RESEARCH ARTICLE
The improvement of doxorubicin activity on breast cancer
cell lines by tangeretin through cell cycle modulation
Edy Meiyanto&Aditya Fitriasari&Adam Hermawan& Sendy Junedi&Ratna Asmah Susidarti
Received: 24 May 2011 / Accepted: 10 June 2011 / Published online: 5 July 2011 #Institute of Oriental Medicine, Kyung Hee University 2011
Abstract Tangeretin, shows cytotoxic effect on COLO 205 colon cancer cells. Combination of tangeretin with tamox-ifen showed synergistic effect and increased the cancer cell sensitivity towards tamoxifen on T47D cells. However, the combination of tangeretin with chemotherapeutic agent doxorubicin on breast cancer cells have not been explored yet. Therefore, the aim of this research is to examine the improvement of cytotoxic effect of doxorubicin by tanger-etin through cell death induction and cell cycle modulation on MCF-7 and T47D cells. The cytotoxic effect of tangeretin, doxorubicin, and their combination on tested cells were carried out by using MTT assay. Cell cycle distribution was determined by flowcytometer FACS-Calibur and the flowcytometry data was analyzed using ModFit LT 3.0 program. Cell death assay were done by double staining method using ethydium bromide-acridin
orange. Single treatment of tangeretin 5–100 μM did not
show cytotoxic effect on MCF-7 and T47D cells. The
combination of tangeretin 50 and 100μM with doxorubicin
200 nM (MCF-7) and 7.5 nM (T47D) increased the cytotoxic effect of doxorubicin on both breast cancer cell lines. This improvement of cytotoxic effect is due to cell death induction and cell cycle modulation. Furthermore, single treatment of tangeretin showed cell death only on T47D cell and caused G1-phase arrest on MCF-7 cell and
G2/M-phase arrest on T47D cell. While doxorubicin induced cell accumulation at G2/M phase in both cancer cell lines. However, combination of tangeretin and doxoru-bicin increased cell death on both cancer cell lines, compared with doxorubicin by itself. The combination also showed G1-phase arrest on MCF-7 cell and increased cell accumulation at G2/M phase on T47D cell. Based on this result, tangeretin is potential to be developed as co-chemotherapeutic agent for breast cancer by inducing apoptosis and cell cycle arrest. However, the molecular mechanism need to be explored further.
Keywords Tangeretin . Co-chemotherapy . Breast cancer . Cell cycle arrest . Cell death
Introduction
Breast cancer is one of the death-cause cancer in the world
(Jemal et al. 2010). Breast cancer is caused by the
uncontrolled proliferation of breast cells which is happened because of their ability to avoid apoptotic mechanism (Yu et
al.2010). One drug for breast cancer therapy is doxorubicin.
The using of doxorubicin has a lot of side effects and also
resistance effects on breast cancer cell (Smith et al. 2006).
Therefore, combination of doxorubicin with chemopreven-tive agent (co-chemotherapy) were needed to increase the activity of doxorubicin by inhibiting proliferation and inducing apoptosis of breast cancer cell.
One of the chemopreventive agents that has been reported to have antiproliferative effect is tangeretin, a
polymethoxy flavone found in citrus species (Citrus
aurantifolia). Tangeretin have been proven to inhibit the growth of estradiol-stimulated T47D cells (Van Slambrouck
et al. 2005); inhibit proliferation of MCF-7 cells after
E. Meiyanto (*)
:
A. Fitriasari:
A. Hermawan:
S. Junedi:
R. A. Susidarti4 days treatment and cause G1-phase arrest on MCF-7 cells
by 24 h, 48 h and 72 h treatment (Morley et al. 2007).
Tangeretin has the smallest IC50value on COLO 205 colon
cancer cells, compared with the other flavonoid com-pounds, such as apigenin, kaempferol, myricetin, quercetin,
luteolin, nobiletin, and rutin (Pan et al. 2002). Tangeretin
also induce apoptosis on HL-60 leukemia cells (Hirano et
al.1995). The combination of tangeretin and tamoxifen in
vitro increased the sensitivity of cancer cell synergistically
towards tamoxifen (Bracke et al.1999).
Those researches showed the potency of tangeretin as chemopreventive agent and became a basic for the development of tangeretin as co-chemotherapeutic agent to increase the cytotoxic activity and reduce the side effects of doxorubicin. Therefore, the purpose of this research is to examine the effect of tangeretin and its combination with doxorubicin on cell cycle and apoptosis of breast cancer cells.
Materials and methods
Materials
Tangeretin was obtained from Sigma Aldrich Chemie GmBH, Steinheim, Germany (Cat No. T8951) while doxorubicin was obtained from P.T. Ferron Par Pharmaceu-tical. A DMSO (Sigma Aldrich Chemie GmBH, Steinheim, Germany) solution of tangeretin was used for in vitro experiment by diluting desired consentration. The final DMSO concentration was not more than 1%.
Cell lines
MCF-7 and T47D cells were kindly provided by Prof. Masashi Kawaichi (Nara Institute of Science and Technol-ogy (NAIST)), Nara, Japan.
Cytotoxic assay (MTT assay)
MCF-7 and T47D cells were cultured in Dulbecco’s
Modified Eagle’s Medium(DMEM) containingFetal Bovine Serum (FBS) 10% (v/v) (FBS qualified, Gibco,
Invitro-gen™ USA) and penisillin-streptomisin 1% (v/v) (Gibco,
Invitrogen Corporation, Grand Island, NY, 14072, USA).
Cells (5×103 cells/well) were transferred to 96-well plate
(Iwaki, Japan) and incubated for 24 h (70–80% confluent).
Cells were treated by tangeretin, doxorubicin, and their combination, and incubated for 24 h. At the end of the incubation, 5 mg/ml solution of MTT [3-(4,5-dimethylth-iazole-2-yl)-2,5-diphenyl tetrazolium bromide] (Sigma, Sigma-Aldrich Corp, St. Louis, MO, USA) were added to each wells and the cells were incubated for 4 h in 37°C.
Viable cells reacts with MTT to form purple formazan crystal. After 4 h, stopper sodium dodesil sulphate 10% in 0,1N sulphuric acid solution were added to dissolve formazan crystal. Cells were incubated over night and protected from light. Cells were shaken for 10 min before
read by ELISA reader atλ595 nm. The absorbance of each
well converted to percentage of viable cells:
% Viable cells¼Treated cells abs Medium control abs
Cells control abs Medium control abs100%
Cell death assay (double staining method)
Cells (5×104 cells/well) were transferred to coverslips
(Nunc, Denmark) in 24-well plate (Iwaki, Japan) and incubated for 24 h (50–60% confluent). Cells were treated by tangeretin, doxorubicin, and their combination, and incubated for 15 h. At the end of the incubation, coverslips containing cells were moved to object glass. Mixture solution of etidium bromide-acridine orange (Sigma, Sigma-Aldrich Corp, St. Louis, MO, USA) were added to the cells to form fluorescence cells. The fluorescence cells were examined immediately by fluorescence microscope (Zeiss MC80). Green fluorescence cells showed viable cells, while red fluorescence cells showed dead cells.
Flowcytometry assay
Cells (5×105cells/well) were transferred into 6-well plate
Result
Cytotoxicity of tangeretin
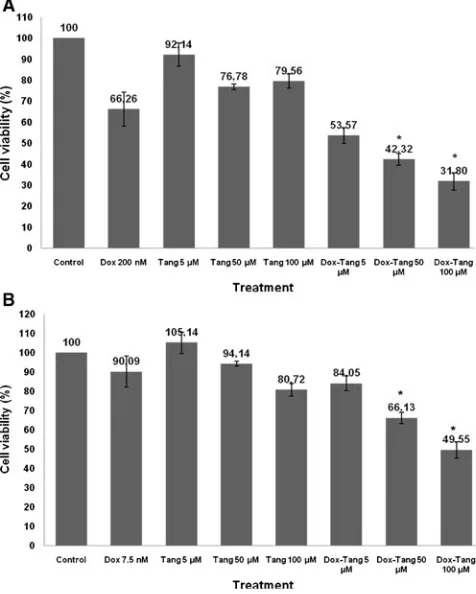
The direct cytotoxicity of tangeretin was assessed by MTT assay of MCF-7 and T47D breast cancer cell lines. Single treatment of tangeretin did not show cytotoxic effect on both cell lines, MCF-7 and T47D with cell viability more
than 100% (Fig. 1). This result showed that the using of
tangeretin to the patient is probably safe without any toxicity to the cells. Surprisingly, combination of
doxoru-bicin with tangeretin 5, 50, and 100 μM showed higher
inhibition of cells growth than single treatment of
doxoru-bicin in both breast cancer cell lines (Fig. 2). On MCF-7
cells tangeretin 50μM and 100μM decreased doxorubicin
treated-cell viability from 66% to 42% and 66% to 31%,
respectively. On T47D cells tangeretin 50μM and 100μM
decreased doxorubicin treated-cell viability from 90% to 66% and 90% to 50%, respectively. This result suggested that this combination give improvement of doxorubicin cytotoxic effect. The decreasing cell viability possibly, could be occurred through inhibiting of cell proliferation and inducing cell death.
Induction of cell death in MCF-7 and T47D cells by tangeretin and combination with doxorubicin
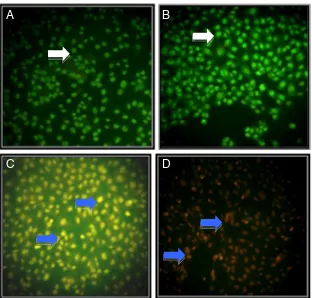
The results from cytotoxic assay of tangeretin and doxoru-bicin were parallel with cell death assay using double staining method. Single treatment of doxorubicin and tangeretin in MCF-7 cells showed chromatin condensation and cell death induction, respectively. Combination of doxorubicin and tangeretin significantly increased the incidence of cell death, compared to single treatment of
doxorubicin (Fig. 3). On T47D cells single treatment of
doxorubicin and tangeretin showed cell death induction, while their combination increased the incidence of cell
death, compared to single treatment of doxorubicin (Fig.4).
Orange fluorescence cells represent death cells that loss cell membrane permeability and form apoptotic bodies that supposed to be apoptosis.
Induction of cell cycle arrest by tangeretin in MCF-7 and T47D cells
Beside cell death induction, improvement of doxorubicin cytotoxic effect by tangeretin could also occurred through cell cycle modulation. Cell cycle analysis of both breast cancer cell lines showed that tangeretin and doxorubicin Fig. 1 Effect of tangeretin to
the proliferation of MCF-7 (left) and T47D (right) breast cancer cells. The assay performed by incubating 5×103cells/well with tangeretin (5–100μM) for 24 h. After 24 h, cells were added by MTT reagent to cal-culate the absorbance which represent viable cells
C
D
A
B
Fig. 3 Effect of tangeretin, doxorubicin, and their combina-tion on MCF-7 cell death. MCF-7 cell were treated by tangeretin, doxorubicin, and their combination for 15 h and stained by etidium bromide-acridine orange.aCell control, bTangeretin 100μM,c Doxo-rubicin 200 nM,dCombination of tangeretin 100μM and doxorubicin 200 nM. Viable cells give green fluorescence ( ), dead cells give orange fluorescence ( )
A
B
C
D
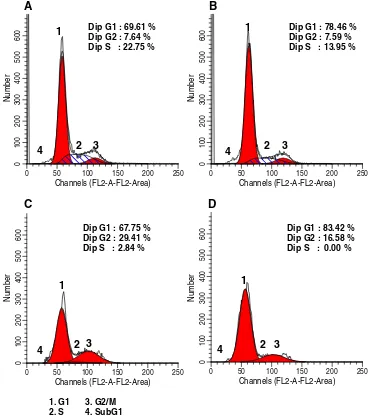
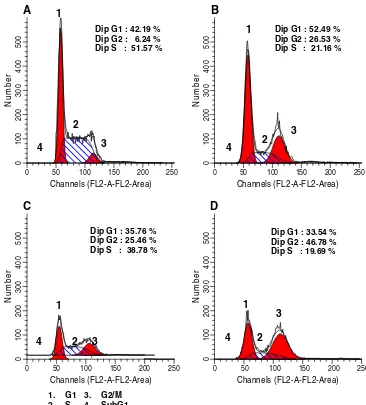
have different cell cycle profile. Single treatment of tangeretin induced cell accumulation at G1 phase on MCF-7 cells and showed cell accumulation at G2/M phase (G2/M-phase arrest) on T47D cells. While, doxorubicin arrested both cell lines at G2/M-phase. However,
combina-tion of tangeretin and doxorubicin showed G1-phase arrest on MCF-7 cells and increased cell accumulation at G2/M
phase on T47D cells (Table1; Figs.5and6). These results
suggested the domination of tangeretin in the improvement of doxorubicin cytotoxic effect.
Cell Treatment Concentration G1 (%) S (%) G2/M (%)
MCF-7 Control – 69.61 22.75 7.64
Tangeretin 100μM 78.46 13.95 7.59
Doxorubicin 200 nM 67.75 2.84 29.41
Tangeretin-Doxorubicin 100μM–200 nM 83.42 0.00 16.58
T47D Control – 42.19 51.57 6.24
Tangeretin 100μM 52.49 21.16 26.35
Doxorubicin 7.5 nM 35.76 38.78 25.46
Tangeretin-Doxorubicin 100μM–7.5 nM 33.54 19.69 46.78 Table 1 Cell cycle distribution
after treatment of tangeretin, doxorubicin, and their combina-tion for 24 h
Discussion
This study explored the effect of tangeretin alone and in combination with doxorubicin on cytotoxicity, cell cycle, and cell death induction of MCF-7 and T47D cells. Our previous study showed that doxorubicin showed strong
cytotoxic effect on MCF-7 and T47D cells with IC50value
of 467 and 15 nM, respectively (Hermawan et al. 2010;
Junedi et al.2010). The higher IC50value on MCF-7 cells
is due to the characteristic of MCF-7 cells which is resistant to doxorubicin by overexpressing anti-apoptotic protein Bcl-2, P-glycoprotein, and phosphorilated Akt (Davis et al.
2003; Valeria and Barrera-Rodrigue 2005; Li et al.2005).
However, T47D cells also showed resistance to doxorubicin
due to p53 mutation (Di Leo et al. 2007; Vayssade et al.
2005). Therefore, to increase the sensitivity of MCF-7 and
T47D cells towards doxorubicin, the combination treatment with tangeretin is needed.
Combination of tangeretin and doxorubicin showed cell death induction that supposed to be apoptosis. Previous study
mentioned that treatment of 30 μM tangeretin induced
apoptosis of human neuroblastoma SH-SY5Y cells by reducing the mitochondrial membrane potential and elevated caspase-3 activity of non treatment cells, while combination of tangeretin and nobiletin showed elevation of caspase-3
activity compared to non treated cells (Akao et al. 2008).
Tangeretin also inhibited the P-glycoprotein function and significantly influenced the cell cycle, whereas they did not induce apoptosis of MOLT4 Daunorubicin-Resistant T
Lymphoblastoid Leukemia Cells (Ishii et al.2010).
Chloro-form fraction of Citrus grandis leaf extract that contains
tangeretin induced apoptosis showed by decreasing of the protein levels of the precursors of caspase-9, -8, -3, and -7
and cleaving of PARP (Kim et al. 2010). Thus tangeretin
probably also induced apoptosis of MCF-7 and T47D cells. The apoptotic mechanism need to be explored details.
A
Fig. 6 T47D cell cycleThere are two main mechanism of apoptosis, intrinsic and extrinsic pathway, through which apoptosis could be occurred in MCF-7 and T47D cells. These apoptotic mechanisms involve the expression of regulatory protein, such as p53, Bcl-2 family, and activation of caspase
family (Kumar et al.2000). MCF-7 cells express p53 and
Bcl-2. Doxorubicin induces apoptosis through intrinsic mechanism by elevating p53 expression which is needed to induce the expression of pro-apoptosis protein (Bax)
(Minotti et al.2004). In addition, tangeretin increases the
expression of p53 protein in colon cancer cell (Pan et al.
2002). Therefore, the increasing of cell death after
combination treatment of tangeretin and doxorubicin on MCF-7 cells probably by apoptosis in p53-dependent pathway. The proposed mechanism is need to be explored further.
While, T47D cells do not express p53. Therefore, apoptotic mechanism of T47D cells is occurred in
p53-independent pathway. Van Slambrouck et al. (2005) showed
that tangeretin inhibit ERK phosphorilation pathway which is involved in T47D cell proliferation. Perhaps, the increasing of apoptotic cell after combination treatment of tangeretin and doxorubicin on T47D cells also occurred through this mechanism. But the exact mechanism need to be explored more detail.
The different effect on MCF-7 and T47D cell cycle are occurred due to different mechanism action of tangeretin in both cells. Cell cycle arrest by tangeretin involves inhibi-tion of CDK2/CDK4 activity and inducinhibi-tion of CDK inhibitor expression, such as p27 and p21, which are
proved by Pan et al. (2002) and Morley et al. (2007) on
colorectal cancer (COLO 205), breast cancer (MDA-MB-435 dan MCF-7), and colon cancer (HT-29). Doxorubicin inhibits the activity of cdc2 which is involved in G2-M
phase transition (Ling et al. 1996). But the exact
mecha-nism need to be explored further.
The significant increasing of cytotoxic effect after combination treatment of tangeretin and doxorubicin could also occurred through inhibition of multidrugs resistance
(MDR) protein. Tanaka et al. (1997) reported that tangeretin
increases vinblastine uptake by inhibiting P-gp efflux pump in Caco-2 cells. Those three mechanisms, MDR protein inhibition, apoptotic induction, cell cycle modulation are strengthened each other to form stronger cytotoxic effect on breast cancer cells.
This result showed the potency of tangeretin to be developed as co-chemotherapeutic agent for doxorubicin by inducing cell death and cell cycle arrest. The use of doxorubicin together with tangeretin is expected to increase the activity and reduce the side effects of doxorubicin. However, the molecular mechanism of cell death induction and cell cycle arrest by this combination need to be explored further.
Conclusion
This research shows that combination of tangeretin and doxorubicin synergically increases the cytotoxic effect of doxorubicin through cell death induction and cell cycle arrest. Based on this result, tangeretin is potential to be developed as co-chemotherapeutic agent for doxorubicin.
Acknowledgement This work was supported by Program DIPA Universitas Gadjah Mada through Research Program Tim Hibah Pascasarjana Multitahun year 2009 and 2010.
References
Akao Y, Itoh T, Ohguchi K, Iinuma M, Nozawa Y (2008) Interactive effects of polymethoxy flavones from citrus on cell growth inhibition in human neuroblastoma SH-SY5Y cells. Bioorg Med Chem 16:2803–2810
Bracke ME, Depypere HT, Boterberg T, Van Marck VL, Vennekens KM, Vanluchene E, Nuytinck M, Serreyn R, Mareel MM (1999) Influence of tangeretin on tamoxifen’s therapeutic benefit in mammary cancer. J Natl Cancer Inst 91(4):354–359
Davis JM, Navolanic PM, Weinstein-Oppenheimer CR, Steelman LS, Wei H, Konopleva M, Blagosklonny MV, McCubrey JA (2003) Raf-1 and Bcl-2 induce distinct and common pathways that contribute to breast cancer drug resistance. Clin Cancer Res 9:1161–1170 Di Leo A, Tanner M, Desmed C, Paesman M, Cardoso F, Durbecq V,
Chan S, Parren T, Aapro M, Sotiriou C, Piccart MJ, Larsimont D, Isola J (2007) p-53 gene mutations as a predictive marker in a population of advanced breast cancer patients randomly treated with doxorubicin or docetaxel in the context of a phase III clinical trial. Ann Oncol 18:997–1003
Hermawan A, Meiyanto E, Susidarti A (2010) Hesperidin Meningkatkan Aktivitas Sitotoksik Doxorubicin pada Sel MCF-7. Majalah Farmasi Indonesia 21(1):8–17
Hirano T, Abe K, Gotoh M, Oka K (1995) Citrus flavone tangeretin inhibits leukaemic HL-60 cell growth partially through induction of apoptosis with less cytotoxicity on normal lymphocytes. Br J Cancer 72(6):1380–1388
Ishii K, Tanaka S, Kagami K, Henmi K, Toyoda H, Kaise T (2010) Effects of naturally occurring polymethyoxyflavonoids on cell growth, p-glycoprotein function, cell cycle, and apoptosis of daunorubicin-resistant T lymphoblastoid leukemia cells. Cancer Invest 28:220–229
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin. doi:10.3322/caac.20073
Junedi S, Susidarti RA, Meiyanto E (2010) Naringenin Meningkatkan Efek Sitotoksik Doxorubicin Pada Sel Kanker Payudara T47D Melalui Induksi Apoptosis. Jurnal Ilmu Kefarmasian Indonesia 8 (2):85–90
Kim H, Moon JY, Mosaddik A, Cho SK (2010) Induction of apoptosis in human cervical carcinoma HeLa cells by polymethoxylated flavone-richCitrus grandisOsbeck (Dangyuja) leaf extract. Food Chem Toxicol 48:2435–2442
Kumar R, Vadlamudi RK, Adam L (2000) Apoptosis in mammary gland and cancer. Endocr Relat Cancer 7:257–269
Li X, Lu Y, Liang K, Liu B, Fan Z (2005) Differential responses to doxorubicin-induced phosphorylation and activation of akt in human breast cancer cells. Breast Cancer Res 7(5):R589– R597
p34cdc2/Cyclin B1 activity induced by doxorubicin in synchro-nized P388 cells. Mol Pharmacol 49(5):832–841
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclins: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56:185–228 Morley K, Ferguson P, Korotpatnick J (2007) Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett 251(1):168–178
Pan MH, Chen WJ, Shiau SYL, Ho CT, Lin JK (2002) Tangeretin induces cell-cycle G1arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis 23 (10):1677–1684
Smith L, Watson MB, O’Kane SL, Drew MPJ, Lind MJ, Cawkwell L (2006) The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Mol Cancer Ther 5 (8):2115–2120
Tanaka T, Makita H, Ohnishi M, Mori H, Satoh K, Hara A, Sumida T, Fukutani K, Tanaka T, Ogawa H (1997) Chemoprevention of
4-nitroquinoline 1-oxide-induced oral carcinogenesis in rats by flavonoids diosmin and hesperidin, each alone and in combina-tion. Cancer Res 57:246–252
Valeria P, Barrera-Rodrigue R (2005) Changes in P-glycoprotein activity are mediated by the growth of a tumour cell line as multicellular spheroids. Cancer Cell Int 5(20), Summary Van Slambrouck S, Parmar VS, Sharma SK, De Bondt B, Foré F,
Coopman P, Vanhoecke BW, Boterberg T, Depypere HT, Leclercq G, Bracke ME (2005) Tangeretin inhibits extracellular-signal-regulated kinase (ERK) phosphorylation. FEBS Lett 579 (7):1665–9
Vayssade M, Haddada H, Faridoni-Laurens L, Tourpin S, Valent A, Bénard J, Ahomadegbe J (2005) p73 functionally replaces p53 in adriamycin-treated, p53-deficient breast cancer cells. Int J Cancer 116(6):860–869