Regulation and subcellular localization of auxin-induced
lipoxygenases
Cunxi Wang
a, Ulla Ja¨rlfors
b, David F. Hildebrand
a,*
aDepartment of Agronomy,Uni6ersity of Kentucky,Lexington,KY40546,USA bDepartment of Plant Pathology,Uni6ersity of Kentucky,Lexington,KY40546,USA Received 25 March 1999; received in revised form 7 July 1999; accepted 7 July 1999Abstract
Auxin [a-naphthaleneacetic acid (NAA) or indole-3-acetic acid (IAA)] can induce the expression of two lipoxygenases (LOXs)
in cultured immature zygotic embryo cotyledons of soybean (Glycine maxL.). One of the IAA/NAA-induced LOX isozymes in cultured embryo cotyledons has been demonstrated to be seedling LOX4 (pI 5.09) and another appears to be LOX5 (pI 5.23). The effects of jasmonic acid (JA), methyl jasmonate (MJ), abscisic acid (ABA) and triiodobenzoic acid (TIBA) on the expression of NAA-induced LOXs were investigated in the cultured embryo cotyledons. Among tested plant regulators, only NAA can induce the expression of LOX4 and LOX5, whereas MJ, JA or ABA did not induce LOX expression in the absence of NAA. However combination of NAA with JA significantly increases the expression of NAA-induced LOXs compared with NAA alone. TIBA, an inhibitor of auxin transport, blocks the expressions of the NAA-induced LOXs in the cultured embryos. Immunogold labeling showed that NAA-induced LOXs are located in vacuoles. Our results suggest that auxin may directly or indirectly trigger the expression of soybean seedling LOXs during soybean germination. We also report that seedling LOX4 and/or LOX5 are located in vacuoles. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Auxin;Glycine max; Lipoxygenase; Subcellular localization
www.elsevier.com/locate/plantsci
1. Introduction
LOX (EC 1.13.11.12) is a nonheme iron-con-taining enzyme that catalyzes the peroxidation of polyunsaturated fatty acids withcis, cis -1,4-penta-diene moieties [1]. LOX has been detected in mul-tiple tissues of all plant species examined. LOX and other enzymes together lead to the formation of oxylipins which appear to be important signal-ing molecules in stress responses and/or involved in certain developmental processes in higher plants [2]. The increase of LOX expression following
stress, such as insect feeding, infection by bacterial and fungal pathogens and wounding ([3], and refs. therein) may lead to higher biosynthesis of oxylip-ins such as JA and thus enhance the ability of plants to respond to stress. An Arabidopsis mu-tant incapable of accumulating wound-induced JA is very susceptible to a common saprophagous fungal gnat [4] and to a fungal root pathogen
Pythium mastophorum[5] but the resistance can be restored with exogenously applied MJ, indicating that LOX products play an essential role in plant defense. It has been documented that specific LOX isozymes responding to pod removal and MJ may serve as a temporary storage protein in soybean [6 – 8]. In addition, LOX and LOX products have been implicated in plant growth, development and senescence [2,9].
A change, usually increase, in LOX protein and activity levels during germination has been re-Abbre6iations: ABA, abscisic acid; IAA, indole-3-acetic acid; JA,
jasmonic acid; LOX, lipoxygenase; MJ: methyl jasmonate; MSO, Murashige and Skoog salts, B5 vitamins, 2% (w/v) sucrose, pH 5.8 and 0.2% (w/v) gelrite; NAA, a-naphthaleneacetic acid; TIBA,
tri-iodobenzoic acid.
* Corresponding author. Tel.:+1-606-257-7319; fax:+ 1-606-257-2185.
E-mail address:[email protected] (D.F. Hildebrand)
ported for a number of species. The increase in LOX activity in soybean after germination is mainly as a result of the expression of different members of the LOX gene family [8,10 – 12]. In cucumber, dry seeds have no LOX activity, but LOX activity and protein start to increase at about 3 days after germination [13]. In barley, LOX1 contributed almost exclusively to total LOX activity in dry seeds, but LOX2 activity rapidly increased at 2 days after germination [14]. The expression of LOX is also regulated during germination of Arabidopsis seeds [15,16]. Even though a number of seedling LOXs from different species have been characterized, it is poorly under-stood what factor(s) triggers the expression of seedling LOXs, what role(s) seedling LOXs play in seed germination as well as where the seedling LOXs are subcellularly located.
Subcellular localization of LOX has been inves-tigated in different plant tissues over a number of years but this has not been addressed satisfactorily as a result of the existence of multiple LOX isozymes. However, evidence has been presented for specific subcellular localization of certain LOX isozymes. In soybean leaves, one LOX, so called vsp94/pvm LOX, is primarily located in vacuoles [6]. The MJ-responsive LOX also accumulates in vacuoles in soybean seedlings [7]. cDNA sequence analysis has shown that Arabidopsis LOX2 and the fungus-induced LOX in rice both have a tran-sit peptide sequence for chloroplast targeting [16,17]. It has been demonstrated that Arabidopsis LOX2 is located in chloroplasts [18]. Interestingly, a chloroplast-located LOX appears to be required for wound-induced JA accumulation in Arabidop-sis [18]. MJ-induced LOXs were also found to be located in chloroplasts of barley leaves [19]. In addition, LOX has also been detected in the cyto-plasm and protein bodies [20], plastids [7], cyto-plasma membranes [21], lipid bodies [22] and nuclei [19]. Developmental expression of LOXs has been investigated in different soybean tissues. At an early stage of soybean seed development (B6 mm
embryo length), no LOX was detectable. As soy-bean embryo development proceeds, both the ac-tivities and protein levels of seed LOXs (LOX1 – 3) reached high levels [23,24]. However, soybean seedling LOXs (LOX4 – 5) are expressed following seed germination, 2 days or earlier after the start of imbibition [10,12]. A high concentration of auxin (NAA or 2,4-D) is commonly used to induce
somatic embryogenesis in soybean and many other plant species [25,26]. Somatic embryos induced by NAA usually exhibit normal morphology and can be more easily converted into plants via somatic embryo germination as compared with somatic embryos induced by 2,4-D [25,26]. Interestingly, NAA or IAA, but not 2,4-D induced the expres-sion of new LOX isozymes with acidic pIs in cultured immature embryo cotyledons of soybean [26]. One of the auxin-induced LOXs has been demonstrated to be seedling LOX4 and another appears to be seedling LOX5 [27]. The cultured embryo cotyledons serve as a good system to subcellularly locate seedling LOXs (LOX4 and LOX5) because they are expressed in the immature embryo tissues only upon induction by auxin but not expressed in absence of auxin. In this study, we tested whether or not auxin-induced LOXs (seedling LOXs) are responsive to other plant regulators such as ABA, MJ and JA. The subcellu-lar localization of auxin-induced LOXs was also investigated.
2. Materials and methods
2.1. Plant materials and culture media
A wild type soybean [(Glycine max L.) Merrc6. ‘Century’] and a soybean LOX triple null mutant were used in this study. The immature zygotic embryo cotyledons of soybean (3 – 5 mm in length) from greenhouse grown plants were used as ex-plants. The tissue culture procedure was per-formed as described by Liu et al. [26]. The media were MSO (Murashige and Skoog salts, B5 vita-mins, 2% (w/v) sucrose, pH 5.8, and 0.2% Gelrite) and MSO adjusted to 10 mg/L NAA commonly used for soybean tissue culture [26] and different concentrations of JA, TIBA, ABA and MJ.
2.2. Protein extraction, LOX acti6ity and pI
measurements, IEF-PAGE, and Western blotting
modified Lowry method using bovine serum albu-min as a standard [28]. LOX activity was mea-sured using the method described by Hildebrand et al. [24]. Native IEF gel electrophoresis was performed as described by Funk et al. [29] with 5% polyacrylamide gels on an LKB flat bed system at 6 W constant power, 20 mA and 3400 vh. Each IEF gel slice (0.5×2.0 mm) was placed in 2 ml deionized water overnight. The pH gradient of IEF gels was measured with a micro pH electrode. LOX pI was determined based on the pH gradient of the IEF gels. Western blotting was performed as described by Liu et al. [26].
2.3. Immunolocalization of LOXs
The cultured embryo cotyledons were cut with a razor blade into approximately 1 mm3blocks, and placed immediately into a vessel containing a freshly prepared fixative [2% (v/v) paraformalde-hyde and 1% (v/v) glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.3] and fixed 2 hin6acuoat room temperature. After being rinsed with 0.1 M sodium phosphate buffer, pH 7.3 for 3×5 min, the tissues were dehydrated through an ethanol series of 30, 50, 70, 90 and 100% (all v/v) for 20 min each, then infiltrated with 25, 50 and 100% L. R. White resin (v/v in ethanol, The London Resin Co. Ltd.) for 1 h each, rinsed one time with pure L. R. White resin and infiltrated overnight at 4°C. Tissue polymerization was performed at 55°C for 24 h. Sections of 0.06 – 0.1 mm were cut with a glass knife and picked up with a loop of platinum wire and transferred to nickel grids, which had been covered with a carbon-coated film. Sections were blocked with 0.1% (w/v) BSA and 5% (v/v) heat denatured normal goat serum in PBS buffer [0.148% (w/v) Na2HPO4, 0.043% (w/v) KH2PO4, 0.72% (w/v) NaCl, 0.13% (w/v) NaN3, pH 7.3] for 1 h and then incubated in either a mixture of soybean seed LOX1+2+3 rabbit antiserum or preimmune serum (1:150 dilution) at room tem-perature for 3 h. After being washed with PBS plus 0.5% (v/v) Tween 20 for 4×10 min, the sections were incubated with goat anti-rabbit im-munoglobulin-gold conjugate (15 nm, Pelco, Ted Pella Inc, Redding CA.) (1:40 in PBS) for 1 h. The sections were then rinsed with PBS plus 0.1% (w/v) BSA and 0.5% (v/v) Tween 20 for 3×5 min, and 3×5 min with deionized water, dried and stained with uranyl acetate for 4 min and then with lead
citrate for 4 min. Sections were examined and photographed with a Hitachi H 600 or Philips 400 transmission electron microscope.
3. Results and discussion
3.1. Effects of different plant regulators on the expression of LOXs in the cultured embryo cotyledons
The immature embryo cotyledons (3 – 5 mm) from the soybean cultivar Century grown in green-house were used as explants. The expression of seedling-specific LOX4 and LOX5 in the NAA-in-duced embryo cotyledons was confirmed by
ri-bonuclease protection assay (Wang and
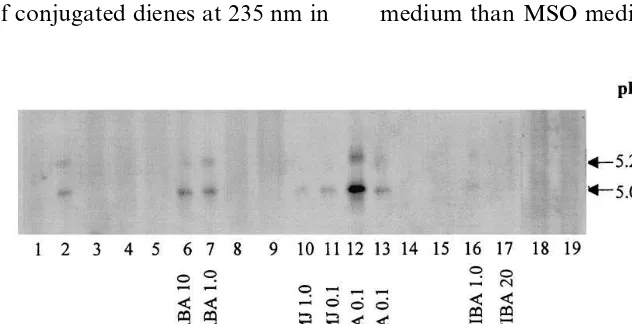
Hildebrand, unpublished data). We previously demonstrated that the NAA-induced pI 5.09 LOX is seedling LOX4 and the NAA-induced pI 5.23 LOX appears to LOX5 [27]. Exogenously applied NAA, ABA, TIBA, JA and MJ in media were used to assess their effects on the expression of LOX in this study. LOX4 and LOX5 cross-react-ing with seed LOX antibodies were seen in tissues cultured on NAA (Fig. 1, lane 2). The expression of LOX4 and LOX5 was not seen in tissues cul-tured on MSO (lane 1) and MSO with either TIBA (lanes 3, 4 and 5), ABA (lanes 8 and 9), JA (lanes 14 and 15) or MJ (lanes 18 and 19) in absence of auxin. However, JA (Fig. 1, lane 12) greatly enhances the expression of LOX4 and LOX5 in presence of NAA. The greatest induction of LOX among the treatments was detected in tissues cultured on 10 mg/L NAA plus 1 mM JA (lane 12). MJ neither enhances the expression of LOX4 nor induces the expression of LOX5 in presence of NAA (lanes 10 and 11). TIBA, an inhibitor of auxin transport [30], blocks the ex-pression of the NAA-induced LOXs (lanes 16 and 17). These data support that during seed germina-tion, auxin may directly or indirectly trigger the expression of seedling LOXs through an unknown mechanism.
MJ was used to treat the Arabidopsis roots [32]. That MJ was incapable of enhancing the expres-sion of LOX4 and LOX5 in the cultured embryo cotyledons of this study is probably as a result of the concentration of MJ (0.1 – 1 mM) used being too low even though this level of JA (0.1 – 1 mM) significantly increased the accumulation of LOX4 and LOX5. A tissue-specific effect of MJ on LOX induction was seen in Arabidopsis [16]. The ex-pression of Arabidopsis AtLox1 is enhanced by MJ in roots but not in leaves, whereas MJ in-creased the expression ofAtLox2 in leaves but not in roots. The fact that the NAA-induced soybean seedling LOX in the embryo cotyledons is not responsive to MJ may be as a result of a tissue-specific effect of MJ on LOX induction. Although the prevalence of MJ in flowers and fruit has not been studied in any depth, it does indicate that MJ and JA could elicit different physiological re-sponses. In most plant tissues examined, including seedlings, JA is much more abundant than MJ [33 – 35].
Developing and mature seeds of the triple LOX null mutant of soybean are essentially devoid of seed LOX [36,37]. The embryo cotyledons from the mutant grown in greenhouse were also used as materials. The expression of the NAA-induced LOXs in the mutant upon induction by NAA is the same as indicated in Century (Fig. 1) and no expression LOX was seen in MSO medium with-out NAA (data not shown). LOX activity was determined by spectrophotometric measurement of the formation of conjugated dienes at 235 nm in
which linoleic acid was used as a substrate. LOX activity in the mutant tissues cultured on 10 mg/L NAA medium is 141mMol hydroperoxy octadeca-dienoic acid mg protein−1min−1while no activity was seen in the cultured tissues in absence of NAA.
3.2. Subcellular localization of the NAA-induced LOXs
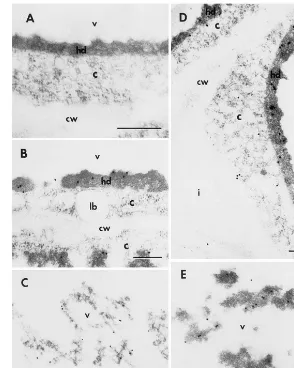
Because the developing cotyledons of the triple LOX null mutant have no LOX protein accumula-tion, LOX found in cultured cotyledons may be induced by auxin. Therefore the embryo cotyle-dons of the triple LOX null mutant cultured on 10 mg/L NAA and MSO media were used for im-munolocalization experiments. No label was seen in a preimmune control (Fig. 2A) in which the tissues were cultured in 10 mg/L NAA for 20 days and in the cotyledons cultured in absence of NAA as well as in uncultured cotyledons (data not shown). After the cotyledons were cultured in 10 mg/L NAA medium for 5 days, LOX appeared in highly dense materials (possibly protein inclusion bodies) in vacuoles (Fig. 2B and C). A higher concentration of label was seen in vacuoles and somewhat less in the cytoplasm of tissues cultured in 10 mg/L NAA medium for 20 days (Fig. 2D and E). No label was seen in other organelles including ER, lipid bodies and cell walls. The water extractable protein content was four fold higher in tissues cultured on 10 mg/L NAA medium than MSO medium based on fresh weight
Fig. 1. Immunoblot of a pH 4 – 7, 5% IEF-PAGE gel illustrating the effects of different plant regulators on LOX expression in immature zygotic embryo cotyledons of soybean cultured for 5 days. The concentration of the regulators (NAA: ppm; other regulators: mM) is indicated on the Figure. A total of 60mg of protein was loaded per each lane (note: pTK 18=LOX4; pTK
Fig. 2. Electron micrographs of cultured immature embryo cotyledons. Immature zygotic embryo cotyledons (3 – 5 mm) of a soybean triple LOX null mutant grown in greenhouse were cultured on 10 mg/L NAA. Sections were cross-reacted with either preimmune serum (A) or soybean seed LOX1+2+3 antibodies (B – E). A: preimmune control in which the tissues were cultured for 20 days. B and C: tissues cultured for 5 days. D and E: tissues cultured for 20 days. A:×94 000; B: ×62 000; C:×54 000; D: ×56 000; and E: ×56 000. All size bars: 0.25mm.
(data not shown). Electron micrographs also showed that the highly dense materials (possibly protein inclusions) appeared in the tissues cultured on 10 mg/L NAA medium (Fig. 2A – E) but not in tissue on MSO medium (data not shown).
Park et al. [11] have isolated two cDNA clones from germinating soybean seedlings, so called pTK18 which encodes soybean LOX4 and pTK11 which appears to encode LOX5. After analysis of both deduced amino acid sequences, they specu-lated that both proteins have putative signal pep-tide motifs for vacuolar targeting. A LOX4 gene was also isolated from a soybean genomic library [8]. Kato et al. [8] claimed that LOX4 has no signal peptide sequence at the amino and carboxyl termini. Multiple LOX isozymes are expressed in germinating soybean cotyledons [12] and in
the intracellular secretory pathway. However, be-cause LOX4 and LOX5 can not be distinguished with soybean seed LOX antibodies, it remains possible that the modest levels of labeling in the cytosol (Fig. 2D) may represent one isoform and that in vacuoles the other.
In conclusion, the expression of pI 5.09 and pI 5.23 LOXs are triggered by auxin but not by ABA, MJ or JA. At least one of the NAA-induced LOXs is located in vacuoles in cultured embryo cotyledons. It was reported that auxin negatively and MJ positively regulates the expression of VSP (vegetative storage protein) and certain wounding-responsive genes in plant tissues [31,38]. The regu-latory pattern of LOX4 in response to auxin, MJ and JA in the cultured cotyledons is distinct from that of VSP in leaf tissues. Although LOX4 and/or LOX5 as well as VSP are located in vacuoles, we can not yet conclude whether LOX4 (or LOX5) serve an enzymatic or storage purpose in the cul-tured embryo cotyledons and germinating soybean seedlings. Further studies such as analysis of levels of JA and C6 aldehydes as well as other lipoxyge-nase products in cultured tissues are needed to help resolve this issue.
Acknowledgements
We thank Ms Yuan Sun and Mr Zhiqiang Yu for their excellent assistance in electron scope, Dr Nordin for use of an electron micro-scope and Dr Wennuan Liu for help with soybean tissue culturing. We also thank Dr K. Kitamura for the soybean LOX mutant. This work was supported by the USDA (grant c9701487) and the Kentucky Agricultural Experiment Station
References
[1] J.N. Siedow, Plant lipoxygenase: structure and function, Annu. Rev. Plant Physiol. Plant Mol. Biol. 42 (1991) 145 – 188.
[2] R.A. Creelman, J.E. Mullet, Biosynthesis and action of jasmonates in plants, Annu. Rev. Plant Physiol. Plant Mol. Biol. 48 (1997) 355 – 381.
[3] D.M. Saravitz, J.N. Siedow, The differential expression of wound-inducible lipoxygenase genes in soybean leaves, Plant Physiol. 110 (1996) 287 – 299.
[4] M. McConn, R.A. Creelman, E. Bell, J.E. Mullet, J. Browse, Jasmonate is essential for insect defense inAra
-bidopsis, Proc. Natl. Acad. Sci. USA 94 (1997) 5473 – 5477.
[5] P. Vijayan, J. Shockey, C.A. Levesque, R.J. Cook, J. Browse, A role for jasmonate in pathogen defense of
Arabidopsis, Proc. Natl. Acad. Sci. USA 95 (1998) 7209 – 7214.
[6] T.J. Tranbarger, V.R. Franceschi, D.F. Hildebrand, H.D. Grimes, The soybean 94-kilodalton vegetative stor-age protein is a lipoxygenase that is localized in par-aveinal mesophyll cell vacuoles, Plant Cell 3 (1991) 973 – 987.
[7] H.D. Grimes, D.S. Koetje, V.R. Franceschi, Expression, activity and cellular accumulation of methyl jasmonate-responsive lipoxygenase in soybean seedlings, Plant Phys-iol. 100 (1992) 433 – 443.
[8] T. Kato, Y. Shirano, H. Iwamoto, D. Shibata, Soybean lipoxygenase L-4, a component of the 94-kilodalton stor-age protein in vegetative tissues: expression and accumu-lation in leaves induced by pod removal and by methyl jasmonate, Plant Cell Physiol. 34 (1993) 93 – 107. [9] H.W. Gardner, Biological roles and biochemistry of the
lipoxygenase pathway, HortScience 30 (1995) 197 – 205. [10] T.K. Park, J.C. Polacco, Distinct lipoxygenase species
appear in the hypocotyl/radical of germinating soybean, Plant Physiol. 90 (1989) 285 – 290.
[11] T.K. Park, M.A. Holland, J.G. Laskey, J.C. Polacco, Germination-associated lipoxygenase transcripts persist in maturing soybean plants and are induced by jas-monate, Plant Sci. 96 (1994) 109 – 117.
[12] T. Kato, H. Ohta, K. Tanaka, D. Shibata, Appearance of new lipoxygenases in soybean cotyledons after germi-nation and evidence for expression of a major new lipoxygenase gene, Plant Physiol. 98 (1992) 324 – 330. [13] K. Matsui, M. Irie, T. Kajiwara, A. Hatanaka,
Develop-mental changes in lipoxygenase activity in cotyledons of cucumber seedlings, Plant Sci. 85 (1992) 23 – 32. [14] W.L. Holtman, G. Van Duijn, N.J.A. Sedee, A.C.
Douma, Differential expression of lipoxygenase isoen-zymes in embryos of germinating barley, Plant Physiol. 111 (1996) 569 – 576.
[15] M.A. Melan, A.L.D. Enriquez, T.K. Peterman, The LOX1 gene of arabidopsis is temporally and spatially regulated in germinating seedlings, Plant Physiol. 105 (1994) 385 – 393.
[16] E. Bell, J.E. Mullet, Characterization of an Arabidopsis
lipoxygenase gene responsive to methyl jasmonate and wounding, Plant Physiol. 103 (1993) 1133 – 1137. [17] Y.L. Peng, Y. Shirano, H. Ohta, T. Hibino, K. Tanaka,
D. Shibata, A novel lipoxygenase from rice: primary structure and specific expression upon incompatible in-fection with rice blast fungus, J. Biol. Chem. 269 (1994) 3755 – 3761.
[18] E. Bell, R.A. Creelman, J.E. Mullet, A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation inArabidopsis, Proc. Natl. Acad. Sci. USA 92 (1995) 8675 – 8679.
[19] I. Feussner, B. Hause, K. Voeroes, B. Parthier, C. Wasternack, Jasmonate-induced lipoxygenase forms are localized in chloroplasts of barley leaves (Hordeum6ul -garecv. Salome), Plant J. 7 (1995) 949 – 957.
labeling with protein A-colloidal gold complexes, Plant Physiol. 76 (1984) 1070 – 1079.
[21] F. Macri, E. Braidot, E. Petrussa, A. Vianello, Lipoxyge-nase activity associated to isolated soybean plasma mem-branes, Biochim. Biophys. Acta 1215 (1994) 109 – 114. [22] I. Feussner, H. Kindl, A lipoxygenase is the main lipid
body protein in cucumber and soybean cotyledons dur-ing the stage of triglyceride mobilization, FEBS Lett. 298 (1992) 223 – 225.
[23] M. Altschuler, W.S. Grayburn, G.B. Collins, D.F. Hilde-brand, Developmental expression of lipoxygenases in soybean, Plant Sci. 63 (1989) 151 – 158.
[24] D.F. Hildebrand, R.T. Versluys, G.B. Collins, Change in lipoxygenase isozyme levels during soybean embryo de-velopment, Plant Sci. 75 (1991) 1 – 8.
[25] P.A. Lazzeri, D.F. Hildebrand, G.B. Collins, Soybean somatic embryogenesis: effects of hormones and culture manipulation, Plant Cell Tiss. Org. Cult. 10 (1987) 197 – 208.
[26] W. Liu, D.F. Hildebrand, W.S. Grayburn, G.C. Phillips, G.B. Collins, Effects of exogenous auxins on expression of lipoxygenases in cultured soybean embryos, Plant Physiol. 97 (1991) 969 – 976.
[27] C. Wang, K.P.C. Croft, D.F. Hildebrand, A soybean seedling lipoxygenase is induced in immature soybean zygotic embryos (Abstract No 661), Plant Physiol. 108 (1995) S129.
[28] A. Bensadoun, D. Weinstein, Assay of proteins in the presence of interfering materials, Anal. Biochem. 70 (1976) 241 – 250.
[29] M.O. Funk, R.T. Carroll, J.F. Thompson, W.R. Dun-ham, The lipoxygenases in developing soybean seeds, their characterization and synthesis in vitro, Plant Phys-iol. 82 (1986) 1139 – 1144.
[30] L. Taiz, E. Zeiger, Auxins: growth and tropisms. In: ‘Plant Physiology’, The Benjamin/Cummings Publishing Company, Inc., Redwood City, 1991, pp. 398 – 425. [31] D.B. Dewald, A. Sadka, J.E. Mullet, Sucrose modulation
of soybean Vsp gene expression is inhibited by auxin, Plant Physiol. 104 (1994) 439 – 444.
[32] M.A. Melan, X. Dong, M.E. Endara, K.R. Davis, F.M. Ausubel, T.K. Peterman, An Arabidopsis thaliana lipoxygenase gene can be induced by pathogens, abscisic acid, and methyl jasmonate, Plant Physiol. 101 (1993) 441 – 450.
[33] H.W. Gardner, D. Weisleder, R.D. Plattner, Hydroper-oxide lyase and other hydroperHydroper-oxide-metabolizing activ-ity in tissues of soybean,Glycine max, Plant Physiol. 97 (1991) 1059 – 1072.
[34] H. Gundlach, M.J. Mu¨ller, T.M. Kutchan, M.H. Zenk, Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures, Proc. Natl. Acad. Sci. USA 89 (1992) 2389 – 2393.
[35] I.T. Baldwin, E.A. Schmelz, T.E. Ohnmeiss, Wound-in-duced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana syl6estris, J. Chem. Ecol. 20 (1994) 2139 – 2157.
[36] M. Hajika, K. Igita, K. Kitamura, A line lacking all the seed lipoxygenase isozymes in soybean [Glycine max(L.) Merrill] induced by gramma-ray irradiation, Jpn. J. Breed 41 (1991) 507 – 509.
[37] W.H. Wang, T. Takano, D. Shibata, K. Kitamura, G. Takeda, Molecular basis of a null mutation in soybean lipoxygenase 2: substitution of glutamine for an iron-lig-and histidine, Proc. Natl. Acad. Sci. USA 91 (1994) 5828 – 5832.
[38] A. Kernan, R.W. Thornburg, Auxin levels regulate the expression of a wound-inducible proteinase inhibitor II – chloramphenicol acetyl transferase gene fusion in vitro and in vivo, Plant Physiol. 91 (1989) 73 – 78.