Detoxification experiments with the seed oil from
Jatropha curcas
L.
Wilhelm Haas, Martin Mittelbach *
Institute of Organic Chemistry,Karl-Franzens-Uni6ersita¨t Graz,Heinrichstrasse 28,A-8010 Graz, Austria
Accepted 14 February 2000
Abstract
Due to its large number of potential utilizations Jatropha curcas L. (physic nut, purging nut), a tropical plant cultivated in many Latin American, Asian and African countries, has become topic of various research projects. Nutritional as well as technical applications, however, are restricted due to the plant’s toxicity. The seed oil contains phorbol esters, a family of compounds known to cause a large number of biological effects such as tumor promotion and inflammation. Therefore it is necessary to find feasable routes for detoxification of the oil.J.curcasseed oil was treated by traditional oil refining processes examining the effect on the content of phorbol esters. Parameters of several refining steps were varied to optimize the grade of detoxification. Almost no effect could be observed with degumming and deodorization, whereas the steps of deacidification and bleaching could reduce the content of phorbol esters up to 55%. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Jatropha curcas; Phorbol esters; Seed oil; Detoxification; Refining
www.elsevier.com/locate/indcrop
1. Introduction
Jatropha curcasL. (physic nut, purging nut), a tropical plant belonging to the family of Euphor-biaceae, is cultivated mainly as a hedge in many Latin American, Asian and African countries. The plant’s frugality to climate and soil, which makes it suitable for erosion control, as well as the manifold technical uses of the oil have led to various research projects all over the world
(Heller, 1986). Nutritional utilizations, however, the use of the seed oil for cooking purposes or of the press cake as animal feed are not possible due to the content of toxic compounds. This property of J. curcas is the subject of many publications (Gu¨bitz et al., 1998).
The seed kernels, which seem to be the part of the plant with the highest potential for utilization, contain 40 – 60% oil (Makkar et al., 1997) with a fatty acid composition (Gu¨bitz et al., 1998) simi-lar to that of oils used for human nutrition. A total of 19 – 27% crude protein can be obtained as cake (Makkar et al., 1997) which could be an ideal protein source with a content of essential amino acids even higher (except lysine) than the * Corresponding author. Tel.:+43-316-3805353; fax:+
43-316-3809841.
E-mail address:[email protected] (M. Mit-telbach)
FAO reference protein (Makkar and Becker, 1997). But the kernels also contain a number of several toxic or antinutritional compounds. Trypsin inhibitors, lectins, saponins and phytate might cause or at least aggravate adverse effects but the short term toxicity of the kernels are ascribed mainly to the phorbol esters content (Makkar et al., 1997).
The term ‘phorbol esters’ is used today to de-scribe a naturally occurring family of compounds widely distributed in plant species of the families Euphorbiaceae and Thymelaeceae. These com-pounds are esters of tigliane diterpenes (Evans, 1986a). The fundamental substance, the alcohol moiety, of this family of compounds is tigliane (Fig. 1), a tetracyclic diterpene. Hydroxylation of this fundamental substance in various positions and connection to various acid moieties by ester bonding characterize the large number of com-pounds termed as phorbol esters. The biological effects of these compounds include tumor promo-tion, cell proliferation, activation of blood platelets, lymphocyte mitogenesis, inflammation (erythema of the skin), prostaglandin production, and stimulation of degranulation in neutrophils (Aitken, 1986). These effects are closely related to the structure of the several compounds — phor-bol itself, the alcohol moiety, is inactive (Evans, 1986b) — and they were found to be correlated with an activation of the protein kinase C which leads to a variety of cellular responses by phos-phorylating target proteins on serine or threonine residues (Azzi et al., 1992).
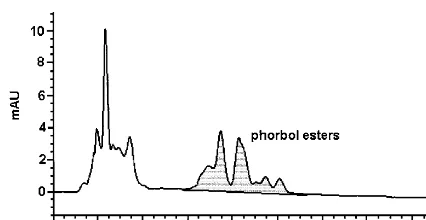
The kernels fromJ.curcascontain at least four different phorbol esters. The structure of the ma-jor compound is 12-deoxy-16-hydroxyphorbol-4% -[12%,14%-butadienyl]-6%-[16%,18%,20% -nonatrienyl]-bi-cyclo[3.1.0]hexane-(13-O)-2%-[carboxylate]-(16-O )-3%-[8%-butenoic-10%]ate (DHPB) (Fig. 2; Hirota et al., 1988). The alcohol moiety of another com-pound was found to be 12-deoxy-16-hydroxy-phorbol (Fig. 3) (Adolf et al., 1984; Biehl, 1987). The acid moiety was proposed to be a highly unsaturated dicarboxylic acid including an epox-ide ring (Biehl, 1987). The structures of the two remaining compounds are not totally clear. Gla¨ser (1991) has mainly concentrated on the quantita-tive analysis of the phorbol esters in the kernels of
J. curcas. Similar work was done by Wink et al. (1997) and Makkar et al. (1997).
These phorbol esters require a detoxification of the oil, even when it is industrially used and there is the possibility of direct contact of persons with the oil. Gross et al. (1997) suggest a method for detoxification of the oil by extraction of the phor-bol esters using ethanol. This method includes a large technical and economical effort and im-mense solvent consumption, so that large-scale detoxification requires an elaboration of alterna-tive processes. In this paper the influence of differ-ent oil refining steps on the contdiffer-ent of phorbol esters is studied.
Traditional edible oil refining consists of four steps (Bokisch, 1993; O’Brian, 1998). Degumming is done to remove phosphatides. Hydratable phos-phatides can be precipitated by adding water to the oil, nonhydratable ones must be destroyed by adding acids. Free fatty acids are removed by neutralization with alkali hydroxides leading to soaps which can be removed. Undesirable coloured impurities are removed by bleaching with an adsorptive reagent, the undesirable com-pounds are adsorbed and can be removed to-gether with the adsorbent by filtration. The final refining step is deodorization where undesirable volatile and odoriferous materials are removed by steam distillation at reduced pressure.
In the present work the effect of each refining step on the phorbol ester content ofJ.curcasseed oil was determined. Parameters of the different steps were varied to reach a maximum reduction of the phorbol ester concentration.
2. Material and methods
When % is used in the following it has to be understood as wt.% (w/w).
Analysis of the oil: water content, 0.1%; free fatty acids, 1.9%; fatty acid distribution, palmitic acid (11.9%); palmitoleic acid (0.3%); stearic acid (5.2%); oleic acid (29.9%); linoleic acid (46.1%); linolenic acid (4.7%); arachidic acid (0.3%); gadoleic acid (0.2%); behenic acid (0.4%); uniden-tified (1.0%).
2.1. Determination of phorbol esters
To determine the phorbol ester content a HPLC method based on a method elaborated by Wink et al. (1997) was used. Sample preparation for measurement was done by extracting 10.0 g of the oil four times with 10.0 g of methanol (techni-cal grade) each. The combined extracts were cen-trifuged and transferred into a 100 ml volumetric flask, which was filled up with methanol.
HPLC analyses were carried out with a Hewlett Packard instrument 1100 (Palo Alto, CA), equipped with a quaterny pump, a vacuum de-gasser, an autosampler, a chem station and a variable wavelength detector. The reversed phase chromatography column was purchased from Merck (Darmstadt, Germany); 125×4 mm, oc-tadecyl as functional group, particle size 5 mm. The column was thermally controlled at 25°C. As eluent a mixture of acetonitrile (HPLC grade, Promochem, Wesel, Germany) and water (HPLC grade, Fluka, Buchs, Switzerland) in the ratio of 80:20 (v/v) was used at a flow rate of 1 ml/min. The detector wavelength was set on 280 nm. A total of 20 ml of samples solution were injected.
A calibration curve was prepared using 4b,9a,12b,13a,20 - pentahydroxytiglia - 1,6 - dien - 3-on-12b-myristate-13-acetate (tetradecanoylphor-bolacetate (TPA), Sigma, UK) as an external standard (Bauer et al., 1983; Wink et al., 1997); the standard was dissolved in methanol (HPLC grade, Fluka, Buchs, Switzerland).
2.2. Refining of the oil
2.2.1. Degumming
A total of 625 g of untreated J. curcasL. seed oil was heated to 80°C under constant stirring at 1000 rpm in a beaker. Then 3% of distilled water, which first was heated to approximately 90°C, and afterwards 0.2% of ortho-phosphoric acid (85%, p.a., Merck, Darmstadt, Germany) were added. The mixture was stirred for 1 h. After cooling, the formed white precipitate was sepa-rated by centrifugation for 0.5 h at 3500 rpm. The degummed oil was dried at 100°C for 0.5 h under reduced pressure with the help of a rotavapor. Fig. 1. Tigliane.
Fig. 2. 12-Deoxy-16-hydroxyphorbol-4%-[12%,14%-butadienyl]-6% -[16%,18%,20%-nonatrienyl]-bicyclo[3.1.0]hexane-(13-O)-2% -[carb-oxylate]-(16-O)-3%-[8%-butenoic-10%]ate (DHPB).
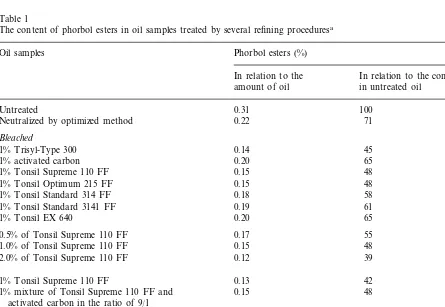
Fig. 4. HPLC chromatogram of the methanol extract of untreatedJatropha curcasseed oil.
2% of a bleaching reagent the flask was evacuated by using a water jet vacuum pump (16 mbar) and the mixture was stirred for 0.5 h. After cooling the bleaching agent was separated by filtration.
Used bleaching reagents: Trisyl-Type: 300 (a bleaching substance based on silicagel, produced by Grace, Worms, Germany), activated carbon, Tonsil Supreme 110 FF, Tonsil Optimum 215 FF, Tonsil Standard 3141 FF, Tonsil EX 640, Tonsil Standard 314 FF (Su¨d-Chemie, Munich, Ger-many), mixtures of Tonsil Supreme 110 FF with activated carbon in a ratio of 6:4 and 9:1.
2.5. Deodorization
A total of 290 g of the bleached oil was heated to 200°C and steam distilled for 2 h.
3. Results and discussion
3.1. Determination of the phorbol esters
The analysis in the present work used an iso-cratic mixture of 80% acetonitrile and 20% water which reduced the retention time of the phorbol esters by about 30 min to 6 – 11 min compared to the method of Wink et al. (1997). This reduction of analysis time correlated with a slight deteriora-tion of the resoludeteriora-tion of the several peaks and therefore the total sum of the phorbol ester peaks was used for quanitification (retention time: 6 – 11 min) (Fig. 4).
Prior experiments have shown, that after sam-ple preparation, which includes extracting the oil four times with the same amount (w/w) of methanol, 5% of the phorbol esters are still re-maining in the oil. This loss was taken into ac-count by compensation with the quotient out of 100/95.
It has to be mentioned that the use of TPA as external standard according to Wink et al. (1997) leads to far higher values than when using DHPB (Gla¨ser, 1991), which, however, is not commer-cially available. As for the present work, only the decrease of the concentration of phorbol esters was interesting, this difference was neglected. 2.3. Neutralization by caustic treatment
After determining the content of free fatty acids of the degummed oil, 30 g resp. 540 g of the oil were heated to 70°C under constant stirring at 1000 rpm in a beaker. Then 1.0; 1.5; 2.0; 2.5 and 3.0 M aqueous NaOH resp. KOH solution was added to the oil, corresponding to an excess of 5, 10, 15, 20 and 25% needed to neutralize the free fatty acids. The mixture was stirred for 10, 20 and 60 min. The appropiate amount of alkaline solu-tion (NaOH resp. KOH) to neutralize the free fatty acids was calculated by the following equa-tion (Bokisch, 1993):
L=d· FFA · 10000
M·N
where L=appropriate volume of N-molar aqueous NaOH solution (l);d=density of the oil (d=0.91 forJ.curcasseed oil of the used varietes, determined at 33°C by Hackel (1994)); M= aver-age molecular weight of the fatty acids (M=278);
N=concentration of the aqueous NaOH solution (mol/l).
After cooling the oil was centrifuged at 3500 rpm to separate the formed soaps and washed with the half amount of distilled water (w/w) for three times and afterwards dried at 100°C for 0.5 h by using a rotavapor.
2.4. Bleaching
3.2. Refining of the oil
The effect of the several steps of the refining process on the content of phorbol esters in theJ.
curcasseed oil was quite varying. The influence of degumming and deodorization was very low, whereas the steps of neutralization and bleaching led to significant reduction of phorbol esters. The content of phorbol esters in the untreated oil was 0.31%. This could not be reduced by degumming. Treatment of the degummed oil by caustic neu-tralization lowered the value to approximately 0.20 – 0.25%. The oil was neutralized with 1.0, 1.5, 2.0, 2.5 and 3.0 M aqueous NaOH or KOH solutions, with a stoichiometric excess of base of 5, 10, 15, 20 and 25%. The mixture was stirred for 10, 20 and 60 min. The optimum conditions in-cluded 20% excess of base and 2.5 M aqueous
KOH at 70°C for 20 min. With these conditions a reduction of phorbol esters from 0.31 to 0.22% could be achieved.
As starting material for experiments on bleach-ing, 20 g of neutralized J. curcasseed oil with a phorbol ester content of 0.22% was used. A series of bleaching agents at 1% concentration were tested with stirring for 30 min at 80°C. The highest reduction of the phorbol ester content was reached by using Trisyl-Type 300 (0.14%), Tonsil Supreme 110 FF (0.15%) and Tonsil Supreme 215 FF (0.15%) (Table 1, Fig. 5). No significant re-duction was achieved with activated carbon (0.20%) or other activated bleaching earths (Ton-sil Standard 3141 FF, Ton(Ton-sil EX 640). For further experiments Tonsil Supreme 110 FF was used.
In the following test series different amounts of Tonsil Supreme 110 FF were used, the other
Table 1
The content of phorbol esters in oil samples treated by several refining proceduresa
Oil samples Phorbol esters (%)
In relation to the In relation to the content amount of oil in untreated oil
0.31
Untreated 100
0.22 71
Neutralized by optimized method
Bleached
1% Trisyl-Type 300 0.14 45
1% activated carbon 0.20 65
1% Tonsil Supreme 110 FF 0.15 48
1% Tonsil Optimum 215 FF 0.15 48
0.18 58
1% Tonsil Standard 314 FF
1% Tonsil Standard 3141 FF 0.19 61
0.20
1% Tonsil EX 640 65
0.5% of Tonsil Supreme 110 FF 0.17 55
1.0% of Tonsil Supreme 110 FF 0.15 48
2.0% of Tonsil Supreme 110 FF 0.12 39
0.13
1% Tonsil Supreme 110 FF 42
0.15
1% mixture of Tonsil Supreme 110 FF and 48
activated carbon in the ratio of 9/1
52 1% mixture of Tonsil Supreme 110 FF and 0.16
activated carbon in the ratio of 6/4
0.13 42
1% Tonsil Supreme 110 FF
two times 1% Tonsil Supreme 110 FF each 0.12 39
0.11 35
three times 1% Tonsil Supreme 110 FF each
0.09
four times 1% Tonsil Supreme 110 FF each 29
Fig. 5. Phorbol ester content in oil samples bleached with different bleaching reagents; (a) untreated oil (reference value=100%); (b) oil, bleached by using Trisyl-Type 300; (c) oil, bleached by using activated carbon; (d) iol, bleached by using Tonsil Supreme 110 FF; (e) oil, bleached by using Tonsil Optimum 215 FF; (f) oil, bleached by using Tonsil Standard 314 FF; (g) oil, bleached by using Tonsil Standard 3141 FF; (h) oil, bleached by using Tonsil EX 640.
Fig. 6. Phorbol ester content in oil samples bleached with different amounts of bleaching earth or bleached several times in series; (a) untreated oil (reference value=100%); (b) oil, bleached by using 0.5 % Tonsil Supreme 110 FF; (c) oil, bleached by using 1.0% of Tonsil Supreme 110 FF; (d) oil, bleached by using 2.0 % of Tonsil Supreme 110 FF; (e) oil, bleached once; (f) oil, bleached two times in series; (g) oil, bleached three times in series; (h) oil, bleached four times in series.
parameters were kept at a constant level (time, 30 min; temperature, 80°C). It is shown (Table 1, Fig. 6) that increasing the amount of bleaching earth leads to a higher reduction of the phorbol ester content.
In another series the influence of temperature was examined. However, no significant relation between temperature and decrease of phorbol es-ter content could be found.
1%. Evaluation of the results (Table 1) shows that adding activated carbon to the bleaching earth does not increase the reduction of phorbol ester content.
In a final test row neutralized oil was bleached four times in series by using 1% Tonsil Optimum 110 FF each at 110°C for 30 min. Results are represented in Table 1 and Fig. 6. Comparison of the results with those out of the test row, in which the amount of bleaching earth was varied (Table 1, Fig. 6: oil, bleached by using 2.0% Tonsil Optimum 110 FF), shows that both alternatives are equal in regard to reducing the phorbol ester content. Although the experiments were carried out at different temperatures (80 and 110°C), the negligible influence of process temperature on the phorbol ester content in the bleached oil allows direct comparison of the results. Nevertheless, the use of a larger amount of bleaching earth in just one process step is assumed to be the better method, because activated bleaching earth can lead to oxidation reactions (Bokisch, 1993). Fi-nally it was determined that optimized bleaching is done with 2% of Tonsil Supreme 110 FF at 110°C for 30 min.
Deodorization is usually done by steam distilla-tion at reduced pressure. Due to the lack of appropriate equipment steam distillation was exe-cuted at normal pressure. However, this proce-dure did not have any influence on the content of phorbol esters.
After finishing the preliminary experiments, a larger amount of untreated J. curcasseed oil was refined according to the optimized refining pro-cess. The content of phorbol esters in untreatedJ.
curcas seed oil was 0.31%. By refining it was reduced to 0.17%; thus, about 45% of the phorbol esters were removed or destroyed. The contribu-tion of the different refining steps to the decrease of phorbol ester content is shown in Fig. 7. The reduction of the phorbol ester concentration was less than expected, which maybe was caused by the scale up of the different steps.
4. Conclusions
It could be shown that by traditional oil refining including degumming, deacidification, bleaching and deodorization the content of phor-bol esters can be reduced by approximately 50%. A total detoxification, however, which is neces-sary for nutritional and several technical applica-tions, could not be achieved under these conditions. The treatment with alkali hydroxides during acidification as well as bleaching with tra-ditional bleaching earth have the most influence on decreasing the amount of phorbol esters. Most probably hydrolysis of the phorbol esters takes place under these conditions, which leads to par-tial detoxification. Further investigations are
rently running to find additional routes for detox-ification J. curcas L., which seems to be very promising as an industrial crop of the future. Perhaps a combination of extraction and refining can lead to an economical solution.
Acknowledgements
The work was financed by the Austrian govern-ment within the scope of a developgovern-ment help project and organized by Sucher & Holzer, Graz, Austria.
References
Adolf, W., Opferkuch, H.J., Hecker, E., 1984. Irritant phorbol derivates from four Jatropha species. Phytochemistry 23 (1), 129 – 132.
Aitken, A., 1986. The biochemical mechanism of action of phorbol esters. In: Evans, F.J. (Ed.), Naturally Occurring Phorbol Esters. CRC Press, Boca Raton, pp. 271 – 288. Azzi, A., Boscoboinik, D., Hensey, C., 1992. The protein
kinase C family. Eur. J. Biochem. 208, 547 – 557. Bauer, R., Tittel, G., Wagner, H., 1983. HPLC-Nachweis und
Isolierung von Phorbolestern aus Crotono¨l. Planta Med. 48/1, 10 – 16.
Biehl, J., 1987. U8ber irritierende Diterpene der Pflanzenfamilie der Euphorbiaceae, insbesondere des Genus Jatropha, und deren ko- und antikarzinogene Wirkung. Ph.D. thesis, University of Heidelberg.
Bokisch, M., 1993. Handbuch der Lebensmitteltechnologie-Nahrungsfette und -o¨le. Ulmer, Stuttgart.
Evans, F.J., 1986a. Environmental hazards of diterpene esters from plants. In: Evans, F.J. (Ed.), Naturally Occurring Phorbol Esters. CRC Press, Boca Raton, FL, pp. 1 – 31. Evans, F.J., 1986b. Phorbol: its esters and derivatives. In:
Evans, F.J. (Ed.), Naturally Occurring Phorbol Esters. CRC Press, Boca Raton, FL, pp. 171 – 215.
Gla¨ser, S., 1991. Untersuchung zu einem mo¨glichen Gesund-heits- und Krebsrisiko durch pflanzliche Arzneimittel sowie
industriell genutzte Rohstoffe aus Euphorbiaceen-Quanti-tative Bestimmung von irritierenden und tumor-pro-movierenden Diterpenestern und Evaluierung durch biochemische biologische Tests. Ph.D. thesis, University of Heidelberg, Heidelberg.
Gu¨bitz, G.M., Mittelbach, M., Trabi, M., 1998. Exploitation of the tropical oil seed plant Jatropha curcas L. Biores. Technol. 67, 73 – 82.
Gross, H., Foidl, G., Foidl, N., 1997. Detoxification of J. curcaspress cake and oil and feeding experiments on fish and mice. In: Gu¨bitz, G.M., Mittelbach, M., Trabi, M. (Eds.), Biofuels and Industrial Products from Jatropha curcas. Dbv, Graz, pp. 179 – 182.
Hackel, S., 1994. Untersuchungen zur Treibstoffgewinnung aus o¨lha¨ltigen Samen des Purgierstrauches (Jatropha curcas L.) in Nicaragua. Ph.D. thesis, University of Graz, Graz. Heller, J., 1986. Physic NutJatropha curcasL. — Promoting the Conservation and Use of Underutilized and Neglected Crops. Institute of Plant Genetics and Crop Plant Re-search, Gatersleber/International Plant Genetic Resources Institute, Rome.
Hirota, M., Suttajit, M., Suguri, H., Yasuyuki, E., Shudo, K., Wongchai, V., Hecker, E., Fujiki, H., 1988. A new tumor promoter from the seed oil of Jatropha curcas L., an intramolecular diester of 12-deoxy-16-hydroxyphorbol. Cancer Res. 48, 5800 – 5804.
Makkar, H.P.S., Becker, K., 1997. Potential ofJ.curcasseed meal as a protein supplement to livestock feed, constraints to its utilisation and possible strategies to overcome con-straints. In: Gu¨bitz, G.M., Mittelbach, M., Trabi, M. (Eds.), Biofuels and Industrial Products from Jatropha curcas. Dbv, Graz, pp. 190 – 205.
Makkar, H.P.S., Becker, K., Sporer, F., Wink, M., 1997. Studies on nutritive potential and toxic constituents of different provenances ofJatropha curcas. J. Agric. Food Chem. 45, 3152 – 3157.
O’Brian, R.D., 1998. Fats and Oils-Formulating and Process-ing for Applications. Technomic, Lancaster.
Patterson, H.B.W., 1976. Bleaching practices in Europe. J. Am. Oil Chem. Soc. 53, 339 – 341.
Wink, M., Koschmieder, C., Sauerwein, M., Sporer, F., 1997. Phorbol esters of J. curcas — biological activities and potential applications. In: Gu¨bitz, G.M., Mittelbach, M., Trabi, M. (Eds.), Biofuels and Industrial Products from Jatropha curcas. Dbv, Graz, pp. 160 – 166.