Hus s e in I. Abde l-Shafy, Mamdo uh F. Abde l-Sabo ur and Rao uf O. Aly
Adso rptio n o f nic ke l and me rc ury fro m drinking wate r simulant by ac tivate d c arbo n
Enviro nme ntal Manage me nt and He alth
9 / 4 [1 9 9 8 ] 1 7 0 –1 7 5
t h e effect of t h e con t a ct t im e, d iffer en t d et en t ion p er iod s ; n a m ely 5, 10, 20, 30 a n d 40 m in -u t es, w er e exa m in ed . T h e r es t of t h e va r ia bles w er e k e p t con st a n t .T h e a m ou n t of GAC r eq u ir ed w a s ca lcu la t ed for m F r eu n d lich ’s eq u a t ion (1) (F r eu n d lich , 1926):
X/ m = K.C 1/n (1)
Wh er e X is t h e a d s or b ed m a s s (Co-C), Co a n d C a r e t h e in it ia l a n d fi n a l m et a l con cen t r a t ion s r esp ect ively, (m ) is t h e m a ss of ca r -b on a n d K a n d 1/ n a r e con st a n t s, w h ich ca n b e d et er m in ed by p lot t in g t h e a d s or b ed m a ss (X/ m ) a s t h e fu n ct ion of t h e eq u ilib r iu m con cen t r a t ion in log-log r ela t ion sh ip. 1/ n is t h e s lop e of t h e lin e t o t h e or d in a t e w h en log C = 0. T h e on ly u n k n ow n in eq u a t ion (1) is t h e va lu e (m ) w h ich h a s b een ca lcu la t ed .
T h e con cen t r a t ion s of N i a n d H g w er e d et er m in ed in t h e a cid ifi ed s a m p les u s in g a t om ic a b sor p t ion s p ect r op h ot om et er.
3. Results and discussion
3 .1 The use of powdered activated carbon
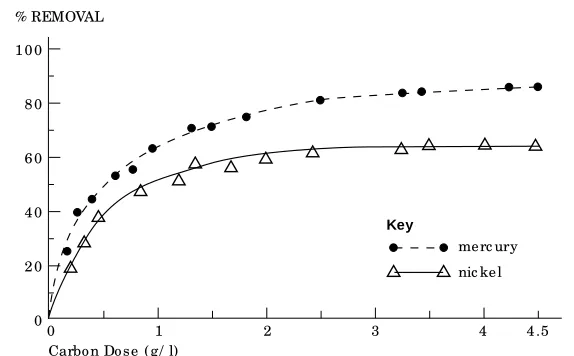
T h e r es u lt s s h ow t h a t a n in it ia l r a p id u p t a k e of t h e m et a ls exa m in ed w a s ob s er ved w it h in t h e fi r s t 15 m in u t es, a ft er w h ich t h e r em ova l efficien cy in cr ea ses s low ly bu t gr a d u a lly u n t il a n eq u ilib r iu m is a t t a in ed a ft er t woa n d woa h woa lf h ou r s. T h e r woa t e of m et woa l woa d s or p -t ion a s a fu n c-t ion of -t h e cor r es p on d in g a m ou n t of ca r b on u s ed a t t h e p r ed et er m in ed eq u ilib r iu m t im e (i.e. a d s or p t ion is ot h er m ), is p r es en t ed in F igu r e 1.
T h e r es u lt s ob t a in ed r evea led t h a t t h e a d s or p t ion of N i a n d H g follow F r eu n d lich ’s eq u a t ion (1). Accor d in g t o t h is eq u a t ion , st r a igh t lin es w er e ob t a in ed by p lot t in g log
X/ m ver su s log C. Ad sor p t ion efficien cy ca n b e d et er m in ed on t h e b a sis of F r eu n d lich ’s con st a n t s. In gen er a l, t h e h igh er t h e K va lu e, t h e gr ea t er t h e ca p a cit y of t h e a d sor b en t . H ow ever, t h e d osa ge of ca r b on r eq u ir ed for t h e r em ova l of eit h er N i or H g is d e p en d en t on b ot h p a r a m et er s (K a n d 1/ n ), in it ia l con -cen t r a t ion of m et a ls Co, a n d fi n a l con cen t r a -t ion r eq u ir ed in -t r ea -t ed w a -t er C.
T h e slop e F r eu n d lich ’s is ot h er m (1/ n ) w a s fou n d t o b e 1.586 for N i a n d 1.206 for H g. Cor r esp on d in g con s t a n t s K w er e 1.02 a n d 1.91 r esp ect ively. It ca n b e con clu d ed t h a t H g is m or e lia ble t o b e a d sor b ed on PAC t h a n N i.
3 .2 The use of granular act ivat ed carbon
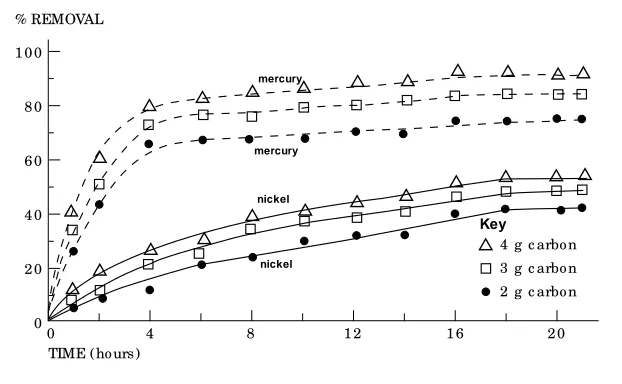
In d r in k in g w a t er t r ea t m en t p la n t s, GAC is u sed in t h e for m of b ed colu m n . Ba t ch exp er im en t t ech n iq u e p er m it s t h e p r ed ict ion of t h e a d sor p t ion p a r a m et er s wh ich con t r ol t h e u p t a k e p r ocess. Tim e r eq u ir ed t o r ea ch eq u ilib r iu m w a s fi r st a ssessed . Resu lt s ob t a in ed (F igu r e 2) sh owed t h a t 18 h ou r s wer e su fficien t t o m a in t a in eq u ilib r iu m . Mea n -wh ile, t h e effect of ca r b on t o n ick el r a t io (C/ N i) a n d ca r b on t o m er cu r y r a t io (C/ Hg) on t h e eq u ilib r iu m a d sor p t ion ca p a cit y a t d iffer -en t con t a ct t im es a r e a lso illu st r a t ed (F igu r e 2). T h ese r esu lt s in d ica t e t h a t h igh er ca r b on t o m et a l (N i or Hg) r a t io in cr ea ses t h e m et a l a d sor p t ion ca p a cit y a n d in cr ea ses t h e in it ia l r em ova l r a t e. However, t h e t im e n ecessa r y for r ea ch in g eq u ilib r iu m a d sor p t ion w a s u n ch a n ged . Su ch r esu lt s a p p r oxim a t e w it h t h e fi n d in gs of ot h er invest iga t or s (Ab d el-Sh a fy a n d Ab o-E l-Wa fa , 1987; Hu a n g a n d Ost ov ic,1978).
T h e a d sor p t ion isot h er m of N i a n d Hg a t t h e p r ed et er m in ed eq u ilib r iu m t im e is given in
1 0 0
8 0
6 0
4 0
2 0
0 % REMOVAL
0 1 2 3 4 4 .5
me rc ury
nic ke l
Key
Carbo n Do s e (g/ l) Figure 1
Hus s e in I. Abde l-Shafy, Mamdo uh F. Abde l-Sabo ur and Rao uf O. Aly
Adso rptio n o f nic ke l and me rc ury fro m drinking wate r simulant by ac tivate d c arbo n
Enviro nme ntal Manage me nt and He alth
9 / 4 [1 9 9 8 ] 1 7 0 –1 7 5 Hus s e in I. Abde l-Shafy, Mamdo uh F. Abde l-Sabo ur and Rao uf O. Aly
Adso rptio n o f nic ke l and me rc ury fro m drinking wate r simulant by ac tivate d c arbo n
Enviro nme ntal Manage me nt and He alth
9 / 4 [1 9 9 8 ] 1 7 0 –1 7 5
F igu r e 3, wh ich d em on st r a t es t h a t in cr ea sin g t h e ca r b on d oses fr om 0.2g/ 1 t o 20.0 g/ 1 r a ises t h e n ick el r em ova l efficien cy fr om 9.7 p er cen t t o 80 p er cen t . Also, by in cr ea sin g t h e ca r b on d oses fr om 0.2g/ 1 t o 7.0g/ 1 r a ises t h e m er cu r y r em ova l fr om 31.4 p er cen t t o 91.4 p er cen t .
It w a s fou n d t h a t t h e a d s or p t ion is ot h er m is con fi r m ed by F r eu n d lich ’s eq u a t ion r a t h er t h a n La n gm u ir (La n gm u ir, 1918) or BE T (Br u n a u er et a l., 1938). T h e cor r es p on d in g (K) va lu es for N i a n d H g w er e 0.219 a n d 1.134 a n d 1.036. T h is in d ica t ed t h e s t r on ger t en d en cy of H g t o b e a d sor b ed on ca r b on com p a r ed t o N i.
3 .3 Continuous flow system
Su ch st u d ies ca n t h r ow ligh t on t h e d a t a n ecessa r y for op t im u m d esign of a d sor b en t s in d r in k in g w a t er p la n t s. To st u dy t h e va r ia -t ion of con -t a c-t -t im e on -t h e a d sor p -t ive ca p a ci-t y,
d iffer en t con t a ct t im es of 5, 10, 20, 30 or 40 m in u t es wer e exa m in ed .
T h e exp er im en t for ea ch con t a ct t im e sy st em t ook eigh t d ay s. T h e op er a t in g con d i-t ion s a r e given in Ta ble I. Br ea k i-t h r ou gh
1 0 0
8 0
6 0
4 0
2 0
0 % REMOVAL
0 4 8 1 2 1 6 2 0
mercury
TIME (ho urs )
mercury
nickel
nickel
4 g c arbo n
3 g c arbo n
2 g c arbo n
Key
Figure 2
Effe c t o f variable time s o n the ads o rptio n o f me rc ury and nic ke l o n granular ac tivate d c arbo n us ing diffe re nt c arbo n do s e s
Table I
Ope rating c o nditio ns c arbo n c o lumn
Contact time (minutes) Flow rate (cm/ minutes)
Five 2 3 .8 8
Ten 1 1 .9 4
Twenty 5 .9 7
Thirty 3 .9 8
Forty 2 .9 9
Notes:c arbon depth = 3 8 c m for eac h c olumn; bed volume = 1 1 9 .4 m3for eac h c olumn; total c arbon weight
= 6 1 .4 g.
1 0 0
8 0
6 0
4 0
2 0
0 % REMOVAL
0 4 8 1 2 1 8 2 0
me rc ury
nic ke l
Key
CARBON DOSE (g/ l)
1 6 1 4 1 0
6 2
Figure 3
Hus s e in I. Abde l-Shafy, Mamdo uh F. Abde l-Sabo ur and Rao uf O. Aly
Adso rptio n o f nic ke l and me rc ury fro m drinking wate r simulant by ac tivate d c arbo n
Enviro nme ntal Manage me nt and He alth
9 / 4 [1 9 9 8 ] 1 7 0 –1 7 5
cu r ves for N i a n d Hg a t d iffer en t con t a ct t im es a r e sh ow n in F igu r e 4. Th e r esu lt s ob t a in ed in d ica t ed t h a t t h e b r ea k t h r ou gh s of N i a n d Hg r efl ect t h e r em ova l efficien cy of ea ch .
T h e b r e a k t h r ou gh p oi n t s of n i ck e l, a t w h i ch t h e con ce n t r a t i on w a s 0.05 m g/ 1 (i .e. t h e p e r m i s s i b le leve l i n d r i n k i n g w a t e r ) w e r e r e a ch e d a ft e r 1.4, 3.5, 13.4, 25.1 a n d 40 h ou r s, cor r e s p on d i n g t o t h e con t a ct t i m e s 5, 10, 20, 30 a n d 40 m i n u t e s. T h e b r e a k t h r ou gh p oi n t s of H g, a t w h i ch t h e con ce n t r a t i on w a s 0.01m g/ l (i .e. t h e p e r m i s s i b le leve l i n d r i n k -i n g w a t e r ) w e r e r e a ch e d a ft e r 0.265, 2.5, 9.0,18.0 a n d 26.0 h ou r s a t t h e con t a ct t i m e s 5, 10, 20, 30 a n d 40 m i n u t e s r e s p e ct ive ly.
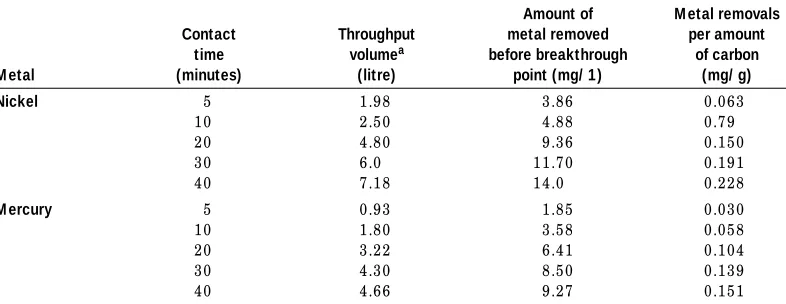
Ta ble II sh ow s t h e r ela t ion b et w een t h e r em oved a m ou n t of N i a n d H g a t d iffer en t con t a ct t im es b efor e b r ea k t h r ou gh p oin t on on e h a n d , a n d t h e a m ou n t of m et a l r em oved p er a m ou n t of ca r b on (m g/ g) on t h e ot h er. T h e ob t a in ed r esu lt s p r oved t h a t w h en ext en d in g t h e con t a ct t im e fr om fi ve t o 40 m in u t es, t h e r em ova l in cr ea s ed fr om 0.063 t o 0.228m g N i/ g ca r b on a n d fr om 0.030 t o 0.151 m g H g/ g ca r b on . T h is fi n d in g is in a gr eem en t w it h p r ev iou s st u d ies (Ab d el-Sh a fy a n d Ab o-E l-Wa fa , 1987; H u a n g a n d Ost ov ic, 1978 a n d Mou r sy a n d Ab d el-Sh a fy, 1984).
T h u s, a lin ea r r ela t ion sh ip w a s ob t a in ed b et w een t h e loga r it h m of con t a ct t im e a n d t h e volu m e of t h e con t a m in a t ed w a t er b efor e t h e b r ea k t h r ou gh p oin t s for N i a n d H g (F igu r e 5).
Conclusion and recommendation
T h er efor e, i t ca n b e con clu d ed fr om t h e over -a ll r es u lt s t h -a t t h e u s e of b ot h PAC -a n d GAC for t h e r em ova l of N i a n d H g fr om d r i n k i n g w a t er i s a p r om i s i n g p r oces s. It i s w or t h m en t i on i n g t h a t t h e ob t a i n ed r es u lt s
r evea led t h a t t h e a d s or b a b i li t y of H g i s m u ch
RESIDUAL METAL CONCENTRATION (mg/ 1 )
Hg
Ni
Key
2 .0 0
1 .5 0
1 .0 0
0 .5 0
0 .0 0
A
1 .7 5
1 .5 0 B
0 1 2 3 4 5 6 7 8
C
D
E 1 .0 0
0 .5 0
0 .0 0
0 .7 5
0 .5 0
0 .2 5
0 .0 0
0 .5 0
0 .2 5
0 .0 0
0 .5 0
0 .2 5
0 .0 0
TIME (days )
5 mins
1 0 mins
2 0 mins
3 0 mins
4 0 mins Figure 4
Bre akthro ugh c urve s o f me rc ury and nic ke l at diffe re nt c o ntac t time s
Table II
Amo unts o f nic ke l and me rc ury re mo ve d at diffe re nt c o ntac t time s
Amount of M etal removals
Contact Throughput metal removed per amount
time volumea before breakthrough of carbon
M etal (minutes) (litre) point (mg/ 1) (mg/ g)
Nickel 5 1 .9 8 3 .8 6 0 .0 6 3
1 0 2 .5 0 4 .8 8 0 .7 9
2 0 4 .8 0 9 .3 6 0 .1 5 0
3 0 6 .0 1 1 .7 0 0 .1 9 1
4 0 7 .1 8 1 4 .0 0 .2 2 8
M ercury 5 0 .9 3 1 .8 5 0 .0 3 0
1 0 1 .8 0 3 .5 8 0 .0 5 8
2 0 3 .2 2 6 .4 1 0 .1 0 4
3 0 4 .3 0 8 .5 0 0 .1 3 9
4 0 4 .6 6 9 .2 7 0 .1 5 1
Hus s e in I. Abde l-Shafy, Mamdo uh F. Abde l-Sabo ur and Rao uf O. Aly
Adso rptio n o f nic ke l and me rc ury fro m drinking wate r simulant by ac tivate d c arbo n
Enviro nme ntal Manage me nt me rc ury fro m drinking wate r simulant by ac tivate d c arbo n
Hus s e in I. Abde l-Shafy, Mamdo uh F. Abde l-Sabo ur and Rao uf O. Aly
Adso rptio n o f nic ke l and me rc ury fro m drinking wate r simulant by ac tivate d c arbo n
Enviro nme ntal Manage me nt and He alth
9 / 4 [1 9 9 8 ] 1 7 0 –1 7 5
La n gm u ir, I. (1918), “T h e a d sor p t ion of ga s es on p la n e su r fa ces of gla ss, m ica a n d p la t in u m ”, J. A m er ica n Ch em ica l S ociety, Vol. 40, p. 1,361. Lu , Y., Su b r a m a n ia n , K.S., Ch a k r a b a r t i, C.L.,
Gu o, R., Ch en g, J ., Ma , X. a n d P ick er in g, W.F. (1993), “Rem ova l of t r a ce Cd (11) by E DTAcoa t ed gr a n u la r a ct iva t ed ca r b on ”, J. E n v ir on -m en ta l S cien ce a n d H ea lth , Vol. 28 A N o. 1, p p. 113-33.
Ma r t in , J .L., Ba t ch elor, B. a n d Ch a p r a , S.C. (1985), “Mod ifi ca t ion of a m et a l a d sor p t ion m od el t o d escr ib e t h e effect of p H ”, J.Wa ter Pollu tion Con tr ol Fed era tion , Vol. 57 N o. 5, p p. 425-7. Ma s ir on i, R. (1969), “T r a ce elem en t s a n d ca r d
io-va scu la r d isea se, B u lletin of W orld H ea lth Orga n iz a tion , Vol. 40, p p. 305-12.
M ou r s y, A.S. a n d Ab d el-Sh a fy, H .I. (1984), “Role of n a t u r a l cla y a n d p ow d er ca r b on on t h e r em ov a l of h yd r oca r b on fr om w a t er ”, E n v ir on m en ta l I n ter n a tion a l Con feren ce, Lon d on , J u ly, p p. 131-5.
P a n d ey, M.P. a n d Ch a u d h u r i, M . (1980), “In or ga n ic m er cu r y -b it u m in ou s coa l s or p t ion in t er a c-t ion in w a c-t er ”, J. Pr og ress in Wa c-ter T ech n ol-og y,
Vol. 12, p p. 697-711.
P a t t er son , J .W. (1975) Wa stew a ter T rea tm en t T ech -n olog y, A-n -n Ar b or Scie-n ce, M I, p. 265. Sa lom on s, W. a n d For st n er, U. (1984), M eta ls in
H yd r ocycle, Sp r in ger -Ver la g, Ber lin -H eid el-b er g, p. 348.