Summary Acer rubrum L., A. saccharum Marsh., Quercus alba L. and Q. rubra L. seedlings subjected to soil and stem base heat treatments showed rapid declines in rates of transpi-ration and photosynthesis. Reductions in photosynthetic rate were partly attributable to mesophyll inhibition. Quercus seed-lings were less able to maintain transpiration and photosynthe-sis after heat treatment than Acer seedlings. Declines in rates of transpiration and photosynthesis of Quercus seedlings were observed 1 h after heat treatment and became more pronounced over time. In contrast, rates of transpiration and photosynthesis of Acer seedlings initially declined in response to heat treat-ment, partially recovered after one or two days, but then declined again six to eight days after the heat treatment. Ob-served changes in leaf water potential after heating were small, suggesting that hydraulic factors were not the primary signal eliciting the gas exchange response to soil and stem heating.
Ultimately, the heat treatments caused stem die-back of most seedlings. For all species, seedlings that resprouted had a greater chance of surviving heat stress than seedlings that did not resprout. Despite the rapid loss of photosynthetic capacity in response to heat treatment in Quercus seedlings, survival was higher in Quercus seedlings than in Acer seedlings, and was associated with a greater capacity for resprouting. We suggest that the reduced allocation of resources toward recov-ery of photosynthesis in existing Quercus stems after heat stress is a physiological mechanism that facilitates resprouting and hence survival of Quercus seedlings after fire.
Keywords: Acer saccharum, Acer rubrum, fire, photosynthesis, Quercus alba, Quercus rubra, resprouting, survival, thermo-tolerance, transpiration.
Introduction
In forest ecosystems, mortality caused by fire is selective against many invading mesic species, suggesting that fire might be used as a tool to control species composition and diversity (Curtis 1959, Crow 1988). Fire is an important factor in the establishment and maintenance of many oak-dominated forests in the eastern USA, forests that otherwise would tend to be replaced by more mesic, fire-sensitive species such as red
maple and sugar maple (Acer rubrum L., A. saccharum Marsh.) (Carvell and Tryon 1951, Lorimer 1989, Abrams and Downs 1990, Nowacki et al. 1990, Johnson 1992). A better understanding of the physiological responses of species to fire would allow fire prescriptions (e.g., season, weather condi-tions during burning) to be adjusted to favor establishment of desired species.
Reich et al. (1990) found that photosynthesis increased several weeks after burning in surviving black cherry (Prunus serotina Ehrh.), northern pin oak (Quercusellipsoidalis E.J. Hill) and blackberry (Rubus allegheniensis Porter) plants, but not in A. rubrum. Long-term increases in insolation and avail-able nutrients after fire may alter physiological processes such as photosynthesis (Ahlgren and Ahlgren 1960, Viro 1974, Reich et al. 1990) that may be associated with mortality or survival soon after fire. In one of the few studies on short-term physiological responses of species to fire, Van Sambeek and Pickard (1976a, 1976b) showed that scorching of leaves of herbaceous species by fire was followed by rapid changes in gas exchange of unheated leaves.
In an attempt to elucidate the mechanisms underlying the physiological responses to fire of species that are differentially tolerant of fire in natural ecosystems, we studied the effects on gas exchange and survival of briefly heating surface roots and stem bases of seedlings of red maple, sugar maple, white oak (Quercus alba L.) and northern red oak (Quercus rubra L.).
Materials and methods
Seedling culture
Seeds of Q. alba, Q. rubra, A. rubrum, and A. saccharum were collected from the Columbia area. Acorns and sugar maple seed were collected in the fall of 1992, and red maple seed was collected in the spring of 1991. Purchased red maple seed (F.W. Schumacher Co., Inc., Sandwich, MA) was used in the spring of 1993. Seeds were stratified and germinated according to standard protocols (USDA Forest Service 1974).
Germinants were planted in a greenhouse in cylindrical 5.6-liter pots (15.3 cm diameter, 30.5 cm tall) filled with a 1/2/1 (v/v) mixture of peat moss, sand and silt loam. After
Effects of soil and stem base heating on survival, resprouting and gas
exchange of
Acer
and
Quercus
seedlings
JULIE A. HUDDLE
1,2and STEPHEN G. PALLARDY
11
School of Natural Resources, University of Missouri, Columbia, MO 65211, USA
2 Present address: Department of Rangeland Ecology and Management, Texas A&M University, College Station, TX 77843, USA
Received June 6, 1995
planting, 30 ml of slow-release 14,14,14 N,P,K fertilizer (Os-mocote, San Milpitas, CA) was placed on the soil surface and 30 ml of vermiculite layered on top to retard evaporation. Pots were watered once a week, and fertilized weekly with modi-fied Hoagland’s solution (Johnson et al. 1957). The tempera-ture of the greenhouse ranged between 10 and 43 °C during the study. Sunlight supplemented with sodium vapor lamps kept midday irradiances at seedling tops at approximately 1000 µmol m−2 s−1 photosynthetic photon flux density (PPFD).
Growth chamber conditions and heating treatments
Before heat treatment, seedlings were moved from the green-house to a growth chamber and allowed to acclimate for at least three days. The growth chamber provided a temperature of 25 °C and a 14-h photoperiod (400 µmol m−2 s−1 PPFD at the top of the seedlings). Mean age at treatment ranged from 118 days for A. saccharum seedlings to 133 days for Q. alba seedlings, with no significant differences among species (P ≤ 0.05).
To simulate the heat of a passing ground fire, one of two aluminum block heaters, one with a plate containing a 1.0 cm slot and a 1.25 cm diameter hole, the other with a plate containing a 2.0 cm slot and a 4.0 cm diameter hole, was used (Figure 1). The block heater with the larger slot width and central ring diameter was fabricated to reduce the number of seedlings for which data had to be discarded because of inad-vertent direct contact between the plate and stem. Two ceramic cartridge heaters (Type 500B, Standard Electric Products Co., Dayton, OH) placed in holes drilled in the block provided
heating. Before the heat treatment was begun, the top of the seedling was enclosed in a 2.54-liter water-cooled cuvette with PPFD maintained at 2000 µmol m−2 s−1 with a multi-vapor lamp (General Electric, Hendersonville, NC). Seedlings were not removed from the cuvette until at least 60 min after apply-ing heat.
After gas exchange had equilibrated, power (80 V, 7.5 amps) was applied to the heaters and the heated block was then placed on the soil. Mean duration of heat treatment ranged from 9.7 to 10.5 min with no significant differences among species. Among species, mean maximum stem base temperature during treatments ranged from 76.6 to 85.8 °C, with no significant differences among species. Among individual seedlings, Tstem ranged from 33 to 180 °C, and appeared to be dependent on small differences in placement of the plate. Although there was substantial variation in stem base temperature, it was not unlike what might be expected in a natural surface fire event. Nearly every heat treatment elicited a gas exchange response, and variation in stem base temperature did not obscure species differences in survival, resprouting or gas exchange responses. Complete sets of gas exchange data were available for between 15 and 20 seedlings per species.
Temperatures at the base of the seedling stem and at three soil depths in the pot were monitored throughout the gas exchange measurements with copper-constantan thermocou-ples (Figure 2). Holes drilled on one side of the pot allowed thermocouples to be inserted at several soil depths (Figure 1). Leaf temperature and air temperature inside the cuvette were also monitored.
Gas exchange measurements
Photosynthesis at 33--35 Pa ambient CO2 partial pressure and transpiration were monitored with an open gas exchange sys-tem described by Ni and Pallardy (1991). Instrument outputs were acquired every 10 s by a computerized data logger. Photosynthesis (A, µmol m−2 s−1), transpiration (E, mmol m−2 s−1) and internal partial pressure of CO
2 (Ci, Pa) were
com-Figure 1. Three-dimensional view of a heating plate and seedling. Wires indicate the point at which two ceramic cartridge heaters were inserted in the heating plate. Note sections of each pot were cut away to facilitate placement of the heating plate and holes were drilled in the side of each pot to provide for placement of thermocouples at several soil depths. The top of the seedling was enclosed in a gas exchange cuvette during the heating treatment.
Figure 2. Examples of temperatures during heating treatments. Sym-bols designate where temperatures were measured: (for Q. rubra 1) m
at stem base, h at 0.5 cm soil depth, , at 2.0 cm soil depth, n at
puted using equations of von Caemmerer and Farquhar (1981). Photosynthesis and E of the heat-treated seedlings were moni-tored for several days after heating during which time seed-lings were watered frequently. Because the pretreatment gas exchange rates varied among seedlings, comparisons were based on ratios of post-treatment to pretreatment values.
Water potential measurements
During the gas exchange measurements, the leaf water poten-tial (Ψleaf) of between six and eleven seedlings per species was measured every 4 to 6 min with two leaf hygrometers (Model L-51, Wescor, INC., Logan, UT) attached to leaves inside the cuvette. The hygrometers were calibrated with KCl solutions of known molality as described by Pallardy et al. (1991).
Assessment of survival and resprouting
Two weeks after heating, seedlings were moved from the growth chamber back to the greenhouse. Watering and fertili-zation were conducted every week as described previously. Resprouting and survival of all heat-treated seedlings (22--25 seedlings per species) were monitored for 12 weeks in the greenhouse.
Statistical analysis
Chi-square tests were conducted to detect statistically signifi-cant differences among species in survival and resprouting (Snedecor and Cochran, 1980). A chi-square test was also used to detect statistically significant differences in survival be-tween resprouting and non-resprouting seedlings.
Although soil temperatures increased in response to the heat treatment, it was not possible to compare results across seed-lings because of variations associated with measurement depth and technical difficulties. Therefore, the maximum tempera-ture measured at the base of the stem (Tstem) was used as a measure of temperature experienced by seedlings.
The statistical tests used to determine the significance of gas exchange responses after heating were based on 95% tolerance limits calculated for 1-min averages of gas exchange parame-ters (A, E, and Ci) measured before treatments were applied (Hald, 1952). Similarly, the 95% tolerance limits of gas ex-change responses 1 h after heat treatment were calculated from
1-min averages between 55 and 65 min after the heat treatment was applied for each seedling. Responses 1 h after heating were scored as 0, if the tolerance limits overlapped, or as 1 if the tolerance limits did not overlap.
Short-term response scores (RS = 0 or 1) were then averaged for each combination of species and three ranges of Tstem (45--75, 75--105 and 105--135 °C). These averages were arcsine transformed to normalize error as described by Box and Cox (1964). The General Linear Models procedure of the SAS version 6.08 software prgram (SAS Institute, Cary, NC) was used to conduct analysis of variance (ANOVA) and to calculate least square means with species and Tstem as treat-ment effects.
To analyze the magnitude of the short-term gas exchange response, percent change in each parameter was calculated for each seedling as follows:
%∆Gs= G1 −G0
G0
100,
where %∆Gs is the short-term percent change in a gas ex-change parameter, G1 is the mean of the parameter 1 h after treatment and G0 is the mean of the parameter before heat was applied. Percent changes in gas exchange were then arcsine transformed to normalize error and analyzed by ANOVA with species, response score 1 h after treatment (RS1 h) and the interaction between species and response score as treatment effects. Calculated least square means were subjected to the t-test to determine whether they were significantly different from zero.
Long-term changes in gas exchange parameters (%∆Gl) were calculated and analyzed as describd for the short-term changes. Data were pooled in two categories (1--2 days after treatment, Dj= 1; and 6--8 days after treatment, Dj= 7) and compared with pretreatment values:
%∆G1= Gj −G0
G0
100,
where Gjis the mean of the gas exchange parameter on day j (Dj) after treatment and G0 is the mean of the gas exchange
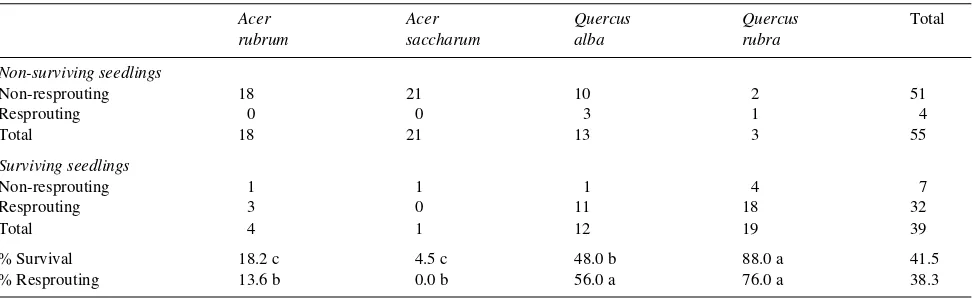
Table 1. Survival and resprouting frequency of each species. Percentages in a row with different letters are significantly different (P≤ 0.05).
Acer Acer Quercus Quercus Total
rubrum saccharum alba rubra
Non-surviving seedlings
Non-resprouting 18 21 10 2 51
Resprouting 0 0 3 1 4
Total 18 21 13 3 55
Surviving seedlings
Non-resprouting 1 1 1 4 7
Resprouting 3 0 11 18 32
Total 4 1 12 19 39
% Survival 18.2 c 4.5 c 48.0 b 88.0 a 41.5
parameter before treatment. These percentages were trans-formed and analyzed by ANOVA with species, Dj and the interaction between species and Djas treatment effects. Each least square mean was tested to detect whether it was signifi-cantly different from zero and back transformed to a percent-age.
Results
Survival and resprouting
Survival among resprouting seedlings was significantly greater (P ≤ 0.05) than among non-resprouting seedlings (88.9 versus 12.1%). Both Quercus species had significantly greater survival and resprouting rates than either Acer species (Ta-ble 1), although only the difference in survival was statistically significant. For all species, mean Tstem did not differ (P ≤ 0.05) between non-surviving, non-resprouting seedlings (82.4 °C) and surviving, resprouting seedlings (87.7 °C).
Short-term gas exchange dynamics
At least 60% of all seedlings showed significant short-term responses (i.e., RS = 1) in Ci, E and A to the heat treatment (Table 2). Although the fraction of seedlings showing signifi-cant changes in Ci and E following heating increased with increasing Tstem, the trend was not significant (P ≤ 0.15). In contrast, the fraction of seedlings showing significant change in A following heat treatment increased significantly (P ≤ 0.01) with increasing Tstem (Table 2).
Heat-induced declines in A and E were usually apparent within 1 h of heat treatment (cf. Figures 3 and 4). Averaged across all species, seedlings showed a mean decline of 23.7% in A 1 h after the heat treatment. Gas exchange of seedlings of both Quercus species generally showed a greater response to
heat treatment than Acer seedlings. For all species, the short-term percent change in photosynthetic rate, %∆As, declined significantly only for seedlings with an RS = 1 (Table 3). Of seedlings with an RS = 1, the relative reduction in A of A. rubrum seedlings was significantly less than that of either of the Quercus species. Internal CO2 partial pressure usually increased, at least transiently, after heat application, except in a few A. rubrum seedlings. For all seedlings, average %∆Ci,s 1 h after heat treatment was 1.4%. Mean %∆Ci,s increased for all combinations of species and response scores after heat treatment (Table 3).
Because the standard error of E increased between the
pre-Table 2. Mean percent of seedlings showing significant responses (i.e., a response score of 1) in Ci, E and A by species and Tstem. Means by
species within a column with different lower case letters are signifi-cantly different (P≤ 0.05); means by Tstem within a column with
different upper case letters are significantly different (P≤ 0.05). All means differed significantly from zero (P ≤ 0.05). P-values of models used to calculate means given on the right.
Ci E A
Species
Acer rubrum 67.4 a 78.7 ab 83.7 ab
Acer saccharum 61.2 a 64.2 b 72.3 b
Quercus alba 86.3 a 95.8 a 95.3 a
Quercus rubra 64.2 a 64.2 b 82.1 ab
Tstem
45 °C 56.7 A 62.2 A 45.2 C 75--105 °C 73.0 A 86.6 A 86.6 B 105--115 °C 89.5 A 82.0 A 100.0 A
P-Values
Species 0.20 0.06 0.12
Tstem 0.15 0.12 <0.01
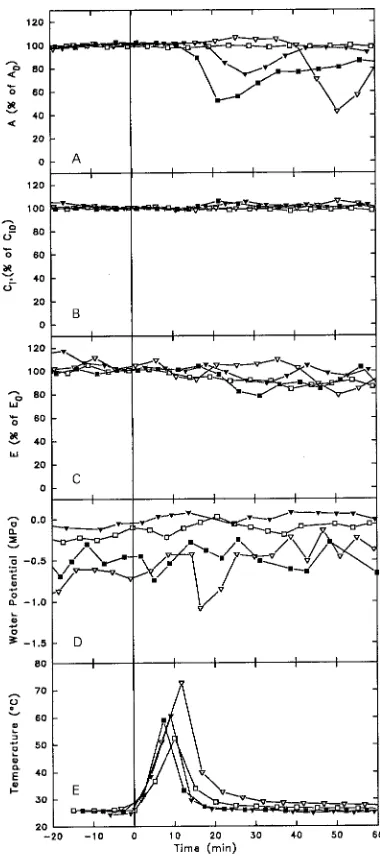
Figure 3. Gas exchange dynamics and maximum stem temperature (Tstem) of Acer seedlings after heat was applied (indicated by vertical
line). A: A expressed as %A0; B: Ci expressed as %Ci0; C: E expressed
as %E0; D: Ψ (MPa); and E: Tstem (°C). Symbols denote individual
treatment period and 1 h after heat treatment (data not shown), only one seedling had an RS = 1 in E. However, across all response score data mean %∆Es was significantly negative for all species (Table 3) and averaged −13.8% for all seedlings. Of the species tested, Q. alba seedlings showed the largest change in E 1 h after heat application. Although differences were not statistically significant, %∆Es of Q. alba seedlings declined more than that of either A. rubrum or A. saccharum seedlings. There were significant correlations (P ≤ 0.05) between Tstem and percent change in A, E and Ci at 1 h after heating. These correlations (negative with A and E, and positive with Ci) indicated a general trend (R2 = 0.18--0.33) toward an increasing
intensity of response with increasing Tstem.
Observed declines in Ψleaf an hour after heat treatment were never greater than −0.5 MPa, and Ψleaf generally drifted up-ward as E decreased (Figures 3 and 4).
Long-term dynamics in gas exchange
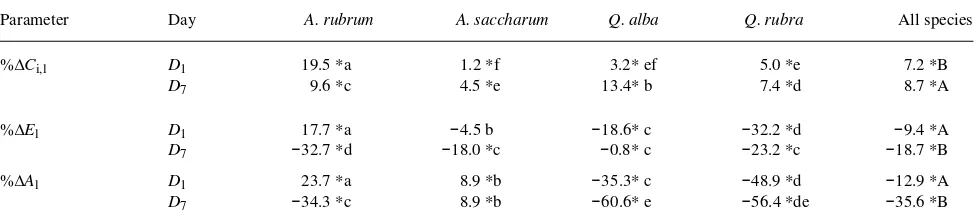
Except for %∆El of A. saccharum seedlings one and two days after heat treatment, all long-term changes of gas exchange parameters from pretreatment values were significantly differ-ent from zero (Table 4). For data pooled over all species, mean %∆Ci,l increased significantly between D1 and D7, whereas both mean %∆El and mean %∆Al decreased significantly over the same interval (Table 4).
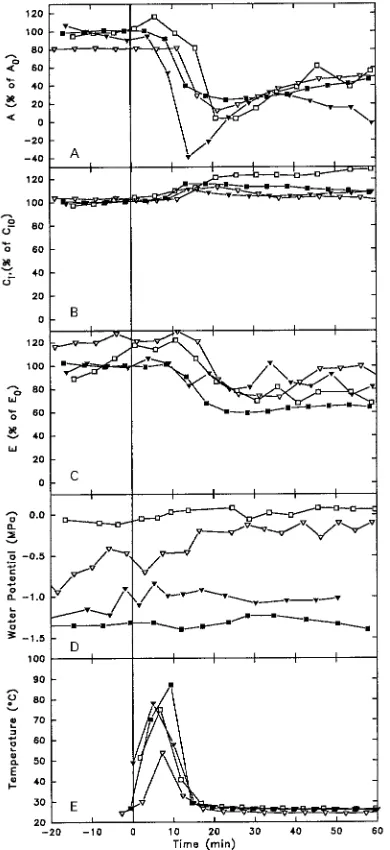
At time D1, seedlings of both Quercus species showed sig-nificant declines in both E and A from pretreatment values (Table 4). In contrast, E and A of Acer seedlings either rose or did not change significantly. Acer rubrum seedlings showed significantly greater relative declines in E than seedlings of all other species about a week after treatment, whereas at time D7 both Quercus species showed significantly greater declines in A from pretreatment values than did either Acer species.
Discussion
Soil and stem base heating caused shoot death, indicating that direct exposure of foliage or the upper stem to heat is not required to cause topkill in seedlings. Seedling survival after heat treatment varied significantly among species and the survival ranking after heat treatment was: Q. rubra > Q. alba > A. rubrum ≈ A. saccharum. Seedlings of both Quercus
Figure 4. Gas exchange dynamics and maximum stem temperature (Tstem) of Quercus seedlings after heat was applied (indicated by
vertical line). A: A expressed as %A0; B: Ci expressed as %Ci0; C: E
expressed as %E0; D: Ψ (MPa); and E: Tstem (°C). Symbols denote
individual seedlings: hQ. alba 107; ,Q. alba 172; j Q. rubra 43;
.Q. rubra 77.
Table 3. Mean short-term (1 h) percent change in Ci, E and A by
species and response score 1 h after treatment. For each combination of species and response score, means with different lower case arabic letters were significantly different; for species, means with different Greek letters were significantly different; for response score, means with different capital arabic letters were significantly different. Aster-isks indicate means significantly different from zero. Arithmetic means are given in parentheses for cells with insufficient numbers of seedlings to calculate reliable least square means. Dashes indicate that no seedlings occurred in a given cell.
Species Response %∆Ci,s %∆Es %∆As
score
Acer rubrum 0 0.03 b −8.3 *a 2.3 a 1 -- -- −16.9 *b Total (0.06) -- −1.7 β
Acersaccharum 0 0.6 b −7.7 *a 0.0 a 1 5.9 *ab -- −33.9 *bc Total 2.6 *α -- −8.6 *β
Quercus alba 0 0.4 b −23.2 *a −26.1 ab 1 10.0 *c -- −53.8 *c Total 3.6 α -- −38.9 *α
Quercus rubra 0 0.0 b −19.0 *a 1.9 a 1 17.0 *a −20.0 a −50.8 *c Total 4.3 *α -- −18.7 *αβ All species 0 0.1 −13.8 * −1.5 B
species showed significantly greater resprouting rates after heating than did seedlings of either Acer species, suggesting that the capacity of Quercus species to resprout after exposure to lethal stem temperatures enables them to survive fire better than Acer species (Huddle 1995).
Decreases in E and A and slight increases in Ci were ob-served after soil and stem base heating was applied, indicating that changes in leaf gas exchange can occur in response to application of heat to tissues remote from the leaf (Sinyukhin and Gorchakov 1966, Van Sambeek and Pickard 1976b). Hy-draulic signals may induce physiological responses in tissues not directly exposed to a variety of stresses, including heat (Malone 1993, Malone et al. 1994). One type of hydraulic signal that might be associated with thermal responses is a change in Ψ. If a hydraulic pulse associated with heating lowered Ψ of cells surrounding guard cells, the latter may lose water through passive water movement, resulting in stomatal closure (Malone 1993). Changes in Ψleaf observed in the pre-sent study do not support this hypothesis. The decreases in water potential never exceeded −0.5 MPa and were usually smaller, and Ψleaf values never declined to the range over which stomatal closure might be expected (Bahari et al. 1985, Ni and Pallardy 1991, 1992). Because Ψleaf remained relatively high after heating and did not drop more rapidly than E or A, we conclude that changes in Ψ were not associated with the heat-induced reductions in A.
An alternative hypothesis is that the declines in E following heat application are the result of a series of events triggered by propagation of action potentials that changes guard cell turgor and causes stomatal closure (Davies 1987, Davies et al. 1991). Studies with herbaceous plants have shown that if a stimulus such as heat, cutting or a series of electrical pulses is sufficient to cause a measurable action potential, a change in gas ex-change follows (Gunar and Sinyukhin 1963, Sinyukhin and Gorchakov 1968, Van Sambeek and Pickard 1976b, Dziu-biñska et al. 1989).
Besides lowering E, heat treatment reduced A. Because stomatal closure reduces availability of CO2, Ci should decline if stomatal closure is largely responsible for reduced A. How-ever, Ci of most seedlings increased after heat treatment,
sug-gesting that increased mesophyll resistance was at least partly responsible for the observed reductions in A.
On the days following heat treatment, seedlings of both Quercus species underwent a progressive and more substantial loss in photosynthetic capacity than seedlings of the Acer species, whereas we had expected that species more capable of surviving fire (i.e., Quercus) would maintain or regain physi-ological function more fully after heating than more poorly adapted species such as Acer. We postulate that the inverse relationship between photosynthetic capacity and survival fol-lowing heat treatment is related to the plant’s capacity to resprout. Our results indicate that the resprouting response, which was more vigorous in Quercus species than in Acer species, is an alternate and effective, but resource-intensive, adaptation to fire. Based on our results and the findings of other studies, we suggest that Quercus spp. respond to heat injury by: (1) abandoning heat-injured shoots; (2) promoting rapid compartmentalization of injured from healthy tissues by the production of a suberin layer (McDougall 1993); (3) promot-ing release from hormonal inhibition associated with apical dominance of living stems (Vogt and Cox, 1970); and (4) promoting reallocation of resources to the resprouting re-sponse.
Physiological and shoot mortality responses to even moder-ate heating were substantial, and thus many surface fires in natural forests could be expected to elicit similar responses in small individuals to those observed in this study. The more vigorous resprouting capacity of Quercus compared to Acer seedlings appears to be a key feature of the successful adapta-tion of Quercus species to fire. This difference could be ex-ploited to promote greater relative abundance of Quercus regeneration in deciduous forests of eastern North America.
Acknowledgments
Research was supported by the McIntire-Stennis Program. The authors thank N. Loewenstein and J. Rhoads for greenhouse assis-tance, John Roberts for technical assisassis-tance, and Dr. G. Krause for suggestions on statistical analysis.
Table 4. Mean long-term percent changes in Ci, E, and A from pretreatment values between one and two days after treatment (D1) and between six
and eight days after treatment (D7). For each species, means with different lower case letters are significantly different (P≤ 0.05); for each day,
means with different upper case letters are significantly different (P≤ 0.05). Means significantly different from zero (P≤ 0.05) are designated with asterisks.
Parameter Day A. rubrum A. saccharum Q. alba Q. rubra All species
%∆Ci,l D1 19.5 *a 1.2 *f 3.2* ef 5.0 *e 7.2 *B D7 9.6 *c 4.5 *e 13.4* b 7.4 *d 8.7 *A
%∆El D1 17.7 *a −4.5 b −18.6* c −32.2 *d −9.4 *A D7 −32.7 *d −18.0 *c −0.8* c −23.2 *c −18.7 *B %∆Al D1 23.7 *a 8.9 *b −35.3* c −48.9 *d −12.9 *A
References
Abrams, M.D. and J.A. Downs. 1990. Successional replacement of old-growth white oak by mixed mesophytic hardwoods in south-western Pennsylvania. Can. J. For. Res. 20:1864--1870.
Ahlgren, I.F. and C.E. Ahlgren. 1960. Ecological effects of forest fires. Bot. Rev. 26:483--535.
Bahari, Z.A., S.G. Pallardy and W.C. Parker. 1985. Photosynthesis, water relations, and drought adaptations in six woody species of oak-hickory forests in central Missouri. For. Sci. 31:557--569. Box, G.E.P., and D.R. Cox. 1964. An analysis of transformations J.
Roy. Stat. Soc., Ser. B. 26: 211--243.
Carvell K.C. and E.H. Tryon. 1961. The effect of environmental factors on the abundance of oak regeneration beneath mature oak stands. For. Sci. 7:98--105.
Crow, T.R. 1988. Reproductive mode and mechanisms for self-re-placement of northern red oak (Quercus rubra)----a review. For. Sci. 34:19--40.
Curtis, J.T. 1959. The vegetation of Wisconsin. Univ. Wisconsin Press, Madison, WI, 657 p.
Davies, E. 1987. Action potentials as multifunctional signals in plants: a unifying hypothesis to explain apparently disparate wound re-sponses. Plant Cell Environ. 10:623--631.
Davies, E., T. Zawafzki and D. Witters. 1991. Electrical activity and signal transmission in plants: how do plants know? In Plant Signal-ling, Plasma Membrane and Change of State. Eds. C. Panel and H. Greppin, Université de Genève, Geneva, Switzerland, pp 119--137. Dziubiñska, H., K. Trebacz and T. Zawadzki. 1989. The effect of excitation on the rate of respiration in the liverwort Conocephalum conicum. Physiol. Plant. 75:417--423.
Gunar, I.I. and A.M. Sinyukhin. 1963. Functional significance of action currents affecting the gas exchange of higher plants. Sov. Plant Physiol. 10:219--226.
Hald, A. 1952. Statistical theory with engineering applications. John Wiley and Sons, Inc., New York, 783 p.
Huddle, J.A. 1995. The effects of fire on species of maple and oak. Ph.D. Diss., Univ. Missouri, Columbia, 235 p.
Johnson, C.M., P.R. Stout, T.C. Broyer and A.B. Carlton. 1957. Com-parative chlorine requirements of different plant species. Plant Soil 8:337--353.
Johnson, P. S. 1992. Oak overstory/reproduction relations in two xeric ecosystems in Michigan. For. Ecol. Manage. 48:233--248. Lorimer, C.G. 1989. The oak regeneration problem: new evidence on
causes and possible solutions. In Proc. Seventeenth Annu. Symp. Hardwood Research Council, Merrimac, Wisconsin, pp 23--40. McDougall, G.J. 1993. Accumulation of wall-associated peroxidases
during wound-induced suberization of flax. J. Plant Physiol. 142:651--656.
Malone, M. 1993. Hydraulic signals. Phil. Trans. R. Soc. Lond. B 341:33--39.
Malone, M., J.J. Alarcon and L. Palumbo. 1994. An hydraulic inter-pretation of rapid, long-distance wound signalling in the tomato. Planta 193:181--185.
Ni, B. and S.G. Pallardy. 1991. Response of gas exchange to water stress in seedlings of woody angiosperms. Tree Physiol. 8:1--9. Ni, B. and S.G. Pallardy. 1992. Stomatal and nonstomatal limitations
to net photosynthesis in seedlings of woody angiosperms. Plant Physiol. 99:1502--1508.
Nowacki, G.L., M.D. Abrams and C.G. Lorimer. 1990. Composition, structure and historical development of northern red oak stands along an edaphic gradient in north-central Wisconsin. For. Sci. 36:276--292.
Pallardy, S.G., J.S. Pereira and W.C. Parker. 1991. Measuring the state of water in tree systems. In Techniques and Approaches in Forest Tree Ecophysiology. Eds. J.P. Lassoie and T.M. Hinckley. CRC Press Inc., Boca Raton, Florida, USA, pp 27--76.
Reich, P.B, M.D. Abrams, D.S. Ellsworth, E.L. Kruger and T.J. Tabone. 1990. Fire affects ecophysiology and community dynamics of central Wisconsin oak forest regeneration. Ecology 71:2179--2190.
Sinyukhin, A.M. and V.V. Gorchakov. 1966. Characteristics of the action potentials of the conducting system of pumpkin stems evoked by various stimuli. Sov. Plant Physiol. 13:727--733. Sinyukhin, A.M. and V.V. Gorchakov. 1968. Role of the vascular
bundles of the stem in long-distance transmission of stimulation by means of bioelectric impulses. Sov. Plant Physiol. 15:400--407. Snedecor, G.W. and W.G. Cochran. 1980. Statistical methods, Seventh
Edn. Iowa State University Press, Ames, IA, 503 p.
USDA Forest Service. 1974. Seeds of woody plants in the United States. USDA Agric. Handbk. 450, U.S. Government Printing Of-fice, Washington, D.C., 883 p.
van Sambeek, J.W. and B.G. Pickard. 1976a. Mediation of rapid electrical, metabolic, transpirational, and photosynthetic changes by factors released from wounds. I. Variation potentials and putative action potentials in intact plants. Can. J. Bot. 54:2642--2650. van Sambeek, J.W. and B.G. Pickard. 1976b. Mediation of rapid
electrical, metabolic, traspirational, and photosynthetic changes by factors released from wounds. III. Measurements of CO2 and H2O
flux. Can. J. Bot. 54:2662--2671.
Viro, P.J. 1974. Effects of forest fire on soil. In Fire and Ecosystems. Eds. T.T. Kozlowski and C.E. Ahlgren. Academic Press, New York, NY, USA, pp 7--46.
Vogt A.R. and G. S. Cox. 1970. Evidence for the hormonal control of stump sprouting by oak. For. Sci. 16:165--171.