Changes in lipid composition during somatic embryogenesis in
leaves of
Cichorium
Axelle Blanckaert
a, Lionel Belingheri
b,*, Jacques Vasseur
a, Jean-Louis Hilbert
aaLaboratoire de Physiologie Cellulaire et Morphogene`se Ve´ge´tales,USTL/INRA,Uni6ersite´ des Sciences et Technologies de Lille, F-59655Villeneu6e d’Ascq Cedex, France
bLaboratoire de Physiologie des Parois Ve´ge´tales,Uni6ersite´ des Sciences et Technologies de Lille,F-59655 Villeneu6e d’Ascq Cedex, France
Received 26 October 1999; received in revised form 13 April 2000; accepted 13 April 2000
Abstract
The present studies were conducted to investigate the changes in lipid and fatty acid composition during the earliest stages of somatic embryogenesis in leaf tissues of theCichoriumhybrid ‘474’. The presence of glycerol in the culture medium during the induction step allowed to separate two phases in the embryogenic process. Firstly, cells are induced for morphogenetic competence (induction phase) and secondly, they express an embryogenic competence (expression phase). The analysis of fatty acid composition of total lipids showed that the percentage of linolenic acid (18:3) decreased while that of linoleic acid (18:2) increased throughout the culture period. A comparison with a non-embryogenic genotype of Cichorium indicated a higher increase of linoleate content in embryogenic genotype. The incorporation of [14C] glycerol into lipid classes was studied in leaf tissues. During
the induction step, label was confined almost exclusively in polar lipids, particularly in phosphatidylcholine (PC). An important increase of labeled triacylglycerols (TAG) amounts was noted during the expression step. The accumulation of 18:2 was observed in PC and also in TAG. These results show that the early stages of somatic embryogenesis are associated with the increase of PC and TAG which are mainly enriched in 18:2. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Cichorium; [14C] glycerol; Fatty acid composition; Lipid classes; Somatic embryogenesis
www.elsevier.com/locate/plantsci
1. Introduction
Lipids are essential constituents of all plant cells and play major roles in higher plants metabolism [1]. While triglycerides are an efficient storage form of reduced carbon, particularly in seeds, phospholipids serve as the primary nonprotein components of plant and are also sources of sec-ond messengers. Somatic embryogenesis is a good model to investigate the morphological and molec-ular events during early development of higher plants. Lipids have been studied in relation to
indirect somatic embryogenesis in species such as
Arachis hypogaea [2],Daucus carota [3,4], Papa6er
orientalis [5] and Vitis 6inifera [6]. These studies
were carried mainly on accumulation and utiliza-tion of triglycerides during the development of somatic embryos but only little information is available on lipid composition and changes during the early stages of the somatic embryogenesis process.
Direct somatic embryogenesis can be obtained in the leaf mesophyll cells of theCichoriumhybrid ‘474’ by a two steps process [7]. The addition of glycerol to the culture medium of foliar fragments during 5 days allowed the synchronization of the first division of embryogenic cells [8]. After 5 days of culture (D5), cells have acquired the embryo-genic competence (induction phase). At this stage, leaves were transferred in the same medium
with-Abbre6iations:DGDG, digalactosyldiacylglygerol; MGDG,
mono-galactosyldiacylglycerol; PC, phosphatidylcholine; PE, phos-phatidylethanolamine; PI, phosphatidylinositol; PL, phospholipids; TAG, triacylglycerols.
* Corresponding author: Tel.:+33-3-20434071; fax: + 33-3-20336302.
E-mail address:[email protected] (L. Belingheri).
out glycerol and cells expressed their embryo-genic competence (expression phase). Multicellu-lar dense embryos appeared 3 days after transfer in the expression medium. The presence of glyc-erol delays the first division of the embryogenic cell, making of this system a valuable tool to investigate the early events taking place during somatic embryogenesis. In previous studies, we have shown the presence of proteins related to somatic embryogenesis during the induction step in leaf tissues [9,10] and in extracellular condi-tioned medium of leaf explants [11,12].
In this paper, we report the changes in total fatty acid composition in leaf tissues of a nonem-bryogenic responsive genotype and a embryo-genic responsive genotype of Cichorium
submitted to embryogenic conditions. The acyl and lipid class composition was investigated in
Cichorium hybrid ‘474’ during the embryogenic process. Moreover, we labelled plant lipids with [14C] glycerol and we carried out a chase
experi-ment during which the labeling of polar and nonpolar lipids was studied.
2. Materials and methods
2.1. Plant material and condition culture
A Cichorium hybrid ‘474’ (Cichorium intybus
L. var. sati6um×Cichorium endi6ia L. var. latifo
-lia) was used as plant material. Plantlets were obtained as described previously [11]. Three leaf fragments of 6-week-old plantlets were precul-tured 5 days at 35°C in darkness in 20 ml of an agitated basal medium supplemented with 60 mM sucrose and 330 mM glycerol. Addition of glycerol allowed activation of the mesophyll cells for embryogenic competence without cell divi-sions [8]. This glycerol pretreatment delayed the first division of the embryogenic cells until trans-fer of the 5-day-old induced leaf fragments on an expression medium devoid of glycerol. The same experience was realised with a non-embryogenic genotype of chicory: Cichorium intybus L. var. Pe´ve`le.
2.2. Lipid extraction
Leaf fragments were collected, dried on filter paper and boiled 4 min in 4 ml distilled water to
ensure inactivation of lipolytic enzymes, if present. Lipids were extracted using a chloro-form/methanol/water solvent system as described by [13]. Total lipids were dissolved in 200 ml chloroform and stored in a freezer.
2.3. Lipid classes separation
The lipids extracted by the above described procedure were separated by thin-layer chro-matography (TLC) using pre-coated silica gel 20×20 cm plates (sil G-25, Macherey – Nagel). Neutral lipids were separated using a solvant sys-tem consisting of hexane/diethyl ether/acetic acid 78:20:4 (v/v/v). Polar lipids were fractionated in chloroform/acetone/methanol/acetic acid/water 50:20:10:10:5 (v/v/v/v/v). Lipids were located by spraying the plates with a solution of primuline (0.001%) in 20% acetone, and viewed under ul-traviolet light. Each lipid classes were identified by cochromatography with authentic standards (Sigma). Individual lipids were then scraped from the plates, recovered by dynamical elution with chloroform/methanol/acetic acid 2:1:0.5 (v/v/v) and analyzed for their fatty acid composition as described below. An appropriate amount of hep-tadecanoic acid was used as an internal standard.
2.4. Fatty acid composition determination
2.5. Labelling of lipids
At day 0 of the induction step, leaf fragments were incubated in 20 ml of the medium described above supplemented with 330 mM glycerol and 9.25 KBq [U-14C] glycerol (5.59 GBq mmol−1;
Amersham Corp). At different days at the em-bryogenic culture, radiolabelled leaves were rinsed with distilled water to remove exogenous isotope. Lipid extraction and lipid classes separa-tion were performed described above. After visu-alization under UV light, the bands were scraped off and their radioactivity was counted in a Beckman LS 2800 scintillation spectrometer (Beckman Instruments Inc. Irvine, CA).
3. Results
3.1. Fatty acids in total lipids
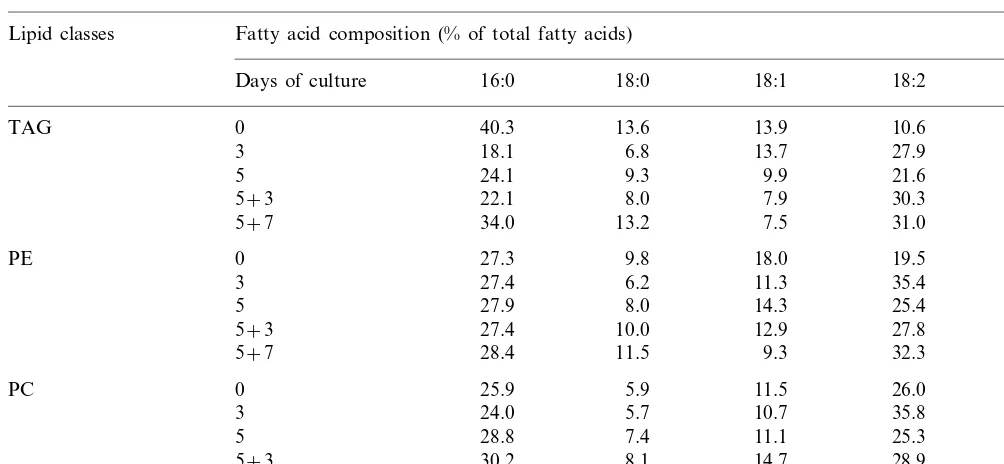
The fatty acid composition of total lipids in leaves of Cichorium ‘‘474’’ submitted to somatic embryogenesis is shown in Fig. 1A. In the con-trol leaves (D0), linolenic acid (18:3) was the ma-jor fatty acid (57.7%), followed by linoleic acid (18:2) (18.3%) and then palmitic acid (16:0) (12.7%). The presence of 18:3 is characteristic of photosynthetic tissues. A considerable decrease of the 18:3 proportion was observed during the in-duction step (38% at 5th day) and the expression step (17% at 5+7th day). Three days after transfer in the expression medium (D5+3), 18:2 became major fatty acid and it reached a max-imun level at day 5+7 (43.7%) when globular embryos were observed, while the proportion of 18:3 was reduced to 15.7%. The saturated fatty acid proportion remained similar during the two phases.
In the non-embryogenic genotype ‘Pe´ve`le’, at D0, the fatty acid composition of total lipid frac-tion is similar to the one observed in the control leaves of Cichorium ‘474’ (Fig. 1B). However, throughout the 12 days of culture period, the relative proportions of total fatty acids in ‘Pe´ve`le’ leaves were different from those obtained for the ‘474’ leaves. The principal difference con-sisted in the changes in relative proportions of polyunsaturated acids. The increase of 18:2 pro-portion is also lower for the ‘Pe´ve`le’ genotype than the embryogenic genotype. At day 5+7, 18:3 and 18:2 were present in equal proportion (about 33%).
3.2. Fatty acids in neutral and polar lipids
Fatty acid compositions in polar and neutral lipid fractions of Cichorium ‘474’ leaves during somatic embryogenesis are presented in Table 1. The principal changes in fatty acid content of polar lipids concerned the C18 unsaturated fatty acids. At day 0, 18:3 was the major component and its proportion reached more than 55% in polar lipids. Its proportion decreased while the amount of 16:0 increased during the induction phase and the 18:2 one increased during the ex-pression phase. At day 12, the polar lipid frac-tion contained more 18:2 and less 18:3.
Table 1
Fatty acid composition of neutral and polar lipids inCichorium‘474’ leaf fragments during somatic embryogenesisa
Lipid classes Fatty acid composition (% of total fatty acids)
16:0 18:0 18:1
Days of culture 18:2 18:3
29.5
Neutral lipids 0 15.8 16.8 11.9 17.3
30.5 14.2 18.6
3 13.9 13.9
24.3 11.6 15.5 17.8
5 22.4
25.4 10.3 22.6
5+3 20.2 12.8
5+7 24.2 11.5 11.8 29.8 13.8
16.6 2.2
Polar lipids 0 3.0 19.1 55.6
19.8 3.6 4.6
3 28.4 39.8
23.3 5.5 5.2
5 21.7 37.4
5+3 24.2 4.5 6.3 29.7 30.5
24.2 4.1 10.5
5+7 38.0 21.3
aThree leaf fragments were incubated for 5 days in an induction medium containing 330 mM glycerol and transferred for 7 days
in an expression medium without glycerol. Neutral and polar lipids were separated by TLC. Data are means of three independent replicates.
Table 2
Distribution of the different lipid classes inCichorium‘474’ leaf fragments during somatic embryogenesisa
Lipid classes Percentages of different lipid classes
Day 3 Day 5
Day 0 Day 5+3 Day 5+7
Neutral lipidsb
22.6 21.1
12.3 23.0
TAG 32.0
16.6 15.4 16.4 18.9
DAG 20.1
25.2 26.1
30.1 21.7
MAG 17.8
35.6 37.4 38.9 31.3
SE 37.5
Polar lipidsc
25.4
MGDG 30.0 23.4 17.2 10.2
18.6 24.3 18.5
DGDG 25.8 16.7
18.0 22.5
16.4 22.8
PC 24.5
10.2 12.0
PE 7.4 13.4 17.7
7.4 10.8
6.5 16.2
PI 14.2
20.4 7.0 11.9 16.7
Others compounds 13.9
aThree leaf fragments were incubated for 5 days in an induction medium containing 330 mM glycerol and transferred for 7 days
in an expression medium without glycerol. Data are means of three independent experiments.
bNeutral lipids comprised triacylglycerols (TAG), diacylglycerols (DAG), monoacylglycerols (MAG) and steryl esters (SE). cPolar lipids were composed of monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG),
phosphatidyl-choline (PC), phosphatidylethanolamine (PE) and phosphatidylinositol (PI). The other compounds were phosphatidic acid, phosphatidylserine and another unidentified polar lipid.
Palmitic acid was the predominant fatty acid in the neutral lipid fraction. The principal change was a progressive increase in the proportion of 18:2. Linoleic acid became the major fatty acid at day 12. There was only a little change in the relative proportions of the principal saturated fatty acids, 16:0 and 18:0.
3.3. Identification of lipid classes in total lipids
phospho-lipids in Cichorium ‘474’ control leaves. Mono-galactosyldiacylglycerol (MGDG) (30%) and di-galactosyldiacylglycerol (DGDG) (25.8%) were the most abundant polar lipids. This result is in gen-eral agreement with other reports on green leaves. The proportions of the two galactolipids declined rapidly especially during the expression phase where, at day 5+7, MGDG and DGDG percent-ages were 10.2 and 16.7%, respectively. It is evi-dent that the galactolipid proportions decreased, while the phospholipid proportions increased. The major phospholipid was phosphatidylcholine (PC) which showed an increase of its ratio from 16.4% at day 0 to 24.5% at day 5+7. The other phos-pholipids were phosphatidylethanolamine (PE) and phosphatidylinositol (PI).
The greatest change in the neutral lipid was observed in the triacylglycerols content (TAG). In the control leaves, TAG were minor components of the neutral lipid (12.3%). During the expression phase, TAG showed a significant increase of its ratio (32%).
3.4. Lipid incorporation of [14C
] glycerol
In order to investigate the incorporation of glyc-erol into lipids, [14C] glycerol was supplied to
culture medium of foliar fragments at day 0. Fo-liar fragments were collected at days 3 and 5 of the induction step and days 5+3 and 5+7 of the expression phase after transfer at day 5 onto a
glycerol-free medium. Following fractionation into neutral and polar lipids, the percentage of each classes was determined by total [14C] incorporated.
Individual classes were separated by TLC and the five major lipid components (PC, PE, MGDG, DGDG and TAG) were identified and quantified by measuring their total radioactivity.
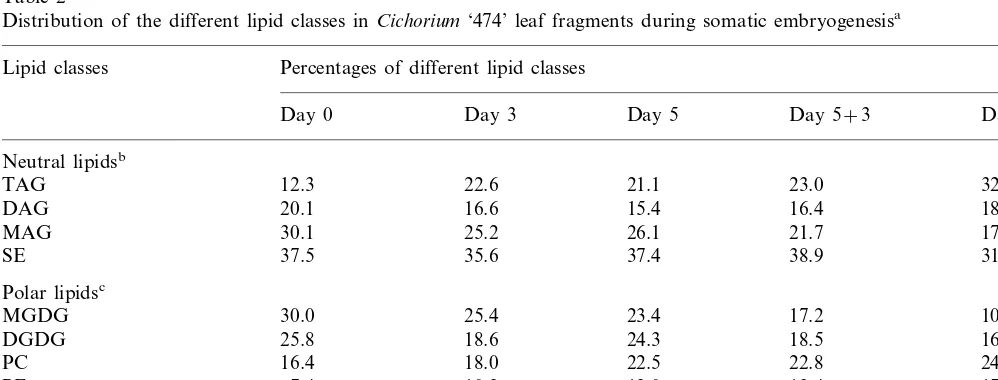
During the induction step, labeled lipid classes were almost exclusively phospholipids and galac-tolipids (Fig. 2). The two major labelled phospho-lipids were PC and PE. The percentage of incorporation of labelled glycerol in PC increased to 45% at the end of the induction phase then decreased during the expression phase. However, PC was always the most strongly labelled individ-ual lipid throughout the culture period. PE was found to be markedly labelled during the expres-sion step. A large enrichment of labelled com-pound was also seen for MGDG and DGDG, known to be plastidial lipids. The detected ra-dioactivity was more important in DGDG than in MGDG overall the culture. This result is in gen-eral agreement with other reports on non-green plastids and could indicate the presence of pro-plastids. It could be noticed that this experience revealed a similar pattern of incorporation among these two galactolipids.
During the induction phase the incorporation of labelled glycerol was lower for TAG than for other lipid classes. The radioactivity drastically increased in TAG from 5% at day 5 up to 17% at day 5+3. So, an important increase of radioactiv-ity in TAG was noted during the expression phase.
3.5. Fatty acids in PE, PC and TAG
The acyl composition of TAG and main mem-brane lipids, PC and PE, was examined and the results are shown in Table 3. At day 0, there were not significant differences in the acyl composition of PC and PE. Both fractions contained 16:0 and 18:2 as their major moieties. Changes in fatty acid patterns of PC and PE were similar during the induction and expression phases. The percentage of 18:2 in both PE and PC was upper to 30% at day 5+7. There was little difference in the relative proportions of saturated fatty acids in these two phospholipids throughout the culture period. These phospholipids have a distinct composition compared to the storage TAG. The triacylglycerol fraction contained relatively high amount of satu-Fig. 2. Incorporation of [U-14C] glycerol into the main lipid
classes in Cichorium ‘‘474’’ leaf tissues during somatic em-bryogenesis. Three leaf fragments were incubated for 5 days in 20 ml of a medium containing 9.25 KBq [14C] glycerol and
transferred for 7 days in a medium without glycerol. The proportion of incorporated14C in each lipid class was
Table 3
Fatty acid composition of different lipid classes inCichorium‘474’ leaf fragments during somatic embryogenesisa
Lipid classes Fatty acid composition (% of total fatty acids)
16:0 18:0
Days of culture 18:1 18:2 18:3
40.3 13.6 13.9
0 10.6
TAG 12.9
18.1 6.8 13.7 27.9
3 24.4
24.1 9.3 9.9
5 21.6 26.0
5+3 22.1 8.0 7.9 30.3 25.1
34.0 13.2 7.5
5+7 31.0 4.7
PE 0 27.3 9.8 18.0 19.5 15.6
27.4 6.2 11.3
3 35.4 13.2
27.9 8.0 14.3
5 25.4 18.6
5+3 27.4 10.0 12.9 27.8 11.4
28.4 11.5 9.3 32.3
5+7 6.9
25.9 5.9
PC 0 11.5 26.0 25.0
24.0 5.7 10.7
3 35.8 21.1
28.8 7.4 11.1
5 25.3 24.0
5+3 30.2 8.1 14.7 28.9 14.5
27.3 9.0 10.0 36.3
5+7 11.6
aThree leaf fragments were incubated for 5 days in an induction medium containing 330 mM glycerol and transferred for 7 days
in a expression medium without glycerol. Lipids were separated by TLC and fatty acid methyl esters were analyzed by GC. Data are means of three independent replicates.
rated fatty acids (54%) and low amount of polyun-saturated acids. In the control leaves, triacylglyc-erols were mainly enriched in 16:0 (40.3%). At day 5+7, the 18:2 proportion was more important and reached 31%, 16:0 being the major fatty acid (34%).
4. Discussion
Somatic embryogenesis is the result of cell dedif-ferentiation and reacquisition of a morphogenetic competence. InCichoriumleaf tissues, somatic em-bryogenesis is direct and offers the opportunity to investigate the changes taking place during the induction and the expression of morphogenetic competence. Due to the metabolic importance of lipids in plant development, we realised a detailed analysis of lipid composition changes at different stages of somatic embryogenesis. The time se-quence of the embryogenic process in leaves of
Cichorium ‘474’ has been described by Dubois et al. and Robatche-Claive et al. [7,8]. At first, cells are induced for morphogenetic competence (induc-tion phase) and then, they expressed embryogenic competence (expression phase). These two steps can be controlled by adding glycerol in the culture
medium during the induction phase [8]. Three days after transfer in the expression medium without glycerol (D5+3), unicellular embryogenic cells were generally segmented into multicellular dense proembryos. At day 5+7, the majority of em-bryogenic cells evolved into big globular proem-bryos inside the leaf. Somatic embryogenesis process involved lipid biosynthesis modifications and the analysis of these changes was performed at five stages: D0, D3, D5, D5+3 and D5+7. A comparison of the fatty acid composition of total lipids in control leaves (D0) of theCichorium
‘Pe´ve`le’. Thus, the high percentage of 18:2 was specific to embryogenic genotype and could be characteristic of the embryogenic process.
The changes in the fatty acid composition of the
Cichorium ‘474’ leaves were not only due to em-bryogenic process but also to culture conditions. The decrease of 18:3 was probably associated with the senescence of leaves. Previously, Krebsky et al. [14] have shown that etiolated Cichorium intybus
accumulate 16:0 and 18:2. Induction of somatic embryogenesis was made in culture conditions de-scribed by Robatche-Claive et al. [8] in darkness. However, in presence of light (12 h/day) there was no significant change in fatty acid composition in the Cichorium ‘474’ leaves throughout the culture period (data not shown). The results with these two genotypes could indicate that the evolution of fatty acids pattern was not only due to the absence of light but was also linked to somatic embryogen-esis. The high percentage of 18:2 after 12 days of culture is also in agreement with the results pub-lished by Dutta and Appelqvist [15] in Daucus carota during somatic embryogenesis. These au-thors showed that 18:2 was the major fatty acid in somatic embryoids at different stages and sug-gested that this high percentage of 18:2 could be associated with somatic embryogenesis.
In the present study, we have also investigated the evolution of the different lipid classes during
Cichorium ‘474’ somatic embryogenesis. Not sur-prisingly, galactolipids and phospholipids, the main structural lipids of chloroplasts and plasma membranes respectively, were predominant in leaves of the Cichorium ‘474’ at day 0. While the percentages of MGDG and DGDG decreased rapidly until day 5+7, phospholipids (PC, PE) proportions increased from the first days of induc-tion phase to the end of expression stage. This relative disappearence of MGDG and DGDG is certainly associated with the breakdown of chloro-plast lipids during leaf fragments culture. In con-trol leaves, the proportion of TAG was low and increased throughout the culture period.
The variations in lipid content in Cichorium
‘474’ during somatic embryogenesis are in well agreement with other published results. Storage lipid modifications during somatic embryogenesis have been well documented [16]. However, these results, expressed in percentages, could not clearly indicate if the increase in TAG content, for exam-ple, is only due to a synthesis of TAG or to a
decrease of the other neutral lipids. Only experi-ments using radioactive tracers could answer this question. We therefore labelled plant lipids with [14C] glycerol, backbone of lipidic molecules, and
then carried out a chase experiment during which the label variations in lipid classes were studied. In the induction phase a rapid incorporation of ra-dioactivity was observed into polar lipids (espe-cially PE and PC). This increase in phospholipids label could be associated with changes in mem-brane composition as well as in memmem-brane forma-tion during the reactivaforma-tion of cells. For example, in culture of oilseed rape, phospholipids predomi-nate in calli cells which were primarily producing membraneous material [17]. During the induction phase low amount of radioactivity was detected into storage lipids. After transfer in the expression medium, there was an important synthesis of TAG which corresponds to the formation of embryos. These results are in accordance with previous data on Daucus carota[18] showing that the incorpora-tion of [14C] acetate was higher in PC than in other
phospholipids. This author also demonstrated that incorporation of labelled acetate showed a rapid decrease in the phospholipids during a chase ex-periment with a concomitant increase in TAG. In
Cichoriumleaves, PC was the most actively synthe-sized phospholipid during the induction phase. During the expression phase, PC could participate in TAG synthesis by donation of its entire DAG portion or by acyl exchange. The accumulation of TAG was related to the embryo development for a number of important species,Arachis hypogaea[2],
Brassica napus [19], Papa6er species [5], Prunus
a6ium [20] and Vitis 6inifera [6]. In oil palm,
histological examination of somatic embryos aris-ing from leaf explants revealed a high content of storage lipids during the early stages of the em-bryogenesic process [16]. Thus some authors even considered that this accumulation of storage lipids, especially TAG, appeared to be an early indicator that in vitro cultured tissues are develop-ing into true somatic embryos.
was observed in several lipid classes. The principal phospholipid, PC, was characterized by a high proportion of 18:2 which reached 36% at day 5+7. This result was in agreement with those obtained in Prunus a6ium [20]. Thus, PC from
embryogenic tissues showed a higher proportion of 18:2 than PC from non-embryogenic calli. Dur-ing somatic embryogenesic process in the Cicho
-rium ‘474’, an increase in the proportion of 18:2 was noted in TAG fraction. At day 5+7, the major fatty acids were 16:0 and 18:2. In Prunus a6ium, TAG were composed mainly of
monopalmitoyl-dilinoleoyl in embryogenic tissues [20]. The accumulation of 18:2 in PC and TAG has been suggested to be markers of somatic em-bryogenesis in Prunus a6ium. Phosphatidylcholine
is the site of the desaturation of oleate into linoleate and could be a precursor of TAG.
Our results demonstrate that important modifi-cations occur in the lipid metabolism related to somatic embryogenesis inCichorium leaves. Using isotopic labelling, it is the first time that labelling kinetics of lipid classes were shown during the very early stages of somatic embryogenesis. Further studies on the molecular species of the neosynthe-sized PC and TAG are required to better charac-terize the embryogenic process. Investigations of the enzymes implied in these changes are necessary and their respective role in the sequence of com-plex events in embryo formation should be determined.
References
[1] C. Somerville, J. Browse, Plant lipids: metabolism, mu-tants and membranes, Science 252 (1991) 80 – 87. [2] V.B. Mhaske, S. Hazra, Appearence of storage lipid
(triglycerides) in somatic embryos of peanut (Arachis hypogaeaL.), In vitro Cell Dev. Biol. 30P (1994) 113 – 116.
[3] P.C. Dutta, L.A,. Appelqvist, Lipids and fatty acid pat-terns in developing seed, leaf, root, and tissue culture initiated from embryos ofDaucus carotaL, Plant Sci. 75 (1991) 177 – 183.
[4] L. Liu, E.G. Hammond, E.S. Wurtele, Accumulation of petroselinic acid in developing somatic carrot embryos, Phytochemistry 37 (1994) 749 – 753.
[5] S. Hara, H. Falk, H. Kleinig, Starch and triacylglycerol metabolism related to somatic embryogenesis inPapa6er
orientalistissue culture, Planta 164 (1985) 303 – 314.
[6] O. Faure, J. Aarouf, Metabolism of reserve products during development of somatic embryos and germina-tion of zygotic embryos in grapevine, Plant Sci. 96 (1994) 167 – 178.
[7] T. Dubois, M. Guedira, J. Dubois, J. Vasseur, Direct somatic embryogenesis in leaves of Cichorium. A histo-logical and SEM study of early stages, Protoplasma 162 (1991) 120 – 127.
[8] A.S. Robatche-Claive, J.P. Couillerot, J. Dubois, T. Dubois, J. Vasseur, Embryogene`se somatique directe dans les feuilles duCichoriumhybride ‘‘474’’: synchroni-sation de l’induction, C. R. Acad. Sci. Se´r. III 314 (1992) 371 – 377.
[9] J.L. Hilbert, T. Dubois, J. Vasseur, Detection of em-bryogenesis-related proteins during somatic embryo for-mation inCichorium, Plant Physiol. Biochem. 30 (1992) 733 – 741.
[10] C. Boyer, J.L. Hilbert, J. Vasseur, Embryogenesis-re-lated protein synthesis and accumulation during early acquisition of somatic embryogenesis competence inCi
-chorium, Plant Sci. 93 (1993) 41 – 53.
[11] S. Helleboid, J.P. Couillerot, J.L. Hilbert, J. Vasseur, Inhibition of direct somatic embryogenesis by a -difl-uoromethylarginine in a Cichorium hybrid: effects on polyamine content and protein patterns, Planta 196 (1995) 571 – 576.
[12] S. Helleboid, G. Bauw, L. Belingheri, J. Vasseur, J.L. Hilbert, Extracellularb-1,3-glucanases are induced dur-ing early somatic embryogenesis in Cichorium, Planta 205 (1998) 56 – 63.
[13] E.G. Bligh, W.J. Dyer, A rapid method of total lipid extraction and purification, Can. J. Biochem. Physiol. 37 (1959) 911 – 917.
[14] E.O. Krebsky, J.M.C. Geuns, M. De Proft, Fatty acids in polar lipids from etiolated Cichorium intybus, Phyto-chemistry 43 (4) (1996) 747 – 751.
[15] P.C. Dutta, L.A, . Appelqvist, The effects of different cultural conditions on the accumulation of depot lipids notably petroselinic acid during somatic embryogenesis in Daucus carotaL, Plant Sci. 64 (1989) 167 – 177. [16] N. Weber, D.C. Taylor, E.W. Underhill, Biosynthesis of
storage lipids in plant cell and embryo cultures, in: A. Fiechter (Ed.), Advances in Biochemical Engineering Biotechnology, 45, Springer-Verlag, Berlin, 1992, pp. 100 – 131.
[17] M. Williams, D. Francis, A.C. Hann, J.L. Harwood, Changes in lipid composition during callus differentia-tion in cultures of oilseed rape (Brassica napus L.), J. Exp. Bot. 42 (1991) 1551 – 1556.
[18] P.C. Dutta, Incorporation of [14C] acetate in different
lipid classes and in the fatty acids of triacylglycerols in somatic embryos and cotyledon slices of Daucus carota
L, Swed. J. Agric. Res. 22 (1992) 117 – 123.
[19] A. Avjioglu, R.B. Knox, Storage lipid accumulation by zygotic and somatic embryos in culture, Ann. Bot. 63 (1989) 409 – 420.
[20] L. Reidiboym-Talleux, G. Grenier-De March, Lipid and fatty acid composition in non-embryogenic calli and embryogenic tissues in wild cherry (Prunus a6ium),
Phys-iol. Plant. 105 (1999) 513 – 520.
![Fig. 2. Incorporation of [U-14C] glycerol into the main lipidclasses in Cichorium ‘‘474’’ leaf tissues during somatic em-bryogenesis](https://thumb-ap.123doks.com/thumbv2/123dok/1032909.928497/5.612.38.256.468.601/fig-incorporation-glycerol-lipidclasses-cichorium-tissues-somatic-bryogenesis.webp)