Structural effects of hierarchical pores in zeolite composite
Jiajun Zheng
a, Xiwen Zhang
a, Yan Zhang
b, Jinghong Ma
a, Ruifeng Li
a,* aKey Laboratory of Coal Science and Technology MOE, Taiyuan University of Technology, Taiyuan 030024, China b
College of Material Science and Technology, Taiyuan University of Technology, Taiyuan 030024, China
a r t i c l e
i n f o
Article history:
Received 12 November 2008 Received in revised form 3 March 2009 Accepted 5 March 2009
Available online 13 March 2009
Keywords: Zeolite composite Hierarchical pores Diffusion Acidity accessibility Catalytic cracking
a b s t r a c t
A zeolite composite with MFI and MOR zeolite structures (denoted as MMZ) was prepared by a two-step hydrothermal crystallization, and characterized by X-ray powder diffraction, nitrogen adsorption– desorption, scanning electron microscopy and in situ IR spectrometry of pyridine and 2,6-dimethyl pyr-idine. The acid catalysis of the composite MMZ was investigated during the catalytic cracking ofn-octane and cumene. The results showed that a hierarchical pore system was created in the zeolite composite improving the accessibility of acid sites and diffusivity in the composite material. The diffusivity in the composite was about seven times higher than in the corresponding physical mixture, and the acid acces-sibility in the composite was 3.4 times as much as in the physical mixture when 2,6-dimethyl pyridine was used as the probe molecule. The conversion of cumene on H-MMZ was three times larger than that on the physical mixture at 423 K.
Ó2009 Elsevier Inc. All rights reserved.
1. Introduction
Zeolites have unique properties in acid catalysis and shape selectivity. Besides providing shape selectivity, the intricate pore and channel systems of zeolites in the molecular size range (0.3– 1.5 nm) lead to diffusion limitations on reaction rates, due to the similarity between the size of the involved hydrocarbons and the micropore diameter[1–3]. Intracrystalline diffusion inside a zeolite micropore at a given temperature and pressure can not be in-creased without changing the internal pore architecture; limiting the effective utilization of acid sites by bulky molecules.
In hydrocarbon transformation over zeolites, the conversion de-pends on both the time the reactant molecules spend inside zeolite crystals and the probability of the reactants accessing the acid sites. An alternative solution to minimize diffusion limitation is to reduce the intracrystalline diffusion path length by decreasing crystal size so as to improve catalytic performance. The beneficial effect of small zeolite crystals on overall reaction rate is twofold. Firstly, smaller crystals have shorter intracrystalline diffusion path length; hence reaction products are released more rapidly. Accordingly, less secondary reactions like coke formation and cracking are observed. Secondly, more micropore entrances per unit mass are present in zeolites with small crystals, which induces a higher accessibility into the zeolite crystals, and therefore, a net increase in overall activity. In the past decade, there have been a considerable number
of attempts to improve the micropore diffusion in zeolites by adjusting their crystal sizes to nanoscale ranges. However, filtering small colloidal particles is not easy, which severely hindered their practical applications. Moreover, the volume and surface area of the micropores in zeolites was decreased because the ordering of the three-dimensional network of the zeolite particles deteriorated when the crystal size decreased to the nanocrystalline region[3]. Therefore, the synthesis of new zeolite materials containing consid-erable intracrystalline or intercrystalline mesopores that provide a better diffusion transport has recently attracted attention of many researchers in the field[4–7]. A practicable route to prepare the materials with an enhanced accessibility is the combination of micropores and mesopores (or macropores) in the same material, since the diffusion in mesopores is several orders of magnitude fas-ter than in micropores. For this goal, some recent studies have ap-plied intercrystalline approaches by which zeolite materials are assembled into ordered mesoporous structures. A more generally applied strategy to attain the materials that combine zeolite micropores with mesopores is the intracrystalline approach, in which mesopores are created in the zeolite crystals. In this way the micropores of the zeolite are effectively shortened and their molecular accessibility is largely enhanced[8–10]. The creation of mesopores in zeolite crystals is equivalent to increasing the external surface area of the zeolite, in this respect a larger number of pore windows is made accessible to the reactants.
The combination of micropores with mesopores in one crystal can be undertaken by steaming[11], acid [1,6]or base leaching
[12]. Mesopores resulting from acid leaching was investigated in Pt/H-mordenite by Donk and co-workers[1]. The experiment results showed that the hydroisomerization activity on Pt/H-mordenite
1387-1811/$ - see front matterÓ2009 Elsevier Inc. All rights reserved. doi:10.1016/j.micromeso.2009.03.009
*Corresponding author. Address: Taiyuan University of Technology, Institute of Special Chemicals, 79# West Yingze Street, Taiyuan 030024, China. Tel./fax: +86 351 6010121.
E-mail address:[email protected](R. Li).
Contents lists available atScienceDirect
Microporous and Mesoporous Materials
was four times higher than on untreated Pt/H-mordenite, because it gave rise to an acceleration of the uptake ofn-hexane under reaction conditions due to a shorter intracrystalline diffusion path length resulting from the mesoporous structure created by acid leaching. Despite intracrystalline mesopores created by acid leaching ap-proach was also reported by other researchers[6], alkali-leaching approach has been the most frequently employed way to create intracrystalline mesopores in zeolite materials. Groen and co-work-ers reported that considerable mesoporosity created in zeolites ZSM-5, BEA, MOR and FER was due to the enlargement of micropores resulting from the silicon extraction by the alkali leaching[2]. Jung also reported that the silicon extraction from micropores by the alkaline treatment created significant mesopores in ZSM-5[13]. Intercrystalline mesopores resulting from polycrystalline accumu-lation were reported in zeolite A[3], which was successfully synthe-sized by using a template method with resorcinol–formaldehyde aerogels. This provides a new way to prepare novel zeolite materials with intercrystalline mesopores.
In the present paper, we report a new method for the synthesis of hierarchically porous zeolites by employing the as-synthesized Mordenite zeolite as silica–alumina source. Intracrystalline mesop-ores are created in Mordenite zeolites by the treatment of basic solution and higher hydrothermal temperature during the sec-ond-step synthesis. In addition, intercrystalline macropores with 50–100 nm are formed because of the polycrystalline aggregation based on crystal growing of zeolite ZSM-5 around Mordenite zeo-lite particles.
2. Experiments
2.1. Synthesis
First, Mordenite zeolite was prepared with the molar composi-tion of the gel: 6Na2O:30SiO2:Al2O3:780H2O. An aliquot of 1.28 g of sodium aluminate (41 wt% Al2O3, 35 wt% Na2O) and 1.89 g of so-dium hydroxide (96 wt%) were mixed in 45 ml water to form a clear solution, then 26.5 ml of silica sol (29 wt%) was slowly added to the solution with vigorous stirring. The mixture was stirred at room temperature for 2 h and transferred into 100 ml autoclave and kept at 443 K for 18–22 h without stirring. The reacted mixture contained the pre-synthesized Mordenite zeolite was directly used in the second-step synthesis. An aliquot of 0.63 g of sodium alumi-nate, 6.2 ml of ethylene diamine (EDA) and 0–1.0 ml of concen-trated sulfuric acid (98 wt%) were added to the reacted mixture, and stirred for 2 h at room temperature. The final molar composi-tion in the mixture of the second-step was: (2.2–4.5)Na2 O:19-SiO2:Al2O3:12EDA:525H2O. The new mixture was then loaded into an autoclave for hydrothermal treatment at 453 K for 48– 72 h under autogenous pressure. The as-synthesized solid product was recovered by filtration, washed with water, dried in air at 373 K overnight and denoted as MMZ. A physical mixture of Mord-enite and ZSM-5 zeolites was denoted as Z + M.
The NHþ
4form of samples was obtained by repeating three times ion exchange at 353 K with 0.5 M NH4NO3solution, for 4 h every time. The protonic form was then obtained by calcining the NHþ
4
-zeolite at 823 K for 5 h and marked as H-MMZ and H-(Z + M).
2.2. Characterization
The XRD patterns were recorded using a Rigaku Dmax X-ray dif-fractometer, which employed Ni-filtered CuK
a
radiation and wasoperated at 40 kV and 80 mA. Crystal size, morphology and macroporosity of the composite MMZ and the corresponding phys-ical mixture Z + M were investigated on a JSM-6301F scanning electron microscope. N2 adsorption at 77 K was performed in a
NOVA 1200e gas sorption analyzer to study the micro- and meso-porosity in the zeolite crystals. The mesopore size distribution was calculated using the Barret–Joyner–Halenda (BJH) pore size model applied to the adsorption branch of the isotherm. The microporous structure was obtained from thet-plot analysis of the adsorption branch of the isotherm. Infrared spectra of pyridine and 2,6-di-methyl pyridine adsorption were obtained on a SHIMADZU FTIR-8400 spectrometer. About 12 mg of sample was pressed into a self-supporting wafer of 10 mm in diameter. The wafer was first evacuated in situ in an IR cell at 573 K for 2 h, and the IR spectra were recorded at room temperature. Pyridine or 2,6-dimethyl pyr-idine was then introduced into the cell at room temperature until the saturated adsorption was reached. Finally, desorption of pyri-dine or 2,6-dimethyl pyripyri-dine was performed at increasing temper-atures under 3103Pa of pressure and the spectra were recorded at various temperatures.
2.3. Catalytic activity
All catalytic experiments were conducted under atmospheric pressure in a fixed-bed quartz tube (i.d. 6 mm) low micro reactor. Prior to each experiment, the H-zeolite was pressed into a cylinder and then broken into 20–40 meshes particles and activated under flowing N2(50 ml/min) at 823 K for 2 h and then kept at desired reaction temperature. n-Octane cracking was carried out at 673 K. The reactant stream of n-octane in N2 (molar ratio of 0.018) was fed into the reactor containing 200 mg of catalyst. Cu-mene cracking was carried out at 423–673 K over 100 mg of cata-lyst, at a molar ratio of cumene and N2kept at 0.006. The total gas flow at the reactor inlet was kept constant at 50 ml/min, and the lines were kept at 433 K by heating tapes. Cracking products were analyzed by an online gas chromatograph (Agilent1790) with a flame ionization detector (FID) and GDX-101 column.
3. Results and discussion
3.1. X-ray diffraction (XRD)
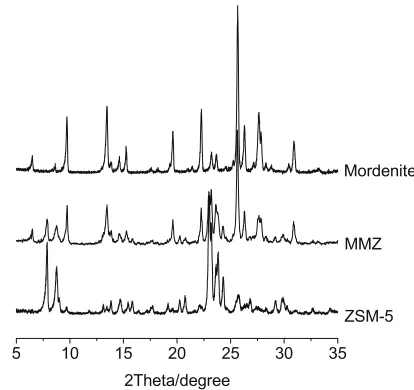
Fig. 1 shows XRD patterns of as-synthesized samples MMZ, Mordenite and ZSM-5. The characteristic diffraction peaks of Mordenite and ZSM-5 zeolites can be observed to occur simulta-neously, indicating the co-existence of Mordenite and ZSM-5 zeo-lite phases in the zeozeo-lite composite.
5 10 15 20 25 30 35
ZSM-5 MMZ
2Theta/degree
Mordenite
3.2. Scanning electron microscopy (SEM)
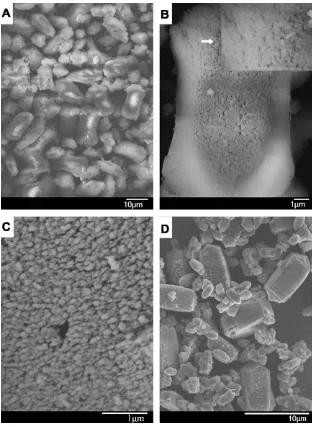
Fig. 2displays SEM images of the zeolite composite MMZ and the physical mixture Z + M. The SEM images of MMZ differ from those of Mordenite or ZSM-5 zeolites. The Mordenite and ZSM-5 zeolite crystals show slab-like particles with the sizes of about 3
l
m10l
m and 1l
m2l
m, respectively (Fig. 2D). The sizesof both Mordenite and ZSM-5 zeolite crystals are smaller than that of the as-synthesized zeolite composite MMZ (about 7
l
m12
l
m). The relatively bigger crystals of the zeolite compositeMMZ resulted from the fact that zeolite crystals of zeolite ZSM-5 were grown around Mordenite zeolite particles.
FromFig. 2A it can be seen that the as-synthesized sample MMZ shows an uneven morphology, indicating an uneven growth rate of ZSM-5 zeolite on Mordenite crystals. ZSM-5 zeolite grows rela-tively faster on the two terminals ends of Mordenite particles than on middle part because of the presence of more lattice defects on these two terminals ends resulting in the dumbbell-like morphol-ogy (Fig. 2B). As shown inFig. 2C, the crystal has a rough surface. Macropores with the sizes of 50–100 nm can be obviously ob-served, which can be assigned to the porous structures composed of ZSM-5 crystals (with the sizes of 50–200 nm) grown onto Mord-enite zeolite surface during the second-step crystallization.
3.3. N2adsorption–desorption isotherms
Fig. 3reveals N2adsorption–desorption isotherms and the cor-responding BJH pore size distribution curves (inset) of the zeolite composite MMZ and physical mixture Z + M. The adsorption– desorption of nitrogen on the mixture Z + M is a type-I isotherm, indicating the presence of micropores only. However, a curve com-bining type-I and type-IV isotherms is observed for MMZ. A larger hysteresis loop occurs afterp/p00.45 in the adsorption–desorp-tion isotherm of the zeolite composite MMZ, which indicates not only the presence of mesopores but a broad pore size distribution also. Moreover, significant increase in N2 adsorption is also ob-served from p/p00.8 for the as-synthesized sample MMZ. According to the SEM results mentioned above, zeolite composite MMZ displays agglomerates of very small crystallites with sizes of 50–200 nm. The sharp condensation in the range ofp/p0= 0.8– 1.0 should therefore be attributed to capillary condensation[14]
in open mesopores obtained by filling the interparticle spaces
[3,15].
As shown inFig. 3(inset), the pore size distribution of the com-posite MMZ illustrates the existence of a mesopore structure with pore size centered around 3.7 nm. However, the BJH pore size distribution derived from the adsorption branch of the isotherm
does not show the similar distribution in the mixture Z + M. The pore centered around 3.7 nm can be attributed to the contribution of alkali leaching on Mordenite zeolite. During the second-step synthesis, the framework of Mordenite zeolite was etched by the synthesis solution and silica was partially solved, which caused the enlargement of micropores, resulting in the formation of mes-opores[13,16].
The value of the BET surface area of the zeolite composite MMZ (SBET= 358 m2/g) is lower than that of the mixture Z + M (SBET= 395 m2/g). The decrease in the BET surface area is most probably because the decrease in the crystal size of ZSM-5 zeolite particles to the nanocrystalline region[3], causes the deterioration of the ordering of the three-dimensional network of ZSM-5 zeolite particles. The dissolution of Mordenite zeolite may also play a role, which results in the breakdown of part micropores[17]. However, the external surface area of the composite MMZ (SEXT= 69 m2/g) is much larger than that of the physical mixture Z + M (SEXT= 18 m2/ g).
These results show that a hierarchical pore system is created in the zeolite composite MMZ, namely, micropore from Mordenite and ZSM-5 zeolites, mesopores centered around 3.7 nm, and mac-ropores with the sizes of 50–100 nm.
3.4. Adsorption results of n-octane
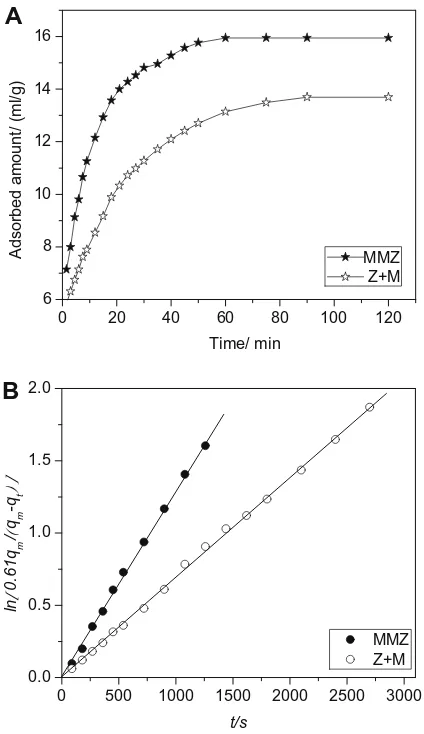
The volume of adsorbedn-octane per unit weight of zeolite ver-sus time for the as-synthesized zeolite composite MMZ along with that of the physical mixture Z + M is depicted inFig. 4A. Comparing these results, we can find that the adsorption capacity of the zeo-lite composite MMZ sample is about 16 ml/g, higher than 14 ml/ g, the adsorption capacity of the physical mixture Z + M. The pres-ence of meso- and macropores in the MMZ sample can contribute to the higher adsorption capacity and to its higher mesoporous vol-ume and lower bulk density.
The uptake curves ofn-octane on the two samples also show that adsorption equilibrium could be reached more quickly on the zeolite composite MMZ than on the mixture of zeolites. This could be attributed to the lower diffusion resistance resulting from the existence of meso- and macropores in the MMZ zeolite com-posite. The formation of hierarchical pores increases the rate of dif-fusion ofn-octane in the MMZ zeolite composite. About 90 min is
required for the saturation of n-octane on the physical mixture Z + M. Whereas it takes only 50 min on the MMZ zeolite composite even though its uptake amount is larger than that of the physical mixture Z + M. Further quantification of the diffusion properties in the different samples is attained using the classic Fick’s law of diffusion[18], which describes the change of the concentration of molecules inside the zeolite crystals as a function of time. 1qt=qmð6
p
2Þ expðp
2Dt=r2Þ ð1Þor
ln½0:61qm=ðqmqtÞ
p
2Dt=r2 ð2Þwhere qt andqm are instant adsorption capacity and equilibrium adsorption capacity, respectively,Dis diffusivity,ris characteristic diffusion length, andtis time. The characteristic diffusion lengthr of the crystals in the zeolite composite MMZ and in the physical mixture Z + M is derived from SEM and estimated as 1
l
m and0.5
l
m (r=Vp/Spfor slab-like particles, whereVpandSpare thevol-ume and external surface area of the particle [19], respectively). Plotting ln½0:61qm=ðqmqtÞversus timetfor the zeolite composite MMZ and the physical mixture Z + M leads to two straight lines, as shown inFig. 4B. This fitting adequately describes the experimental data covering the uptake curve up toqt/qm= 0.8 and leads to a char-acteristic diffusion time ofr2/D= 7.35103s and 1.48104s for
Fig. 3.N2adsorption–desorption isotherms and the corresponding BJH pore size
distribution curves (inset) of the zeolite composite MMZ and physical mixture Z + M.
0 500 1000 1500 2000 2500 3000 0.0
Fig. 4.The adsorption kinetics curves ofn-octane over physical mixture Z + M and
the zeolite composite MMZ and the physical mixture Z + M, respec-tively. Combined withr= 1
l
m (the zeolite composite MMZ) and0.5
l
m (the physical mixture Z + M), yields a diffusivity D ofapproximately 1.361016m2s1for the zeolite composite MMZ and 1.701017m2s1for the physical mixture (Z + M). Showing a lower diffusion resistance for the MMZ zeolite composite. Com-paring with the mixture Z + M, the characteristic diffusion path length in the composite is shortened because of the presence of meso- and macropores, which causes the improved gas transport properties[19]and thus faster diffusion ofn-octane.
3.5. Accessibility of Brönsted-acid sites
To investigate the accessibility of Brönsted-acid sites, 2,6-di-methyl pyridine (DMPy) is selected as a probe molecule because it is widely used as a sterically hindered base. DMPy has already been used in the investigation of the acid sites located on the exter-nal surface of zeolites[20]as well as in the characterization of the strength of Brönsted-acid sites[21]. The size of DMPy molecular is about 6.7 Å, which is bigger than the dimensions of pores in ZSM-5 (5.35.6 Å) and close to the dimensions of pores in Mordenite (6.57.0 Å). Theoretically the DMPy molecule can enter the micropores of Mordenite zeolite, but diffusion inside the pores would be very limited, and therefore not all the acid sites inside the crystal are accessible. The creation of meso- and macropores in the MMZ zeolite composite should facilitate the diffusion of bulky molecule and increase the accessibility of the sites. The aim of the DMPy IR results is to verify whether the hierarchical porosity in the zeolite composite could lead to the improvement of the accessibility of the Brönsted-acid sites.
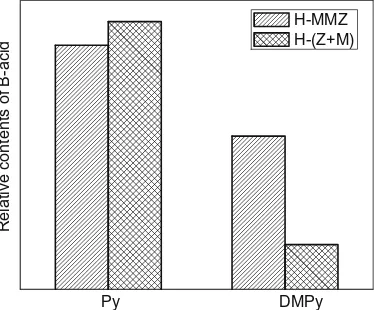
Fig. 5summarizes IR results of pyridine (Py) and DMPy adsorp-tion at saturaadsorp-tion in terms of the integrated absorbance (IA) of the bands at 1545 cm1(Py), 1630 and 1650 cm1(DMPy) which gives the Brönsted site (B) accessibility for Py and DMPy in H-MMZ and H-(Z + M). A high IA indicates high accessibility of Brönsted for Py observed on both samples. However, when DMPy is used as probe molecule, higher amount of accessible sites is only found on H-MMZ. In comparison with Py as the probe molecule, the accessible Brönsted-acid site of DMPy in H-MMZ zeolite composite is about 63 percent, however, it is only 17 percent in the physical mixture H-(Z + M).
The Brönsted-acid site accessibility of Py in the physical mix-ture H-(Z + M) is slightly higher than that in the zeolite composite H-MMZ. However, when DMPy is used as probe molecule, the acid accessibility in H-MMZ is 3.4 times as much as in H-(Z + M). That
can be ascribed to the formation of meso- and macropores in the zeolite composite H-MMZ. As discussed previously, the presence of meso- and macropores in the zeolite composite crystal facili-tates the diffusion of bulky molecules and increases the accessibil-ity of Brönsted-acid sites.
3.6. Catalytic tests
The effects of the existence of meso- and macropores on cata-lytic performances of zeolite composite H-MMZ were investigated byn-octane and cumene catalytic cracking, and compared with those of the physical mixture H-(Z + M), as shown inFigs. 6 and 7, respectively.
It can be seen that H-MMZ has a higher activity than H-(Z + M) forn-octane catalytic cracking as well as for cumene cracking un-der the same reaction conditions.Fig. 6shows that the initial con-version ofn-octane on H-MMZ is about 73 wt% (1 h), higher than the 62 wt% conversion (1 h) on H-(Z + M); In addition, the conver-sion of cumene on H-MMZ (57 wt%) is about three times as much as on H-(Z + M) (about 20 wt%) at 423 K (Fig. 7). The higher conver-sion ofn-octane or cumene on the zeolite composite H-MMZ could be caused by both the faster diffusion of reactant in its channel and higher accessibility of Brönsted-acid sites resulting from the existence of the meso- and macropores in the composite. The
Relative
Fig. 5.Relative contents of Brönsted sites accessibility for Py and DMPy in H-MMZ
and H-(Z + M), determined from the areas of the peaks around the bands at 1545 cm1(Py), 1630 and 1650 cm1(DMPy).
Fig. 7.The conversions of cumene on H-(Z + M) and H-MMZ at different reaction
hierarchical pore system in the composite makes the reactants easy to access acid sites[10]. The existence of meso- and macrop-ores could also accelerate the elution of cracked products from the composite materials.
The introduction of the hierarchical porosity in the zeolite com-posite has a major impact on catalytic activity. Higher activity of the H-MMZ zeolite composite results from the enhanced accessi-bility of Brönsted-acid sites, however, the largest beneficial effect is the alleviated diffusion limitation by the presence of meso-and macropores.
4. Conclusions
In summary, a zeolite composite MMZ has been successfully synthesized by using Mordenite zeolite as silica–alumina source. The results from SEM observation reveal that the meso- and mac-ropores presented in the composite MMZ have the porous win-dows of 50–100 nm, which is attributed to the polycrystalline aggregation of zeolite ZSM-5. N2adsorption–desorption isotherms show that zeolite composite MMZ has a mesopore structure with pore size centered around 3.7 nm, which is associated with the al-kali leaching on Mordenite zeolites. The existence of meso- and macropores in the zeolite composite facilitate the molecular trans-port by shortening its micropores and its acid sites are more acces-sible, which gives the catalyst a higher activity.
Acknowledgment
Author R. Li thanks Professor E.E. Wolf (University of Notre Dame) for his valuable advice and edit for the revised manuscript. This work is supported by the ‘‘973” project (No. 2005CB221204) and SinoPEC (No. 107009).
References
[1] S. van Donk, A. Broersma, O.L.J. Gijzeman, J.A. van Bokhoven, J.H. Bitter, K.P. de Jong, J. Catal. 204 (2001) 272.
[2] J.C. Groen, L.A.A. Peffer, J.A. Moulijn, J. Pérez-Ramírez, Micropor. Mesopor. Mat. 69 (2004) 29.
[3] Y. Tao, H. Kanoh, K. Kaneko, Langmuir 21 (2005) 504.
[4] K. Egeblad, M. Kustova, S.K. Klitgaard, K. Zhu, C.H. Christensen, Micropor. Mesopor. Mat. 101 (2007) 214.
[5] B.T. Holland, Micropor. Mesopor. Mat. 89 (2006) 291.
[6] M. Tromp, J.A. van Bokhoven, M.T. Garriga Oostenbrink, J.H. Bitter, K.P. de Jong, D.C. Koningsberger, J. Catal. 190 (2000) 209.
[7] [a] J.C. Groen, L.A.A. Peffer, J.A. Moulijn, J. Pérez-Ramírez, Colloid Surface A 241 (2004) 53;
[b] J.C. Groen, J.A. Moulijn, J. Pérez-Ramírez, Micropor. Mesopor. Mat. 87 (2005) 153;
[c] J.C. Groen, A. Brückner, E. Berrier, L. Maldonado, J.A. Moulijn, J. Pérez-Ramírez, J. Catal. 243 (2006) 212.
[8] V.V. Ordomsky, V.Y. Murzin, Y.V. Monakhova, Y.V. Zubavichus, E.E. Knyazeva, N.S. Nesterenko, I.I. Ivanova, Micropor. Mesopor. Mat. 105 (2007) 101. [9] R. Mann, Catal. Today 18 (1993) 509.
[10] N.S. Nesterenko, F. Thibault-Starzyk, V. Montouillout, V.V. Yuschenko, C. Fernandez, J.-P. Gilson, F. Fajula, I.I. Ivanova, Micropor. Mesopor. Mat. 71 (2004) 157.
[11] K.-H. Lee, B.-H. Ha, Micropor. Mesopor. Mat. 23 (1998) 211.
[12] M. Ogura, S. Shinomiya, J. Tateno, Y. Nara, E. Kikuchi, M. Matsukata, Chem. Lett. 8 (2000) 882.
[13] J.S. Jung, J.W. Park, G. Seo, Appl. Catal. A Gen. 288 (2005) 149.
[14] M. Bjørgen, F. Joensen, M.S. Holm, U. Olsbye, K.-P. Lillerud, S. Svelle, Appl. Catal. A Gen. 345 (2008) 43.
[15] A. Simon-Masseron, J.P. Marques, J.M. Lopes, F. Ramôa Ribeiro, I. Gener, M. Guisnet, Appl. Catal. A Gen. 316 (2007) 75.
[16] J.C. Groen, J.C. Jansen, J.A. Moulijn, J. Perez-Ramirez, J. Phys. Chem. B 108 (2004) 13062.
[17] Y. Bouizi, L. Rouleau, V.P. Valtchev, Chem. Mater. 18 (2006) 4959.
[18] H.G. Karge, J. Weitkamp, Molecular Sieves Science and Technology – Adsorption and Diffusion, Springer, Berlin, 2008.
[19] J.C. Groen, W. Zhu, S. Brouwer, S.J. Huynink, F. Kapteijn, J.A. Moulijn, J. Pérez-Ramírez, J. Am. Chem. Soc. 129 (2007) 355.
[20] A. Corma, C. Rodellas, V. Fornes, J. Catal. 88 (1984) 374.