, 168C, 208C and 248C and two photoperiods 9 and 17 h .
daylight . In our system, the best combination was 248C and 9 h daylight for growth as well as for
Ž .
gonad quality. The gonadal indices obtained in dry weight were over 9% at 168C and over 12% at 248C, which are better than what is found in the field for this population. q2000 Elsevier
Science B.V. All rights reserved.
Keywords: Gonad; Growth; Temperature; Photoperiod; Sea urchin; Paracentrotus liÕidus
)Corresponding author. Laboratoire de Biologie Marine Pentagone-Universite de Mons-Hainaut, Avenue du´
Champ de Mars 6, B-7000 Mons, Belgium. Tel.:q32-65-373441; fax:q32-65-373434.
Ž .
E-mail address: [email protected] C. Spirlet .
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
1. Introduction
Increasing demand for sea urchin roe has led in the last decade to over-fishing natural
Ž .
populations Conand and Sloan, 1989; Le Gall, 1990 . Several possible solutions have Ž
been tested: reseeding natural habitats with farmed juveniles Agatsuma and Momma,
. Ž
1988; Gomez et al., 1995 ; mariculture Fernandez and Caltagirone, 1994; Fernandez,
. Ž
1996 ; raising sea urchins in immerged cages, alone Fernandez, 1996; Robinson and
. Ž
Colborne, 1998 ; or with other animals polyculture with salmons notably, Kelly et al., .
1998 ; and finally land-based, closed-system echiniculture allowing control of each Ž
phase of the echinoid biological cycle Le Gall and Bucaille, 1989; Le Gall, 1990; .
Grosjean et al., 1998 .
Whatever the culture method, temperate sea urchins exposed to natural conditions of temperature and photoperiod have a seasonal sexual cycle that restrains the crop to only
Ž
2 or 3 months per year Byrne, 1990; Lozano et al., 1995; Fernandez, 1996; Spirlet et .
al., 1998a . Many experimental attempts have already been made to manipulate the gonadal cycle by modifying these exogenous parameters. Most of them were successful
Ž
in obtaining out-of-season gametogenesis Leahy et al., 1981; Pearse et al., 1986; .
McClintock and Watts, 1990; Walker and Lesser, 1998 . However, the experiments were always run on specimens collected from the field, and hence, sexually in phase to begin
Ž
with. Moreover, the shift in the reproductive cycle took at least 7 months Walker and .
Lesser, 1998 .
Ž .
With adult sea urchins originating from cultivation see Grosjean et al., 1998 , the first problem is to obtain individuals in phase with regard to their reproductive cycle. As gonads also act as storage organs, it is possible to induce the consumption of most of
Ž .
their content by starving the animal Lawrence, 1985; Pearse and Cameron, 1991 . This
Ž .
attribute, experimentally observed, was successfully used by Spirlet et al. 1998b to study the impact of feeding strategies on gonadal production and to obtain marketable
Ž .
sea urchins all year long see Grosjean et al., 1998 . However, it has not been used yet Ž
to determine the best values of exogenous parameters mainly, temperature and photope-.
riod for optimizing the production of marketable gonads.
Several criteria have to be considered in echiniculture. The reproductive state of the gonads has to be in the correct range, from stage 3 to stage 5 according to Spirlet et al. Ž1998a ; the gonads preferably need to be fleshy and firm without an abundance of. gametes spilling when they are consumed; and, as previously stated, they also need to be sexually synchronized to ensure a large quantity of exploitable individuals all at once.
Ž .
This study has two objectives: 1 to determine the influence of the two major abiotic
Ž .
parameters temperature and photoperiod on gonadal growth and gametogenesis of Ž .
cultured sea urchins in a closed-circuit facility; and 2 to control the reproductive cycle and determine the best combination of temperature and photoperiod to obtain individuals ready for marketing as soon as possible.
2. Material and methods
The Paracentrotus liÕidus used were laboratory reared from two successive
fertiliza-Ž .
Ž .
collected on the rocky shore of Morgat Brittany, France . The individuals selected were
Ž . Ž .
30"2 mm "SE of ambital diameter excluding spines and aged 2 years and 3
months at the beginning of the experiment. Indeed, this size class presents the best
Ž .
gonadal index GI and therefore, a priori, the best potential of growth for the gonads ŽJangoux et al., 1996 ..
The experiment was conducted in four specific rearing structures described by
Ž .
Grosjean et al. 1998 . They consisted of three superposed toboggans overhanging a Ž
reserversettling tank. These experimental structures were thermoregulated one tempera-.
ture per unit and each toboggan was isolated so that photoperiod regulation was independent. A centrifugal pump insured circulation of the water. A water renewal of 200% per day maintained seawater quality during the experiment.
Ž
Sea urchins were starved 2 months beforehand i.e., until most of the nutrients .
present in the gonads were resorbed to make sure they were sexually in phase at the beginning of the experiment. Starvation occurred at the lowest temperature used in the
Ž . Ž .
study 128C , to prevent subsequent mortality Jangoux et al., 1996 .
Before initiating the experiment, five batches of six individuals were weighed in
Ž .
water immersed weight, IW, see below and dissected for control measurements. The
Ž .
immersed weighed was then standardized Jangoux et al., 1996, Grosjean et al., 1999 as shown in Eq. 1:
2.80y1.00
SIWsIW
ž
/
Ž .
12.80yMdr1000
with SIW being the standard immersed weight in g, Md the water mass density in grl, estimated from sea water temperature and salinity at the time of measurement and 2.80
Ž
the mean density of the sea urchin test allometry between the IW and the dry weight of the skeleton calculated on 356 reared animals after digestion of the soft tissues with
. sodium hypochloride 10% under gentle agitation .
The gonads were extracted and fresh weighed. One was fixed in Bouin’s fluid to be analyzed histologically, while the remaining gonads were dried for 48 h at 708C and dry weighed. The value was corrected for the missing gonad.
Ž
For the experiment, eight further sets of 30 individuals five replicates of six .
individuals were subjected for 45 days to eight treatments involving combinations
Ž . Ž
between four temperatures 128C, 168C, 208C and 248C and two photoperiods 7 h days .
or short day period, SD; 17 h days or long day period, LD . Initial immersed weights were determined for each batch. From the first day on, the individuals were fed ad
Ž .
libitum with extruded food, in the form of cylindrical pellets 8=15 mm . This food Ž
contained mainly wheat, fish, soybean, and minerals see Klinger et al., 1998 for exact .
composition . New food was distributed every other day after leftovers from the previous distribution were collected. The latter were dried and weighed to estimate the
Ž .
quantity of ingested food ingestion rate . Additional portions of food were placed in the same experimental structures, but away from the echinoids, and treated similarly to estimate the loss due to their degradation in seawater. The total ingested food was calculated with Eq. 2:
with P being the portions distributed, L the leftovers, and k a correction factor that Ž
takes into account the partial degradation of the food in the water k was estimated for .
each treatment .
At the end of the experiment, the immersed weight of the echinoids was determined
Ž .
prior to dissection. Somatic growth SG was calculated as the difference between the initial and final dry weights. The initial dry weight was estimated from initial standard
Ž .
immersed weight IW . Eq. 3 shows the relationship between the SIW and the dry
Ž .
weight of soma Grosjean et al., 1999 is:
DWsomas
Ž
1.68=SIW.
q0.21Ž .
3where DWsoma is expressed in g of dry weight and SIW is in g.
Ž .
The gonadal growth GG in g of dry weight was assessed as the difference between the final measured value and the initial value estimated from the control batch and corrected by the following calculation in Eq. 4:
GWb
GWiniŽest.s SIWini
Ž .
4SIWb
where GW and SIW are the values for the measured initial batch, SIWb b ini and GWiniŽest. is the estimated dry weight of the gonads in g when feeding starts.
Ž .
Hence, GG is calculated as follows Eq. 5 :
GGsGWfinyGWiniŽest..
Ž .
5The differential allocation of resources to soma and gonads is evaluated by means of
Ž .
the final GI expressed in dry weight Eq. 6 :
GW
GIds =100
Ž .
6SWqGW
where GI is the gonadal index in dry weight in percent, GW is the dry weight of thed
Ž . Ž .
gonads g and SW is the dry weight of the soma g . To compare with other studies,
Ž . Ž .
wet weight of gonadal index GIw is also evaluated Eq. 7 :
GWw
GIws =100.
Ž .
7TFW
With GIw being the gonadal index in fresh weight in percent, GW being the freshw
weight of the gonads in g and TFW being the total fresh weight of the sea urchin also in g. As a reminder, total fresh weight is independent of the fresh weight of gonads since the volume unoccupied by the gonads is replaced by coelomic fluid of equal density while total animal volume does not change. As the SIW is independent of the gonad weight and much more accurate than fresh weight, the TFW is evaluated from the
Ž .
allometric relationship presented as Eq. 8 Grosjean et al., in press :
Ž .
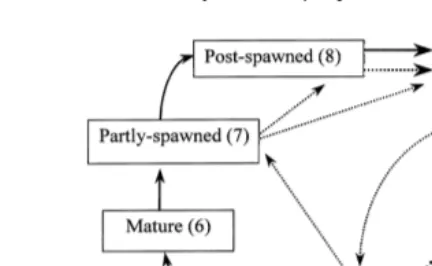
Fig. 1. Complete gametogenic cycle of P. liÕidus. In closed-circuit cultivation standard conditions , the
growing phase circled by a dotted line is actually by-passed: the gonads tend to start gametogenesis directly once they have enough nutrients, storing them only occasionally.
The maturity stages were diagnosed by histology and classified following an eight
Ž . Ž . Ž .
stage scale Spirlet et al., 1998a . The maturity index MI Eq. 9 of a batch was calculated as:
8
w
x
MIs
Ý
C ni irnŽ .
91
with C the maturity coefficient going from 1 to 8, n the number of individualsi i
presenting that coefficient, and n the total number of individuals in the batch.
Table 1
P. liÕidus. Two-way ANOVA analysis of the effect of temperature and photoperiod on somatic growth,
gonadal growth and food ingestion
SS df F-ratio P
Gonadal growth
Temperature 44.400 3 66.313 -0.001
Photoperiod 0.736 1 3.299 0.079
Temperature=Photoperiod 4.004 3 5.980 0.002
Error 7.142 32
Somatic growth
Temperature 73.123 3 53.857 -0.001
Photoperiod 2.473 1 5.464 0.026
Temperature=Photoperiod 3.268 3 2.407 0.085
Error 14.483 32
Food ingestion
Temperature 537.452 3 34.477 -0.001
Photoperiod 0.335 1 0.064 0.801
Temperature=Photoperiod 37.904 3 2.431 0.083
As no difference was noted between males and females in previous studies on wild
Ž .
populations of P. liÕidus in Morgat Spirlet et al., 1998a and cultivated specimens
Žpersonal observation , the data were pooled in all analysis Mann–Whitney U-test,. Ž .
Ps0.05 .
The treatments and the effect of temperature and photoperiod were compared using
Ž .
two-way ANOVA and Tukey test for temperature t-test for photoperiod , food inges-tion, for somatic production and gonadal growth. Normality and uniformity of variances were ensured, respectively, by x2 and Bartlett’s test. As distributions were sometimes
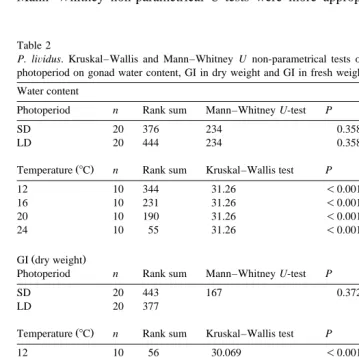
asymmetrical and could not always be considered normal, one-way Kruskal–Wallis and Mann–Whitney non-parametrical U-tests were more appropriate for the gonad index
Table 2
P. liÕidus. Kruskal–Wallis and Mann–Whitney U non-parametrical tests of the effects of temperature and
photoperiod on gonad water content, GI in dry weight and GI in fresh weight Water content
2
Photoperiod n Rank sum Mann–Whitney U-test P x approximately df
SD 20 376 234 0.358 0.846 1
LD 20 444 234 0.358 0.846 1
Ž .
Temperature 8C n Rank sum Kruskal–Wallis test P df
12 10 344 31.26 -0.001 3
Photoperiod n Rank sum Mann–Whitney U-test P x approximately df
SD 20 443 167 0.372 0.797 1
LD 20 377
Ž .
Temperature 8C n Rank sum Kruskal–Wallis test P df
12 10 56 30.069 -0.001 3
Photoperiod n Rank sum Mann–Whitney U-test P x approximately df
SD 20 406 204 0.914 0.012 1
LD 20 414 204 0.914 0.012 1
Ž .
Temperature 8C n Rank sum Kruskal–Wallis test P df
12 10 58 22.594 -0.001 3
16 10 275 22.594 -0.001 3
20 10 217 22.594 -0.001 3
Ž . 2 Ž .
GI . Watson’s U -test was used for the polar transformed values of MI Zar, 1996 . The factor we defined as ‘‘temperature’’ is in fact the combined effect of temperature
Ž .
and rearing structure. Indeed, the number of structures available four did not permit replicates. Thus, although the structures were identical and the seawater was the same, the effect of temperature cannot be technically dissociated from a possible effect of the structures.
3. Results
Ž
Observations of several cohorts of P. liÕidus specimens in culture i.e., follow-up of
.
gonad growth and MIs, unpublished data led us to the conclusion that gametogenesis Ž was continuous. In addition, the lack of variation in the external parameters i.e.,
.
absence of seasonality causes the growth phase to be by-passed as shown in Fig. 1. Consequently, the gonads are poor in stored nutrients. Finally, the individuals are not sexually synchronized.
Statistical analyses of the results are presented in Tables 1 and 2. The quantity of Ž food ingested during the experiment was homogeneous for the higher temperatures Fig.
.
2 . Only at the 128C treatments did the animals show significantly lower feeding. For the
Ž .
128C treatments, individuals exposed to the SD treatment winter photoperiod ate less
Ž . Ž .
than those exposed to LD summer photoperiod . For higher temperatures over 128C , ingestion was similar for all treatments.
Fig. 2. P. liÕidus. Food ingestion vs. temperature and photoperiod. Bold horizontal lines at the same level
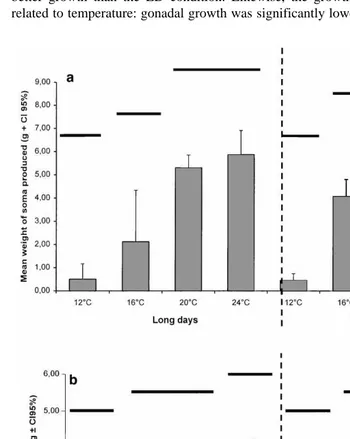
The results of somatic and gonadal growth are reported in Fig. 3. Somatic growth was
Ž .
very low at 128C and increased significantly with temperature Fig. 3a . The somatic growth was also affected by photoperiod: SD condition at 168C and at 248C gave a better growth than the LD condition. Likewise, the growth of gonads was positively related to temperature: gonadal growth was significantly lower at 128C and significantly
Ž . Ž .
Fig. 3. P. liÕidus. Mean values of the a somatic production and b gonadal production vs. temperature and
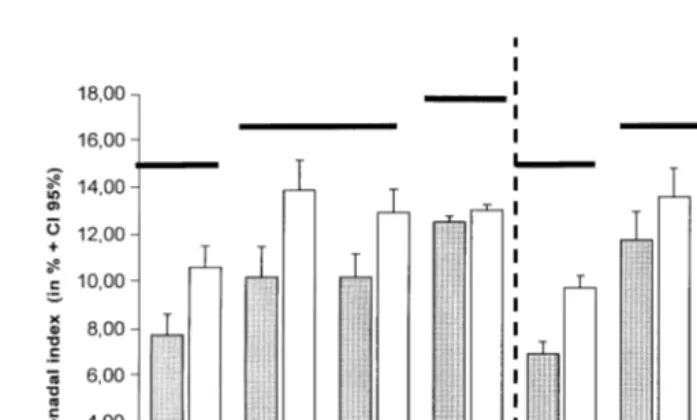
Fig. 4. P. liÕidus. Mean values of GI calculated in fresh and dry weight vs. temperature and photoperiod. Bold
horizontal lines at the same level mean no significant difference.
Ž .
higher at 248C with no difference between 168C and 208C Fig. 3b . Difference between photoperiod regimes was significant only at 248C: gonadal production was more
Ž
important for the SD treatment. Fig. 4 presents final values of GI dry weight GI-DW .
and fresh weight GI-FW . The GI was significantly lower at 128C and higher at 248C, while 168C and 208C were equivalent for both fresh and dry weight measurements. For the SD-248C treatment, the GI-DW was unexpectedly superior to the GI-FW. This result
Ž .
led us to assess the mean water content of the gonads expressed in percent and allowed us to note an effective change in that parameter depending upon treatments. The data are reported in Table 3. Water content and temperature were inversely related: water content was low when temperature was high. The initial group, measured after the starving and
Table 3
P. liÕidus. Mean values of MI and water content of the gonad in percent, all shown with the confidence
interval value at 95%
Ž . Ž .
Photoperiod Temperature 8C MI Water content %
Initial values 1.4"0.2 75.1"2.1
before the experiment, had about 75"2% water which was significantly higher than the
Ž .
values obtained after treatment except 128C . Although some relationship must exist between the maturity stage and the water content of the gonads, this does not explain the unusual low moisture percentage obtained at 248C. This was much lower than the range obtained for wild individuals with all maturity stages pooled.
Ž . Ž .
Concerning sexual maturity, no stage 6 fully mature or stage 8 post-spawned were observed, suggesting the individuals did not reach full maturation during the experiment.
Ž 2 .
All treatments were significantly different from the initial one Watson U , Us0.225 ,
Ž .
meaning that every batch had gone forward in the reproductive process Fig. 5 . This Ž .
difference was marked by 1 gonads filled with reserve material, represented by a heavy Ž .
network of nutritive phagocytes; and 2 the presence of some or many sexual cells. Ž
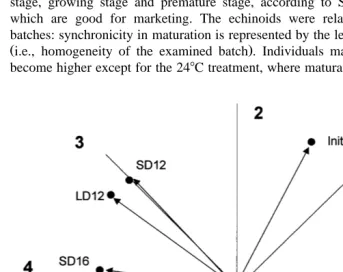
Basically, all sea urchins had reached a maturity stage, going from 3 to 5 recovering . stage, growing stage and premature stage, according to Spirlet et al., 1998a , all of which are good for marketing. The echinoids were relatively in phase among the batches: synchronicity in maturation is represented by the length of the vectors in Fig. 5 Ži.e., homogeneity of the examined batch . Individuals matured faster as temperature. become higher except for the 248C treatment, where maturation speed was equivalent to
Fig. 5. P. liÕidus. Circular representation of the MI after polar transformation of the data. The vector
Ž . Ž .
characteristics represent the mean value of the MI direction and the homogeneity of the values length . LD and SD are long and short day, respectively. The numbers 12, 16, 20 and 24 are8C temperature. Lines 1 to 8
Ž .
Ž 2 .
that observed at 168C Watson U -test, Us0.225 . There was no significant difference between SD and LD photoperiod treatments, although maturation was faster at 128C and 168C for the LD treatment.
4. Discussion
Populations of P. liÕidus in a land-based cultivation system lack the regular annual
Ž .
reproductive cycle that they have in the field Spirlet et al., 1998a . This phenomenon
Ž .
has already been observed by Leahy et al. 1981 : initially, synchronous populations of
Strongylocentrotus purpuratus eventually drift apart in phase, within a period of 9 to 12
months, when held in a maintenance system in the absence of seasonal environmental stimuli. Different stages of the reproductive cycle are present at any one time, but there
Ž . Ž
is no well-defined spent phase empty gonads or growing phase gonads filling with
. Ž .
reserve material . Leahy et al. 1981 experimented with two possible ways to drive Ž .
asynchronous individuals into a phased condition: 1 spawn the echinoids repeatedly at Ž .
1–2 months intervals until all late gametes are exhausted; 2 force a population of females into a phased state where they are gravid and contain large reserves of vitellogenic eggs by withholding spawning for one or two periods of 6 months or more.
Ž . Ž
These methods are too long. Knowing 1 that the gonads are storage organs see . Ž .
Fernandez, 1996 for P. liÕidus notably , 2 that the individuals need to be sexually in
Ž . Ž .
phase, 3 that the desired stage of the reproductive cycle is the growing stage, and 4 that productivity implies the shortest delay possible, starvation seemed to be the most effective synchronizing method. Our study shows that after 2 months of starvation,
Ž . Ž
gonads are in the spent stage see Spirlet et al., 1998a , with a very low GI -2% in dry .
weight . The virtual absence of mortality and the absence of care needed during starvation make the method very attractive. After starvation, the gonads grow and mature in synchronicity, whatever the treatment.
Ž
Gonadal production is usually evaluated from the GI see Byrne, 1990; Lawrence et al., 1991; Urgorri et al., 1994; Guettaf and San Martin, 1995; Lozano et al., 1995;
.
Fernandez, 1996 for P. liÕidus . Thus, gonadal growth cannot be dissociated from
somatic growth. If two individuals produce the same mass of gonad and different masses
Ž .
of soma, the one with the largest overall growth gonadal and somatic will present the lowest GI and will be regarded as less productive. With initial sexual synchrony, similar size and GI, the differences in final GI express allocation of resources: the gonads Žhigher GI or the soma lower GI . The gonad retrieval rate method calculated as the. Ž . Ž
.
slope of the regression of gonad weight against total weight is another useful method
Ž .
used by Byrne et al. 1998 . Nonetheless, the quality of the gonads is best described with a combination of measures including GI, growth and MI. Variation of the water content of the gonads appears to be a complementary indicator of gonad quality.
For P. liÕidus in land-based cultivation system, photoperiod has less influence than
temperature on the reproductive cycle and growth. Although, some effect was detected on somatic growth, temperature seems to be the key factor in the metabolism and reproductive cycle of P. liÕidus in culture.
Ž .
affect the digestion efficiency andror the nutrient conversion process. This means that,
Ž .
in culture, the food conversion efficiency is low at low temperature. Klinger et al. 1986 showed for LytechinusÕariegatus that for the same feeding rate, the sea urchin was less
Ž
efficient at processing food at 168C than at 238C. Several authors notably Lawrence et .
al., 1991 and Fernandez, 1996 for P. liÕidus have demonstrated the correlation between
gonadal growth, development and food intake.
Surprisingly, the most important growth was obtained with the 248C-SDs treatment,
Ž .
an unnatural condition. Ulbricht and Pritchard 1972 stated that intertidal species, exposed to important temperature changes, present a ‘‘constant’’ metabolism while subtidal species, exposed to constant low temperature, are much more sensitive to
Ž .
temperature variation. McBride et al. 1997 found gonadal production of S.
francis-canus to be independent of temperature and explained that increased catabolism at
higher temperature balanced the increase of food intake. Conversely, in our experiment, 248C could possibly correspond to the peak of growth. Our results are in disagreement
Ž .
with those of Le Gall et al. 1990 who found that the peak of somatic growth was reached between 208C and 228C, and then decreasing abruptly until total mortality at 298C.
In echinoids, photoperiod has been shown to influence mainly the gametogenic cycle ŽMcClintock and Watts, 1990; Pearse and Cameron, 1991 . This factor had never been. tested as a potential somatic and gonadal growth enhancer, combined or not with other factors. Previous studies proved its ability to shift a seasonal gametogenic cycle in some
Ž . Ž
species Walker and Lesser, 1998 and thus to produce marketable gonads i.e., in the .
growing or premature stages with a long-term exposure to inverted natural photoperiod. In the present case, it does not have a significant influence on growth except at high
Ž .
temperature 248C . One must keep in mind that longer treatment might change this conclusion.
The fact that sea urchins generate soma and gonads separately is widely documented. Resource allocation goes to maintenance, then to the digestive tract, a first stage in
Ž .
stocking nutrients Lawrence et al., 1991; Fernandez, 1996 , finally to the gonads, where the nutrients accumulate in the scope of producing gametes and insure reproduction ŽPearse and Cameron, 1991 . At 12. 8C, somatic production is negligible while some
Ž .
gonads are produced. In only 45 days a relatively short time for echinoids , all batches with the exception of the low temperature treatment showed notable growth of both soma and gonads. The absence of intermediate measurements makes it impossible to know if the compartments’ growth occurred successively or simultaneously. However,
Ž .
the total somatic growth grossly between 10% and 25% per batch is substantial. Hence, it is reasonable to consider that somatic growth was progressive, along with the
Ž
growth of gonads, perhaps after a short period of recovery after starvation Bishop and
. Ž .
Watts, 1992 . The GI increase is also remarkable see Fig. 4 , especially when compared to wild populations in Brittany, for which GI measurements in the field do not exceed
Ž .
8% see Spirlet et al., 1998a .
As previously mentioned, we have no indication that soma and gonads grow separately in time. The reason why a SD treatment gives better growth results is not easily interpretable. Many field observations showed that echinoids in temperate climate
Ž
.
liÕidus , when the days are short. However, feeding is more important then and growth
is proportionate. In our experiment, with equivalent amounts of food ingested, photope-riod had a direct effect on growth. To our knowledge, this has not yet been reported.
Ž Temperature had an important influence on the composition of the gonad water
.
content . Moreover, gonad water content decreased with increasing temperature. Conse-quently, the SD-248C combination gave the best results in terms of growth, and also the richest gonads, in nutritional terms, as they contained only about 63% water. This compares to the initial batch, which contained about 75% water, and to field specimens
Ž .
for which the mean values were between 70% and 80% personal observation . Many studies have shown that temperature influences and regulates gametogenesis in
Ž .
most echinoids living in temperate climate see Pearse and Cameron, 1991 for a review .
Ž .
In field populations, Spirlet et al. 1998a suggested that temperature acted as an enhancer of the gametogenic process but probably not as a trigger signal. This observation is confirmed here: the rate of gametogenesis increases with temperature until it reaches 208C. In the Northern Atlantic coasts, as described by several authors ŽByrne, 1990; Spirlet et al., 1998a , spawning takes place in late Spring and Summer. when temperature reaches between 168C and 208C. This range must be optimal for fertilization and favourable for survival and development of the larvae. At 248C, we observed fleshier but less mature gonads, meaning that high temperature can inhibit
Ž .
andror interrupt the gametogenic process. Cochran and Engelmann 1975 have shown that S. purpuratus loses the ability to spawn when temperature exceeds 178C and that reproductive activity is turned off by elevated temperature. Gametogenesis in some other
Ž
echinoids Pseudocentrotus depressus, Yamamoto et al., 1988; Anthocidaris
cras-.
sispina and Hemicentrotus pulcherrimus, Sakairi et al. 1989 , is triggered by a drop in
temperature. It does not occur if the temperature is either constant or high. It would be reasonable to consider that high temperature represents a stress and is unfavourable for the gametogenic process, and that the echinoids accumulate nutrients because these are not converted into gametes.
Acknowledgements
We are grateful to J.M. Lawrence who supplied the artificial food. This research has Ž
been supported by a EC research grant attributed to C. Spirlet ref. ERB 4001 GT92
. Ž
0223 , in the framework of the Sea Urchin Cultivation contract no. AQ 2.530 BFE EC .
‘‘FAR’’ Research Program . This paper is a contribution to the Centre Interuniversitaire
Ž .
de Biologie Marine CIBIM .
References
Agatsuma, Y., Momma, H., 1988. Release of cultured seeds of the sea urchin Strongylocentrotus intermedius
ŽA. Agassiz , in the Pacific coastal waters of southern Hokkaido: I. Growth and reproductive cycle. Sci..
Rep. Hokkaido Fish. Exp. Stn. 31, 15–25.
Bishop, C.D., Watts, S.A., 1992. Biochemical and morphometric study of growth in the stomach and intestine
Ž .
Byrne, M., 1990. Annual reproductive cycles of the commercial sea urchin Paracentrotus liÕidus from an
exposed intertidal and a sheltered subtidal habitat on the west coast of Ireland. Mar. Biol. 104, 275–289. Byrne, M., Andrew, N.L., Worthington, D.G., Brett, P.A., 1998. Reproduction in the diatematoid sea urchin
Centrostephanus rodgersii in contrasting habitats along the coast of New South Wales, Australia. Mar.
Biol. 132, 305–318.
Cochran, R.C., Engelmann, F., 1975. Environmental regulation of the annual reproductive season of
Ž .
Strongylocentrotus purpuratus Stimpson . Biol. Bull. 148, 393–401.
Conand, C., Sloan, N.A., 1989. World fisheries for echinoderms. Mar. Invertebr. Fish., 647–663.
Fernandez, C., 1996. Croissance et nutrition de Paracentrotus liÕidus dans le cadre d’un projet aquacole avec
alimentation artificielle. These de doctorat, Universite de Corse, 278 pp.` ´
Fernandez, C., Caltagirone, A., 1994. Growth rate of adult sea urchins Paracentrotus liÕidus in a lagoon
Ž .
environment: the effect of different diet types. In: David, Guille, Feral, Roux Eds. , Echinoderms Through´
Time. Balkema, Rotterdam, pp. 655–660.
Gomez, J.L.C., Tallon, J.G.M., Rodriguez, L.G.M., 1995. Experiments of sowing juveniles of Paracentrotus
Ž . Ž .
liÕidus Lamarck in the natural environment. In: Emson, Smith, Campbell Eds. , Echinoderm Research
1995. Balkema, Rotterdam, pp. 255–258.
Grosjean, P., Spirlet, C., Gosselin, P., Vaitilingon, D., Jangoux, M., 1998. Land-based closed cycle
Ž .
echiniculture of Paracentrotus liÕidus Lamarck Echinodermata: Echinoidea : a long term experiment at a
pilot scale. J. Shellfish Res. 17, 1523–1531.
Grosjean, P., Spirlet, C., Jangoux, M., 1996. Experimental study of growth in the echinoid Paracentrotus
Ž . Ž .
liÕidus Lamarck, 1816 Echinodermata . J. Exp. Mar. Biol. Ecol. 201, 173–184.
Grosjean, P., Spirlet, C., Jangoux, M., 1999. Comparison of three body-size measurements for echinoids. In:
Ž .
Candia Carnevali, M.D., Bonasoro, F. Eds. , Proceedings of the 5th European Echinoderm Conference. Balkema, Rotterdam, pp. 31–35.
Guettaf, M., San Martin, G.A., 1995. Etude de la variabilite de l’indice gonadique de l’oursin comestible´
Ž .
Paracentrotus liÕidus Echinodermata: Echinoidea en Mediterranee nord-occidentale. Vie Milieu 45,´ ´
129–137.
Jangoux, M., Larsonneur, C., Catoira Gomez, M., 1996. Sea urchin cultivation, final report of contract no. AQ 2.530 BFE. FAR research program of the ECC, 96 pp.
Kelly, M.S., McKenzie, J.D., Brodie, C.C., 1998. Sea urchins in polyculture: the way to enhanced gonad
Ž .
growth?. In: Mooi, R., Telford, M. Eds. , Echinoderms: San Francisco. Balkema, Rotterdam, pp. 707–711.
Klinger, T.S., Hsieh, H.L., Pangallo, R.A., Chen, C.P., Lawrence, J.M., 1986. The effect of temperature on feeding, digestion, and absorption of LytechinusÕariegatus. Physiol. Zool. 59, 332–336.
Klinger, T.S., Lawrence, J.M., Lawrence, A.L., 1998. Digestion, absorption and assimilation of prepared feeds
Ž .
by echinoids. In: Mooi, R., Telford, M. Eds. , Echinoderms: San Francisco. Balkema, Rotterdam, pp. 713–721.
Ž .
Lawrence, J.M., 1985. The energetic echinoderm. In: Keegan, B.F., O’Connor, B.D.S. Eds. , Echinodermata. Balkema, Rotterdam, pp. 47–67.
Lawrence, J.M., Fenaux, L., Corre, M.C., Lawrence, A., 1991. The effect of quantity and quality of prepared
Ž .
diets on production in Paracentrotus liÕidus Echinodermata: Echinoidea . In: Scalera-Liaci, L., Canicatti,
Ž .
C. Eds. , Echinoderm Research. Balkema, Rotterdam, pp. 107–110.
Leahy, P.S., Hough-Evans, B.R., Britten, R.J., Davidson, E.H., 1981. Synchrony of oogenesis in laboratory
Ž .
maintained and wild populations of the purple sea urchin Strongylocentrotus purpuratus . J. Exp. Zool. 215, 7–22.
Ž .
Le Gall, P., 1990. Culture of echinoderms. In: Barnabe, G. Ed. , Aquaculture 1 Ellis Horwood, New York, pp. 443–462.
Le Gall, P., Bucaille, D., 1989. Sea urchins production by inland farming. In: De Pauw, N., Jaspers, E.,
Ž .
Ackerfors, H., Wilkins, N. Eds. , Aquaculture — a biotechnology in progress. European Aquaculture Society, Breden, Belgium, pp. 53–59.
Le Gall, P., Bucaille, D., Grassin, J.B., 1990. Influence de la temperature sur la croissance de deux oursins´
comestibles Paracentrotus liÕidus et Psammechinus granularis. In: De Ridder, C., Dubois, P., Lahaye,
Ž .
M.C., Jangoux, M. Eds. , Echinoderm Research. Balkema, Rotterdam, pp. 183–187.
Ž .
of Paracentrotus liÕidus Echinodermata: Echinoidea in two contrasting habitats. Mar. Biol. Prog. Ser.
122, 179–191.
McBride, S.C., Pinnix, W.D., Lawrence, J.M., Lawrence, A.L., Mulligan, T.M., 1997. The effect of temperature on production of gonads by the sea urchin Strongylocentrotus franciscanus fed natural and prepared diets. J. World Aquacult. Soc. 28, 357–365.
McClintock, J.B., Watts, S.A., 1990. The effects of photoperiod on gametogenesis in the tropical sea urchin
Ž . Ž .
Eudaris tribuloides Lamarck Echinodermata: Echinoidea . J. Exp. Mar. Biol. Ecol. 139, 175–184. Pearse, J.S., Cameron, R.A., 1991. Echinodermata: Echinoidea. In: Reproduction of marine invertebrates.
Ž .
Giese, A.C., Pearse, J.S., Pearse, V.B. Eds. , Echinoderms and Lophophorates VI Boxwood Press, pp. 513–662.
Pearse, J.S., Pearse, V.B., Davis, K.K., 1986. Photoperiodic regulation of gametogenesis and growth in the sea urchin Strongylocentrotus purpuratus. J. Exp. Zool. 237, 107–118.
Robinson, S.M.C., Colborne, L., 1998. Roe enhancement trials of the green sea urchin using an artificial food
Ž .
source. In: Mooi, R., Telford, M. Eds. , Echinoderms: San Francisco. Balkema, Rotterdam, p. 803. Sakairi, K., Yamamoto, M., Ohtsu, K., Yoshida, M., 1989. Environmental control of gonadal maturation in
laboratory reared sea urchins: Anthocidaris crassispina and Hemicentrotus pulcherrimus. Zool. Sci. 6, 721–730.
Spirlet, C., Grosjean, P., Jangoux, M., 1998a. Reproductive cycle of the echinoid Paracentrotus liÕidus:
analysis by means of the maturity index. Invertebr. Reprod. Dev. 34, 69–81.
Spirlet, C., Grosjean, P., Jangoux, M., 1998b. Optimizing food distribution in closed-circuit cultivation of
Ž .
edible sea-urchins Paracentrotus liÕidus: Echinoidea . Aquat. Living Resour. 11, 273–277.
Turon, X., Giribet, G., Lopez, S., Palacin, C., 1995. Growth and population structure of Paracentrotus li´ Õidus
ŽEchinodermata: Echinoidea in two contrasting habitats. Mar. Ecol. Prog. Ser. 122, 193–204..
Ulbricht, R.J., Pritchard, A.W., 1972. Effect of temperature on the metabolic rate of sea urchin. Biol. Bull.
Ž .
Mar. Biol. Lab. Woods Hole 142, 178–185.
Urgorri, V., Reborada, P., Troncoso, J.S., 1994. Dispersion, demografia y produccion gonadal de una´ ´
Ž .
poblacion de Paracentrotus li´ Õidus Lamarck, 1816 . Memoria final de resultados del P.I. FEUGA,
University Santiago de Compostela.
Walker, C.W., Lesser, M.P., 1998. Manipulation of food and photoperiod promotes out-of-season gametogene-sis in the green sea urchin Strongylocentrotus droebachiengametogene-sis: implication for aquaculture. Mar. Biol. 132, 663–676.
Yamamoto, M., Ishine, M., Yoshida, M., 1988. Gonadal maturation independent of photic conditions in laboratory reared sea urchins, Pseudocentrotus depressus and Hemicentrotus pulcherrimus. Zool. Sci. 5, 979–988.