Summary A 7-m tall white ash tree (Fraxinus americana Marsh.) was dissected, and hydraulic parameters of the xylem were determined by inducing a steady-state flow of water through the stem segments and monitoring volume and velocity flow rates. Leaf-specific conductivity (LSC) was highest in the main stem and lowest in some of the leaf-bearing lateral shoots. The LSC was higher in the main stem than in branches and higher in primary than in secondary branch axes. Terminal leaf-bearing shoots were larger and had a significantly greater mean LSC than subjacent lateral shoots. A significant reduction in LSC was associated with the transition between 1- and 2-year-old growth. In branches of the same age, there was a close correspondence among LSC, branch position and branch size. The average LSC of leaf-bearing shoots from south-facing branches was 43% greater than that of shoots from north-facing branches.
Within-crown variation in LSC was associated with vari-ation in velocity flow rate (V). By contrast, the ratio of poten-tially functional xylem area to supported leaf area (Apf/Al) was relatively stable throughout the crown. Stratification of stems by Strahler order accounted for approximately 70% of the total variation in LSC. These results suggest that (1) there exists a systematic pattern of variation in LSC distribution within the crown of white ash, (2) within-crown variability in LSC is primarily the result of variation in mean vessel diameter, and (3) there is a physiological linkage between LSC and crown morphology that is maintained through a positive feedback mechanism during branch ontogeny.
Keywords: hydraulic architecture, hydraulic dominance, hy-draulic segmentation, leaf-specific conductivity.
Introduction
Xylem tissue in trees serves several biological functions in-cluding mechanical support and vascular transport. Because the relative importance of these functions varies according to branch location, there may exist some measure of order in the structure of the internal water transport system and its relation-ship to the morphology of a tree’s crown.
Long distance transport of water and mineral nutrients is a vital function of the xylem. A practical measure of the xylem’s
hydraulic supply capacity is leaf-specific conductivity (LSC) (Zimmermann 1978). For example, the LSC of a stem segment can be used to relate the average transpirational water loss (E) from leaves supported by the segment to the decline in water potential (dΨ) per unit path length (dx) within the segment (dΨ/dx = E/LSC). Thus, the distribution of LSC within a tree influences patterns of Ψ throughout the crown and can impose constraints on such physiological processes as transpiration and photosynthesis (Tyree and Sperry 1988, Shumway et al. 1993, Yang and Tyree 1993).
Within-tree patterns of LSC distribution have been quanti-fied for several angiosperm and conifer tree species (Zimmer-mann 1978, 1983, Tyree etal. 1983, Ewers and Zimmermann 1984a, 1984b, Tyree etal. 1991). In most species examined, LSC increases with increasing stem diameter and is higher in the main stem than in branches. Another consistently observed feature of tree hydraulic architecture is the increased hydraulic resistance associated with leaf and branch junctions (Isebrands and Larson 1977, Zimmermann 1978, Tyree etal. 1983, Ewers and Zimmermann 1984a, 1984b).
Zimmermann (1983) considered that LSC distribution pat-terns in tree crowns were evidence of hydraulic segmentation, defined as a structural feature that confines cavitation events to relatively expendable plant parts in favor of parts more impor-tant to plant survival. Hydraulic segmentation is considered an architectural advantage in dicotyledonous tree species because leaves and small diameter stems are more readily replaced than older, supporting stem structures.
Xylem also provides mechanical support to the crown. The distribution of xylem anatomical features within a tree’s crown likely reflects a compromise between hydraulic and mechani-cal support functions. Because the multiple roles of xylem depend directly on its anatomical characteristics, we hypothe-sized that crown development will result in an organized pat-tern of xylem anatomical features. The objective of this study was to describe this pattern of organization in white ash ( Frax-inus americana Marsh.) by characterizing variations in several xylem hydraulic parameters throughout an entire tree crown. We also examined the relationship between functional and morphological attributes.
Systematic variation in xylem hydraulic capacity within the crown of
white ash (
Fraxinus americana
)
BRIAN J. JOYCE
1and KIM C. STEINER
21 School of Forest Resources, 206 Forest Resources Laboratory, The Pennsylvania State University, University Park, PA 16802, USA
2 School of Forest Resources, 213 Ferguson Building, The Pennsylvania State University, University Park, PA 16802, USA
Received September 19, 1994
Materials and methods
Plant material and branch segmentation
An 18-year-old white ash tree that was approximately 7 m in height was selected for study from within a plantation popula-tion in Center County, PA. The entire crown and main stem of the tree was destructively sampled. Starting in early Septem-ber, all branches in the crown were labeled, and leaf areas were measured. Stem segments were coded starting with the distal end of the lowest branch. The first internode of at least 0.075 m in length was identified as segment one, and the leaves sup-ported by that segment were collected and placed in a high humidity bag. This procedure was continued basipetally for all lateral and terminal branch segments. Segment codes were used to identify parent and descendent segments associated with branch trifurcations (ash has opposite leaves). Leaf area (Al) supported by each current-year shoot was measured to the nearest 0.01 cm2 with a model LI-3000 surface area meter (Li-Cor Inc., Lincoln, NE). After all stem segments were identified on the tree (390 total), detailed drawings were made of each branch system noting the relative position of all labeled segments and the compass quadrant orientation of each branch from the main stem: NE (1--90°), SE (91--180°), SW (181--270°), and NW (271--360°). The Strahler order (McDonald 1983) of each segment was also noted.
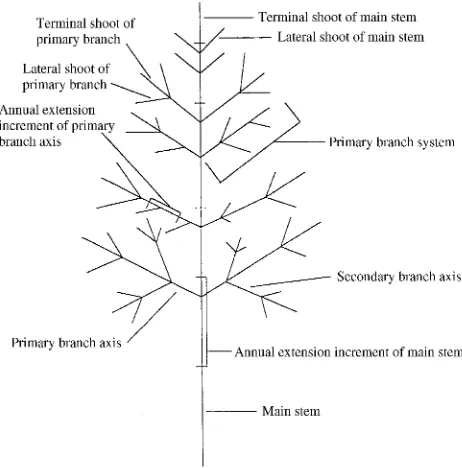
To facilitate within-crown comparisons, stem segments were assigned to a hierarchy of crown components (Figure 1): the main stem with its primary branches, and primary branch systems comprising a central axis and secondary branches.
Measurement of xylem hydraulic parameters
Xylem hydraulic parameters were measured during the dor-mant season from November 3 to February 11. On each sam-pling day, an entire branch system was cut from the tree with hand pruners and transported to the laboratory. Segments ap-proximately 0.15 m long were excised from labeled internodes and cut to 0.075 m in length with a miter trimmer, to assure a smooth stem surface without crushing the vessels. Segments were immersed in a water bath at 20 °C until measurements were made. A 1.0-cm-wide band of bark and phloem tissue was removed from the ends of the segments, and the segments were fitted with plastic tubing with a neoprene seal.
To remove air emboli, stem segments were then attached to an apparatus similar in design to that described by Kelso et al. (1963), and pressurized at their proximal ends with filtered (0.2 µm retention) and de-aerated, distilled water at a gradient of 0.75 MPa m−1 until a steady-state flow condition was achieved. The pressure gradient was then reduced to 0.14 MPa m−1, and the system was allowed to reach steady-state flow. Because there was no decrease in flow with time, a perfusing agent was not used (see Zimmermann 1978). Steady-state mass flow rate (M, kg s−1) was then measured gravimetrically by collection of induced flow in beakers over a 15-min interval and converted to volume flow rate (Q, m3 s−1). At the end of the 15-min interval, 0.5 M KCl was injected with a syringe into the water stream as it entered the segment. Electrical resistance was monitored at the segment output. The average velocity flow rate (V, m s−1) was estimated from segment length and the elapsed time between injection of KCl and the average change in electrical resistance of the output stream. The potentially functional transverse xylem area (Apf, m2) (Shumway et al. 1991) was determined as Apf = Q/V (Preston 1952, Heine 1971). Because air emboli were removed under positive pres-sure, the derived Apf parameter represents a maximum conduc-tive xylem area and may include vessels that were dysfunctional in situ. Leaf specific conductivity (LSC, m2) was calculated as: the stem segment (m2), and P is the pressure difference across the segment (MPa). Most published values of LSC do not include units of viscosity and must be multiplied by 10−12 m3 kg−1 MPa s (at 20 °C) to obtain the same units for LSC.
For each stem segment, total xylem (less pith) cross-sec-tional area (Ax) (m2) was measured on a thin, transverse section from the segment’s midpoint by means of a digital caliper and stereomicroscope.
Statistical methods
Paired comparison analysis (see Sokal and Rohlf 1969) was used to test differences in both hydraulic and morphological variables between branches with opposing compass directions and between terminal and lateral leaf-bearing shoots. Each Figure 1. Diagrammatic representation of the descriptive terminology
individual branch pair (originating from the same node) and each terminal-lateral shoot pair was treated as a within-crown replicate. Comparisons of xylem characteristics between par-ent and descendant segmpar-ents of branch trifurcations were made by paired t-tests (α = 0.05). Trifurcation comparisons were made using the mean of the descendant segment values and the parent segment value. Paired t-tests were also used to test differences in hydraulic parameters among branches aris-ing from a common annual extension increment of the main stem, between segments immediately above and below the transition between the 1-year-old and 2-year-old extension growth of branch axes and between different year extension increments of primary and secondary branch axes. Analysis of variance (ANOVA) and mean separation procedures were con-ducted with Statistical Analysis Systems software (SAS Inc., Cary, NC). Variance component analysis (Steel and Torie 1980) was used to calculate the proportion of total variance in the measured variables explained by Strahler order.
Results
General distribution of xylem hydraulic and morphological parameter values
Values of LSC and V varied considerably (CV = 1.16 and 1.01, respectively) within the crown, but the Apf/Al ratio was less variable (CV = 0.53). Both LSC and V were lowest in
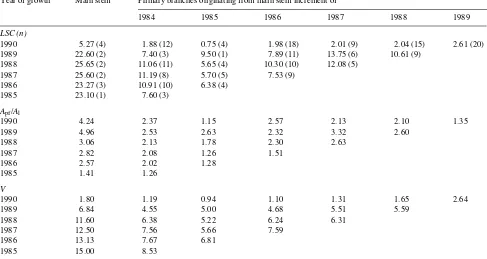
first-or-der (ultimate) segments and increased significantly (P < 0.05) with increasing branch order (Table 1). The Apf/Al ratio showed little variation (P > 0.05) among branch orders. Strahler order accounted for 72.53, 0.68 and 84.87% of the total variability in LSC, Apf/Al and V, respectively. Values of LSC for the main stem were higher than those for primary branch axes as a result of substantially greater V and Apf/Al values in the main stem (Table 2). The mean LSC of the main stem (mean = 20.91) was more than 2.9 times that of the proximal segments of adjacent branches (mean = 7.80). Values of LSC were similar among segments along the main stem until the last extension incre-ment (1990), where there was a sharp decrease from 22.60 to
Table 1. Mean (± 1 SE) leaf specific conductivity (LSC) × 1016 in m2, potentially functional xylem area to leaf area ratio (Apf/Al) × 106 (unitless), and velocity flow rate (V) × 102 in m s−1 for all segments from white ash crown and for segments stratified by Strahler order. Means within a column appearing with the same letter are not signifi-cantly different (P > 0.05) from one another using Fisher’s LSD test.
Strahler n LSC Apf/Al V
order
All orders 390 4.58 (0.27) 2.21 (0.59) 2.94 (1.51) Order 1 257 1.72 (0.07) a 2.21 (0.78) a 1.18 (0.47) a Order 2 102 9.06 (0.52) b 2.21 (1.10) a 5.63 (2.10) b Order 3 26 12.60 (1.18) c 2.10 (1.10) a 8.00 (4.71) c Order 4 5 18.62 (3.10) d 2.30 (2.90) a 12.75 (13.78) d
Table 2. A comparison of mean leaf specific conductivity (LSC) × 1016 in m2, potentially functional xylem area to leaf area ratio (Apf/Al) × 106 (unitless), and velocity flow rate (V) × 102 in m s−1 between the sequence of annual extension increments that comprise the main stem and the central axes of primary branches originating from the same annual increment of the main stem (n = number of segments representing each year of growth). Primary branch values within a column represent successive annual segments along those branch axes. All values within a row represent segments of the same age but at different crown positions.
Year of growth Main stem Primary branches originating from main stem increment of
1984 1985 1986 1987 1988 1989
LSC (n)
1990 5.27 (4) 1.88 (12) 0.75 (4) 1.98 (18) 2.01 (9) 2.04 (15) 2.61 (20) 1989 22.60 (2) 7.40 (3) 9.50 (1) 7.89 (11) 13.75 (6) 10.61 (9)
1988 25.65 (2) 11.06 (11) 5.65 (4) 10.30 (10) 12.08 (5) 1987 25.60 (2) 11.19 (8) 5.70 (5) 7.53 (9)
1986 23.27 (3) 10.91 (10) 6.38 (4) 1985 23.10 (1) 7.60 (3)
Apf/Al
1990 4.24 2.37 1.15 2.57 2.13 2.10 1.35
1989 4.96 2.53 2.63 2.32 3.32 2.60
1988 3.06 2.13 1.78 2.30 2.63
1987 2.82 2.08 1.26 1.51
1986 2.57 2.02 1.28
1985 1.41 1.26
V
1990 1.80 1.19 0.94 1.10 1.31 1.65 2.64
1989 6.84 4.55 5.00 4.68 5.51 5.59
1988 11.60 6.38 5.22 6.24 6.31
1987 12.50 7.56 5.66 7.59
1986 13.13 7.67 6.81
5.27. Values of V declined steadily along the main stem with a pronounced decrease (from 6.84 to 1.80) at the 1990 extension increment. The Apf/Al ratio increased acropetally along the main stem, with only a slight decline (4.96 to 4.24) at the 1990 extension increment. Similar trends for LSC, V and Apf/Al occurred along the axes of primary branches that originated in 1985 from the 1984 increment of the main stem.
Differences in xylem hydraulic parameters between the cen-tral and lateral axes of primary branch systems were similar to the observed differences between the main stem and the central axes of primary branches from the main stem (Table 3). Values of LSC, V and Apf/Al of primary branch axes were consistently higher than those of secondary branch axes.
In all branches sampled, the transition between 1- and 2-year-old extension growth represented a point of reduction in hydraulic supply capacity, i.e., there were significant reduc-tions (P < 0.05) in mean LSC and V across the transition (Table 4). The Apf/Al ratio did not differ significantly in seg-ments from above and below the transition.
Xylem hydraulic parameters in terminal versus lateral leaf-bearing shoots
The values of LSC varied in a consistent manner between terminal and lateral leaf-bearing shoots. The mean LSC of shoots arising from terminal buds was significantly greater (P < 0.01) than that of shoots arising from axillary buds imme-diately below the terminal (Table 5). The difference is attribut-able to a difference in V, because there was no difference (P > 0.05) in Apf/Al. Terminal shoots were longer than lateral shoots and had a greater (P < 0.05) mean Ax.
Variation in LSC among branches arising from a common annual extension increment
Within an annual extension increment of the main stem, there was a pattern of hydraulic dominance (higher LSC) expressed by branches in distal (superior) positions compared to their subordinates. Because of the small number of comparisons (n = 5 pairs) and the large amount of variation that existed among the various extension increments, a significant
differ-Table 3. A comparison of mean leaf specific conductivity (LSC) × 1016 in m2, potentially functional xylem area to leaf area ratio (Apf/Al) × 106 (unitless), and velocity flow rate (V) × 102 in m s−1 between the central axis and lateral branches of 2-, 4- and 6-year-old primary branch systems. Each branch age group is represented by three branches (n = number of segments in sample). For a given variable within a branch age group, means within a row appearing with the same letter are not significantly different from one another (P > 0.05) using paired t-tests.
Year of growth n (axis) n (branch) LSC Apf/Al V
Axis Branch Axis Branch Axis Branch
6-Year-old primary branches
1990 10 39 1.90 a 1.12 b 2.37 a 1.77 b 1.23 a 0.93 a
1989 2 9 6.30 a 2.97 b 2.53 a 1.89 a 4.55 a 2.89 a
1988 9 12 10.14 a 2.27 b 2.25 a 1.67 b 6.41 a 2.85 b
1987 6 7 11.00 a 3.64 b 2.13 a 1.39 b 7.58 a 3.66 b
1986 5 1 10.32 6.30 2.15 1.44 6.82 5.80
1985 4 7.08 1.22 8.10
4-Year-old primary branches
1990 10 38 2.61 a 1.21 b 2.98 a 2.13 a 1.24 a 0.83 b
1989 7 7 9.43 a 4.12 b 2.42 a 1.99 b 5.35 a 2.85 b
1988 6 1 11.42 1.00 2.30 1.04 6.96 1.27
1987 4 9.67 1.98 6.56
2-Year-old primary branches
1990 12 17 2.07 a 0.98 b 2.10 a 2.42 a 1.74 a 0.68 b
1989 7 11.15 2.73 5.14
Table 4. Mean leaf specific conductivity (LSC) × 1016 in m2, velocity flow rate (V) × 102 in m s−1, and potentially functional xylem area to leaf area ratio (Apf/Al) × 106 (unitless) for segments immediately above and below the transition between the 1-year-old and 2-year-old growth of primary branch axes and results of paired t-tests (n = 19).
LSC V Apf/Al
Above transition 1.44 1.37 2.13 (1-year-old growth)
Below transition 8.17 4.78 2.34 (2-year-old growth)
P-value < 0.001 < 0.001 > 0.300
Table 5. A comparison of hydraulic parameters for terminal and lateral leaf-bearing shoots and results of paired comparison F-tests (n = 18 pairs). Table values are leaf specific conductivity (LSC) × 1016 in m2, velocity flow rate (V) × 102 in m s−1, and potentially functional xylem area to leaf area ratio (Apf/Al) × 106 (unitless). Lateral means were computed using values from the two laterals below each terminal.
Shoot position LSC V Apf/Al
Terminal 2.13 1.49 2.04
Lateral 1.26 0.90 2.05
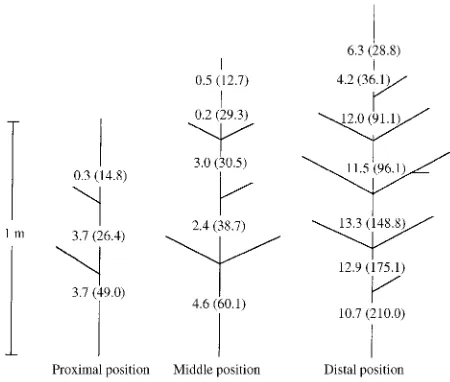
ence in LSC between distal and proximal branches within an annual increment was not detected (P > 0.05); however, when-ever a branch system in a more distal branch position was more vigorous (i.e., greater length, larger basal diameter, more ex-tensive branching) than a branch in a proximal position, it consistently had a higher LSC (Figure 2).
LSC in branches with opposing orientations
Values of LSC at the base of terminal shoots of branches from the southwest (SW) quadrant (mean = 3.02) were greater (P < 0.05) than those of branches from the northeast (NE) quadrant (mean = 2.14), and LSC values at the base of shoots from the southeast (SE) quadrant (mean = 2.35) were greater (P < 0.05) than those of shoots from the northwest (NW) quadrant (mean = 1.61). The mean LSC at the base of terminal shoots with a southern exposure (SW and SE quadrants combined) was 43% greater (P < 0.05) than that of shoots with a northern exposure (NW and NE quadrants combined). The average LSC for all primary and secondary branch segments was also greater (P < 0.05) for branches with a southern exposure (mean = 4.32) than for branches with a northern exposure (mean = 3.10). Although not significantly different, both V and Apf/Al values were con-sistently higher in south-facing branches than in north-facing branches.
Comparison of parent and descendant segments
Measured values of LSC, V and Apf/Al for parent and descen-dant segments for 17 trifurcations of third-order branches are shown in Table 6. There were no significant differences (P > 0.05) between the parent and terminal descendant segments for any of the parameters. In contrast, significant differences were detected between the parent and lateral descendant segments for all three parameters. Mean lateral segment LSC was 63.22% lower than the mean parent LSC as the result of a
reductions in both V and Apf/Al. The average V for lateral segments was 62.38% lower than for parent segments, and the average Apf/Al ratio was 29.63% lower.
Discussion
Variations in LSC values within the tree crown and within its component parts resulted primarily from variation in V; by comparison, the Apf/Al ratio was much less variable. Variations in both LSC and V were systematically related to branch order. Stratification by Strahler order accounted for slightly more than 70% of the total variation in both LSC and V.
The highest LSC values occurred in the main stem. The hydraulic dominance of the main stem over the primary branches appeared to arise in the year that shoots were formed by the elongation of terminal (stem axis) and axillary (branch) buds on the main stem and was attributable to differences in both V and Apf/Al (Table 2). Because velocity flow rate is proportional to the square of mean vessel diameter (Zimmer-mann and Brown 1971), the observed pattern of xylem hydrau-lic capacity must be associated with a greater mean vessel diameter in the main stem relative to that in the branches as has been previously observed in both conifers (Ewers and Zimmer-mann 1984a, 1984b) and angiosperms (Zimmermann 1978).
The pattern of LSC distribution within the main stem and its branches was repeated within the primary branch axes and their secondary branches. As with the main stem, the pattern of LSC dominance in the primary branch axis compared to branches from that axis appeared to arise in the first year of shoot elongation and persisted over subsequent years (Ta-ble 3).
A substantial reduction in the hydraulic capacity of the xylem was associated with the lateral segments of trifurcations of primary branches. Although differences in LSC between parent and lateral descendent segments were associated with reductions in both components of LSC (Apf/Al and V), they resulted primarily from differences in V (Table 6), suggesting that mean vessel diameter is smaller in lateral segments than in parent segments.
The reduction in LSC at the transition between the 1- and 2-year-old growth resulted from a decrease in V, indicating a reduction in mean vessel diameter across the transition. Simi-lar reductions have been observed in the xylem of Quercus alba L. and Quercus rubra L. (Cochard and Tyree 1990), Figure 2. Leaf specific conductivity (LSC in m2× 1016) and transverse
xylem area (Ax in m2× 106) (in parentheses) along the central axes of three primary branches arising from the proximal, middle and distal positions along a common annual extension increment (1986) of the main stem.
Table 6. A comparison of hydraulic parameters between parent (P) and terminal (DT) and lateral descendant (DL) stem segments of 17 trifur-cations of third-order branches and results of paired t-tests. Table values are leaf specific conductivity (LSC) × 1016 in m2, velocity flow rate (V) × 102 in m s−1, and potentially functional xylem area to leaf area ratio (Apf/Al) × 106 (unitless).
Variable P DT P-value DL P-value
where vessel diameters were significantly smaller in 1-year-old shoots than in 2-year-1-year-old shoots. Substantial reductions in LSC at this transition have also been reported for several diffuse-porous species (Zimmermann 1978) and may result from the developmental lag between cambial reactivation and secondary xylem development observed in elongating shoots (Larson 1976). The slight acropetal increase in Apf/Al observed at the transition provided some compensation for the relatively large decrease in V across this growth node.
Because the terminal growth increment of a main axis con-sistently had a lower LSC than older portions of the same axis, the terminal increment itself was analogous to a branch from the main axis. However, the analogy is imperfect because in every instance the terminal extension increment had higher LSC and Ax values and greater extension growth than lateral shoots of the same age located immediately below the termi-nal. We have found a similar pattern of hydraulic dominance within the crowns of eight other white ash trees (unpublished data). A similar pattern of hydraulic dominance of the main axis was also observed in Abies balsamea (L.) Mill. (Ewers and Zimmermann 1984b), where LSC was higher in the termi-nal leader than in lateral segments of equal diameter, and was associated with a strong expression of apical control (as de-fined by Zimmermann and Brown 1971). In contrast, the plagiotropic leader of Tsuga canadensis (L.) Carr. did not exhibit hydraulic dominance or a strong expression of apical control (Ewers and Zimmermann 1984a).
Sellin (1987) suggested that hydraulic architecture plays a significant role in apical control and overall crown form. He observed that, in Norway spruce (Picea abies (L.) Karst.), the terminal shoot grows faster and for a longer period than the lateral shoots, and that this growth habit is repeated in the branches, resulting in a conical crown. In white ash, we found that the terminal shoots exhibited greater elongation and di-ameter growth than lateral shoots, and the terminal shoots also had lower resistance to water transport, suggesting a mechanis-tic linkage between xylem hydraulic capacity and shoot devel-opment.
The relationship between hydraulic dominance and mor-phology observed in the leaf-bearing shoots was also evident among primary branches arising from a common annual exten-sion increment of the main stem. Typically, distal (upper) branches arising from a given increment had a higher LSC and were better developed (greater diameter and length, and more extensive branching) than their subordinates. Because LSC values are scaled to leaf area, LSC and branch size are poten-tially independent. Thus, an association between high LSC and large size suggests the existence of a physiological connection between LSC and stem size during branch ontogeny.
Initial differences in LSC and diameter between terminal and lateral shoots and among lateral shoots originating from the same extension increment may be attributable to differ-ences in the size and phenology of the buds from which the shoots developed. Gill (1971) found that current-year incre-ments of white ash have large terminal buds and decreasingly smaller lateral buds at successively lower nodes. We found a similar order of dominance in the size and LSC of shoots
arising from buds in these respective positions, indicating that there is a strong linkage between the size of the bud and subsequent LSC development (cf. Larson 1976).
We suggest that a high LSC and large stem size in dominant branches are maintained by a positive feedback mechanism, whereby initial patterns of LSC dominance that develop in the year of shoot formation are sustained over subsequent cycles of growth and dormancy. Presumably, a branch with an initial superiority in LSC will be better able to acquire water and nutrients and to sustain higher rates of transpiration than a branch having a low LSC. Leaves supported by such branches may also photosynthesize at higher rates (Ewers and Zimmer-mann 1984a) with an accompanying increase in net assimila-tion rate. A porassimila-tion of this photosynthate will be reinvested in the stem and its developing buds, thus favoring LSC domi-nance in subsequent years. Thus, the observed patterns of hydraulic dominance suggest a physiological connection be-tween the LSC of a shoot, the leaves supported by that shoot, the buds that develop on that shoot, and the LSC of shoots elongating from those buds.
A similar type of physiological connection between leaf and shoot is suggested by the observed differences in LSC between shoots of north- and south-facing branches. In ash in central Pennsylvania, south-facing shoots supported leaves having a high evaporative demand relative to leaves supported by north-facing shoots (Joyce and Steiner, unpublished data). The find-ing that these shoots developed a high LSC suggests that a functional balance is maintained between transpirational de-mand and xylem hydraulic supply capacity. Similarly, Shum-way et al. (1993) found that LSC in seedlings of Q.rubra and Liriodendron tulipifera L. developed in close correspondence with the water regime and evaporative potential of the environ-ment.
The observation of relatively stable Apf/Al values tends to support the notion of a pipe model of tree architecture (Shi-nozaki et al. 1964), although the concept of a unit pipe supply-ing each unit of leaf area is anatomically inaccurate. The similar Apf/Al values between large and small diameter stems were the result of large stems having fewer but wider vessels (as suggested by V values) than small stems. Therefore, the number of vessels supplying a unit of leaf area must decrease basipetally. This anatomical plasticity presumably helps to maintain a functional balance between xylem hydraulic supply capacity and transpirational demand during crown develop-ment. Aloni (1991) suggested that such changes in xylem anatomy were necessary to compensate for increased transport distances and supported leaf areas associated with large branches.
was associated with the 1-year-old leaf-bearing shoots. Within the population of leaf-bearing shoots, a finer level of segmen-tation was evident between terminal and subordinate, lateral axes. This pattern of hydraulic architecture insures that water will flow more readily to the terminal shoots and the main axes (main stem and primary branches), increasing the likelihood that the crown’s apices will survive a drought. As a result, these portions of the crown are preferentially maintained during drought, thereby contributing to the tree’s long-term survival under stress. During two dry summers, we observed that drought-induced leaf death on ash occurred first on lateral and terminal shoots of branches in lower portions of the crown and on lateral shoots only of branches in the upper crown. On a few trees, all leaves had dried except those on the current year’s extension of the main stem.
Zimmermann’s segmentation hypothesis should be consid-ered together with vulnerability segmentation (Tyree and Ew-ers 1991). The large diameter vessels associated with the ring-porous xylem anatomy of white ash conduct water effi-ciently, but may be prone to drought-induced cavitation. Be-cause embolized vessels were refilled under high pressure, the LSC values calculated in this study represent a potential maxi-mum and may be an overestimation of the in situ values.
Variability in LSC within the sampled crown was due pri-marily to variation in V, indicating differences in vessel diame-ter distribution throughout the crown. The distribution of V values suggested a basipetal increase in mean vessel diameter along the main axes and a larger mean vessel diameter in the main stem relative to branches. Basipetal increases in the mean diameter of conducting elements have been documented in angiosperms (Zimmermann 1978, Zimmermann and Potter 1982) and conifers (Ewers and Zimmermann 1984a, 1984b), and support the hormonal gradient hypothesis of xylem differ-entiation proposed by Aloni and Zimmermann (1983).
The observed systematic patterns of LSC distribution, cor-responding to similar patterns of V distribution, reveal that regulated changes in xylem anatomy (i.e., vessel diameter distribution) accompany crown development. Because Stra-hler order describes relative crown position and presumably is associated with variation in hydraulic and mechanical de-mands within the crown, the observed trend of increasing V with increasing branch order reflects an anatomical plasticity that enables the xylem to compensate for changes in hydraulic and mechanical requirements during crown development. In addition, large decreases in mean vessel diameter (as sug-gested by velocity values) at certain points within the crown lead to a segmented pattern of hydraulic architecture, influenc-ing the growth habit and potential survival of the tree.
References
Aloni, R. 1991. Wood formation in deciduous hardwood trees. In Physiology of Trees. Ed. A.S. Raghavendra. Wiley, New York, pp 175--197.
Aloni, R. and M.H. Zimmermann. 1983. The control of vessel size and density along the plant axis: a new hypothesis. Differentiation 24:203--208.
Cochard, H. and M.T. Tyree. 1990. Xylem dysfunction in Quercus: vessel size tyloses, cavitation and seasonal changes in embolism. Tree Physiol. 6:393--407.
Ewers, F.W. and M.H. Zimmermann. 1984a. The hydraulic architec-ture of eastern hemlock (Tsuga canadensis). Can. J. Bot. 62:940--946.
Ewers, F.W. and M.H. Zimmermann. 1984b. The hydraulic architec-ture of balsam fir (Abies balsamea). Physiol. Plant. 60:453--458. Gill, A.M. 1971. The formation, growth and fate of buds of Fraxinus
americana L. in central Massachusetts. Harvard Forest Paper No. 20, Harvard University, Petersham, MA, 16 p.
Heine, R.W. 1971. Hydraulic conductivity in trees. J. Exp. Bot. 22:503--511.
Isebrands, J.G. and P.R. Larson. 1977. Vascular anatomy of the nodal region in eastern cottonwood. Am. J. Bot. 64:1066--1077. Kelso, W.C., C.O. Gertjejanson and R.L. Hossfield. 1963. The effect
of air blockage upon the permeability of wood to liquids. Univ. Minn. Agric. Res. Stn. Tech. Bull., 242 p.
Larson, P.R. 1976. The leaf--cambium relation and some prospects for genetic improvement. In Tree Physiology and Yield Improvement. Eds. M.G.R. Cannell and F.T. Last. Academic Press, New York, pp 261--282.
McDonald, N. 1983. Trees and networks in biological models. John Wiley and Sons, New York, pp 160--167.
Preston, R.D. 1952. Movement of water in higher plants. In Deforma-tion and Flow in Biological Systems. Ed. A. Frey-Wyssling. North Holland Pub. Co., pp 257--321.
Sellin, A. 1987. Hydraulic conductivity of the water transport system in Norway spruce. Fiziologiya Rastenii 34:545--553 (Translated in English).
Shinozaki, K., K. Yoda, K. Hozumi and T. Kira. 1964. A quantitative analysis of plant form: the pipe model theory. I. Basic analyses. Jpn. J. Ecol. 14:97--105.
Shumway, D.L., K.C. Steiner and M.D. Abrams. 1991. Effects of drought stress on the hydraulic architecture of seedlings from five populations of green ash. Can. J. Bot. 69:2158--2164.
Shumway, D.L., K.C. Steiner and T.E. Kolb. 1993. Variation in seed-ling hydraulic architecture as a function of species and environ-ment. Tree Physiol. 12:41--54.
Sokal, R.R. and F.J. Rohlf. 1969. Biometry. W.H. Freeman and Co., San Francisco, 776 p.
Steel, R.G.D. and J.H. Torrie. 1980. Principles and procedures of statistics. McGraw-Hill, New York, 633 p.
Tyree, M.T. and F.W. Ewers. 1991. The hydraulic architecture of trees and other woody plants. New Phytol. 119:345--360.
Tyree, M.T. and J.S. Sperry. 1988. Do plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress. Plant Physiol. 88:574--580.
Tyree, M.T., M.E.D. Graham, K.E. Cooper and L.J. Bazos. 1983. The hydraulic architecture of Thuja occidentalis. Can. J. Bot. 61:2105--2111.
Tyree, M.T., D.A. Snyderman, T.R. Wilmot and J.-L. Machado. 1991. Water relations and hydraulic architecture of a tropical tree (Schef-felera morotoni): data, models and a comparison of two temperate species. Plant Physiol. 96:1105--1113.
Yang, S. and M.T. Tyree. 1993. Hydraulic resistance in Acer sac-charum shoots and its influence on leaf water potential and transpi-ration. Tree Physiol. 12:231--242.
Zimmermann, M.H. 1978. Hydraulic architecture of some diffuse-po-rous trees. Can J. Bot. 56:2286--2295.
Zimmermann, M.H. and C.L. Brown. 1971. Trees: structure and func-tion. Springer-Verlag, New York, 336 p.