Differential expression of proteoglycans biglycan and decorin
during neointima formation after stent implantation in normal and
atherosclerotic rabbit aortas
Tomoyuki Yamakawa
a,*, Hong-zhi Bai
a, Junichi Masuda
b, Yoshiki Sawa
a,
Ryota Shirakura
c, Jun Ogata
d, Hikaru Matsuda
aaDepartment of Surgery,Course of Inter6entional Medicine(E1),Osaka Uni6ersity Medical School,2-2Yamada-oka,Suita,Osaka565,Japan bDepartment of Laboratory Medicine,Shimane Medical Uni6ersity,Izumo,Shimane693,Japan
cDi6ision of Organ Transplantation Biomedical Research Center,Osaka Uni6ersity Medical School,2-2Yamada-oka,Suita,Osaka565,Japan dNational Cardio6ascular Center Research Institute,Suita,Osaka565,Japan
Received 10 June 1999; received in revised form 25 October 1999; accepted 5 November 1999
Abstract
Proteoglycans decorin and biglycan, which bind to TGF-b, are thought to participate in regulation of extracellular matrix accumulation in arterial intimal hyperplasia. To investigate the correlation of these proteoglycans with the cellular localization and phenotypic modulation of smooth muscle cells (SMCs), we analyzed the spatial and chronological distribution of these proteoglycans and two cytokines, TGF-band IL-1b, in the process of neointima formation after stent implantation in the aortas of rabbits fed a high-cholesterol diet (atherosclerotic group) or a regular diet (control group). We implanted metallic stents in the rabbit aortas and harvested the aortas 4 – 56 days later for immunohistochemical and mRNA in situ hybridization analyses. In the control group, TGF-band biglycan expression was in correspondence with the chronology and localization of embryonic SMCs. In the atherosclerotic group, TGF-b and biglycan expression was sustained throughout the experimental period, which was in accord with the prolonged expression of embryonic SMCs. Decorin, which did not occur in neointima in the control group, appeared in the atherosclerotic aortas in the confined area of vascular SMCs surrounding the macrophages around the stent wire. These results indicate that biglycan and decorin kinetics during neointima formation after arterial injury are distinct, despite their similar construction; biglycan synthesis correlates with embryonic SMCs. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Stent; Restenosis; Extracellular matrix; Proteoglycan; Imunohistochemistry; In situ hybridization
www.elsevier.com/locate/atherosclerosis
1. Introduction
Several clinical studies have demonstrated that intra-coronary stent implantation in conjunction with percu-taneous transluminal coronary angioplasty (PTCA) has an advantage over PTCA alone in maintaining long-term patency, and may thus reduce the incidence of restenosis after angioplasty [1 – 3]. Such a reduction in restenosis is thought to be due to the larger acute gain
obtained by stent implantation. However, the late loss of diameter due to intimal hyperplasia is even greater after stenting than after PTCA. Thus, restenosis after stenting remains an unsolved problem. We recently analyzed the spatial and chronological distribution of cellular components during neointima formation after stent implantation in normal rabbit aortas [4] and we observed that the compression injury due to stent im-plantation led to neointima formation through tran-sient proliferation and phenotypic modulation of smooth muscle cells (SMCs). The targets of angio-plasty, however, are highly atherosclerotic vessels, and the mechanism of the response to vascular stent injury in such vessels is not yet well examined.
* Corresponding author. Tel.: +81-6-68793154, fax: + 81-6-68793159.
E-mail address:[email protected] (T. Yamakawa).
Accumulation of extracellular matrix (ECM) is an-other major event in the intimal hyperplasia following arterial injury, as are the migration and proliferation of SMCs [5]. Atherosclerotic lesions also contain a great amount of various ECM components. Recent studies revealed that ECM not only maintains the structural integrity of the tissues, but also interacts with cellular components and regulates their functions [6,7].
Proteoglycans are a family of noncollagenous glyco-proteins constituting ECM. The functions of proteogly-cans are varied, and some of them are known to interact with particular cytokines [8]. Recent studies on fibroproliferative lesions such as in liver fibrosis, [9] pulmonary fibrosis [10] and atherosclerosis [11] have focused on two kinds of leucine-rich small proteogly-cans, biglycan and decorin, which can bind to trans-forming growth factor-beta (TGF-b), the most potent inducer of ECM synthesis [12,13]. Decorin, in particu-lar, neutralizes the effect of TGF-b activities in vitro and in vivo [14,15]. These proteoglycans may therefore have a role in the modulation of ECM synthesis and accumulation.
Atherosclerotic lesions also contain a large number of macrophages secreting various kinds of cytokines such as TGF-b and interleukin-1 (IL-1). Recently IL-1 was reported to enhance the production of decorin in arterial SMCs in vitro, [16] suggesting the dynamic interactions of macrophages with ECM formation in atherosclerotic lesions.
The above findings prompted us to examine the spatial and chronological distribution of the expressions of decorin and biglycan in the association of TGF-b and IL-1 expression in normal and atherosclerotic rab-bit aortas after vascular stent implantation in order to clarify the ECM kinetics in the process of neointima formation.
2. Materials and methods
2.1. Stent description
The stent used was the same as that used in our previous study [4], a 12-mm-long self-expandable device with an expanded diameter of 7.0 mm, made of 0.2-mm stainless steel wire (SUS304) with five bends in a zigzag pattern (known as Gianturco’s Z type stent).
2.2. Animals
Thirty-six adult male New Zealand White rabbits weighing 3.5 – 4.0 kg (Kbl-NZW, Nagano, Japan) were used in this study and divided into two groups. Eigh-teen rabbits had been fed a diet containing 0.5% choles-terol (ORC4, Oriental Yeast, Tokyo, Japan) to induce atherosclerotic lesions (atherosclerotic group), and the
others had been fed regular chow (control group). The stents were implanted in the aortas of 12 rabbits from the atherosclerotic group after 3 months on the choles-terol diet and in 15 animals from the control group. The diets did not change until the time of sacrifice. The procedures were in accordance with the Guide for Care and Use of Laboratory Animals issued by the US Institute of Laboratory Resources.
2.3. Stent implantation
The stents were implanted as described in our preced-ing paper [4]. In brief, general anesthesia was achieved with an intramuscular injection of xylazine (5 mg/kg) and ketamine (5 mg/kg), followed by an additional intravenous administration of 5 mg xylazine. A midline incision was made in the ventral area of the neck after infiltration with 1% xylocaine, and the right common carotid artery was exposed and used for arterial access in all animals. After heparin administration (300 U/kg), a 4F intra-arterial sheath was placed in the descending thoracic aorta, slightly beyond the area of interest, over a 0.028-in. tight J-wire. The stent was loaded into the sheath just before deployment in the contracted configuration, and pushed to the distal end of the sheath by an inner catheter. Finally, the sheath was withdrawn with the inner catheter being fixed to release the stent and allow it to expand at the proper place. This procedure was repeated to implant another stent at a site just proximal to the first site. The right common carotid artery was ligated, and the neck inci-sion was closed with interrupted sutures. Angiography was performed before and immediately after the stent implantation with contrast medium (Iohexol, Daiichi Pharmaceutical, Tokyo, Japan) to confirm the stent location. No anticoagulant agents were administered except for the initial bolus injection of heparin.
2.4. Animal sacrifice and tissue preparation
micro-scope, and the aorta was divided into three parts, each 4-mm long, before being embedded in paraffin. The paraffin sections were stained with hematoxylin eosin as the routine stain, with Elastica van Gieson as the elastin stain, and immunohistochemistry as described below. The specimen with the other stent was frozen in an OCT compound (Miles Scientific, Naperville, IL) in a cryomold in liquid nitrogen after removal of the stent wires, and used for the immunohistochemical staining for myosin heavy chain (MHC) isoforms, and mRNA in situ hybridization as described below. The adjoining sites proximal and distal to the stent implantation site were also examined in paraffin-embedded and frozen sections.
2.5. Immunohistochemistry on paraffin-embedded sections and frozen sections
Paraffin-embedded sections were immunostained us-ing the immunoperoxidase streptavidin – biotin complex system with nickel chloride (NiCl) color modification as previously described [4]. Monoclonal antibodies against smooth muscle-specifica-actin (1A4, DAKO, Glostrup, Denmark), rabbit macrophages (RAM11, DAKO), proliferating cell nuclear antigen (PCNA: PC10, DAKO), and decorin (6B6, Seikagaku, Tokyo, Japan) were used on the paraffin sections as specific markers for SMCs, macrophages, proliferating cells, and decorin, respectively. Triple immunostaining was per-formed to identify the cell types of proliferating cells with an application of the immunoalkalinephosphatase streptavidin – biotin complex system (DAKO) in combi-nation with the enhanced polymer one-step staining (EPOS) system (DAKO) with and without NiCl color modification. This immunostaining procedure enabled us to discriminate three immunostaining reaction prod-ucts in three different colors as follows; macrophages in red, SMCs in brown, and PCNA-positive nuclei in black.
For the identification of SMC phenotypes, MHC isoforms (SM1, SM2, SMemb) were immunostained on frozen sections as described previously [4]. SM1 is
expressed in both adult and embryonic phenotypes of SMCs, whereas SM2 has been observed only in adults, and SMemb only in embryonic SMCs. Thus SM2 can be used as a marker for the adult phenotype and SMemb is specific for the embryonic phenotype of SMCs. These antibodies were generated and kindly provided by Dr R. Nagai (Gumma University, Japan).
2.6. cDNA cloning and digoxigenin-labeling of cRNA probes
Each cDNA was cloned using the reverse transcrip-tion-polymerase chain reaction (RT-PCR) method. In brief, total cellular RNA was extracted from the ho-mogenized rabbit aorta with an acid guanidine thio-cyanate – phenol – chloroform extraction method using ISOGEN (Nippon Gene, Tokyo, Japan). Single-stranded cDNA was then synthesized with random ninemer oligonucleotide as a primer. One microgram of the total cellular RNA was incubated at 30°C for 10 min and at 42°C for 30 min with AMV reverse tran-scriptase XL (TAKARA Shuzo, Otsu, Japan) and the random ninemer in a total volume of 20 ml. After incubation, the total reaction mixture was diluted with 100 ml of a PCR buffer. Respective antisense and sense primers were designed for the targeted cDNAs; decorin, biglycan, TGF-b, and IL-1b; the nucleotide sequences of the primers are listed in Table 1. Rabbit-specific cDNA of TGF-b and biglycan were not released. Therefore, they were derived from the human sequence entry.
The reaction mixture for PCR contained 100 pmol each of the sense and antisense primers, and the reac-tion was started by adding 2.5 U of Taq DNA poly-merase (TAKARA Shuzo). The conditions for PCR were 1 min at 95°C, 1 min at 55°C, and 1.5 min at 72°C for 35 cycles. Each PCR product was digested with two proper restriction enzymes (Table 1) and ligated into a plasmid pBluescript SKII+(TOYOBO, Osaka, Japan). The obtained sequences of decorin and IL1-b cDNAs were identical to published sequences. TGF-b and biglycan cDNAs were sequenced and we found
ho-Table 1
Strategy of cDNA cloning by RT-PCRa and subcloning into plasmids
Fragment size (bp)
IL-1b GGTCCCMTTACATGMGA Aat-I/Eco-RI Sma-I/Eco-RI 550
AGAG GC
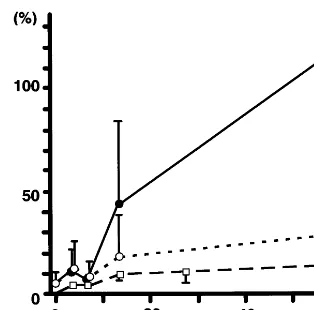
Fig. 1. Chronological changes in intimal thickening expressed as the intimal index (intimal area/medial area) after stent implantation. The stent-implanted site of control animals (); the stent-implanted site of the atherosclerotic animals ( ); the immediately distal portion of the stent-implanted site of atherosclerotic animals ().
2.8. Measurement of the intimal area
The measurement of the intimal area was performed on the middle part of the three paraffin sections taken from the aorta with a stent, because the stent wires aligned in almost the same distance in these sections. The sections of the aorta just distal to the stent implan-tation sites were also measured as an internal control of primary atherosclerotic lesions. Intimal and medial ar-eas were mar-easured in each section stained with Elastica van Gieson stain using a computerized image-analysis system (Image Command 5098, Olympus, Tokyo, Japan). The area of the stent wires was excluded from the measurement. In the atherosclerotic group, we mea-sured the intimal areas including both newly-formed intima after stenting, and the primary atherosclerotic lesion that had been formed prior to stenting, since their boundaries were undelineable.
3. Results
3.1. Chronological changes of fibrofatty lesion formation without and after stent implantation
3.1.1. General findings
After the rabbits had been fed the cholesterol diet for 3 months, their serum cholesterol levels rose from 1.24090.36 – 28.62912.29 mmol/l, while triglyceride levels showed no significant changes (0.57709227 – 0.24390.236 mmol/l). On gross examination, the tho-racic aortas of the cholesterol-fed animals sacrificed without stenting presented extensive atherosclerotic sions on the luminal surface. These atherosclerotic le-sions were considered to be an advanced stage of fatty streaks which are called ‘fibrofatty lesions’ [18].
The process of neointima formation after stent im-plantation in the aortas of the atherosclerotic group was compared with that of the control animals which is described in our previous paper [5]. In the control animals, the intimal index increased gradually until day 14, and no further increase was seen thereafter, whereas in the atherosclerotic group, the intima expanded markedly and became circumferential, covering all of the luminal surface of the aorta by day 14, continuing to thicken up to day 56. The intimal areas of both groups were measured and expressed as the intimal index, and the chronological changes of both groups are compared in Fig. 1. The intimal index at the stent-implanted site was much greater than that of the internal controls measured at the immediately distal portion of the stent-implanted site at each point, and the differences became greater as time elapsed.
Cell type-specific immunohistochemistry revealed that fibrofatty lesions without stenting were made up of an intimal accumulation of lipid-laden macrophages mologies of 94.4 and 89.1% compared to the human
sequences, respectively.
Each plasmid was amplified in Escherichia coli and obtained using CsCl ultracentrifuge methods, then lin-earized with restriction enzymes. Digoxigenin-labeled antisense and sense RNA probes were generated by T3 or T7 RNA polymerase, respectively.
2.7. Messenger RNA in situ hybridization
Messenger RNA in situ hybridization was performed on the serial 3 – 4-mm thick frozen sections of stent implanted tissues essentially as described by Hirota et al. [17]. In brief, the sections were fixed with phosphate-buffered 4% paraformaldehyde for 10 min, acidified with 0.2 M HCl for 10 min, and acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine, pH 8.0, for 10 min. They were air-dried after dehydration with a graded series of ethanol, and hybridized with both antisense and sense probes at 50°C for 16 h. Hybridized digoxigenin-labeled probes were detected using a nu-cleic acid detection kit (Boehringer Mannheim, Mannheim, Germany). After the color reaction, the slides were rinsed with 10 mM Tris – HCl, pH 8.0, 1
mM EDTA, fixed with phosphate-buffered 4%
positive for RAM11, and intermingled with a -actin-positive SMCs. In the lesions, macrophages were domi-nant (Fig. 2A). By day 14 following the stent implantation in the atherosclerotic group, the stent wire was covered by a fibrocellular layer containing dense a-actin-positive SMCs, and the layer thickened further after day 56. A large number of RAM-11-positive macrophages accumulated adjacent to the stent wires, and some of them were scattered in the layer above the fibrocellular tissue composed of SMCs on days 14 and 56 (Fig. 2B).
3.1.2. Immunohistological findings
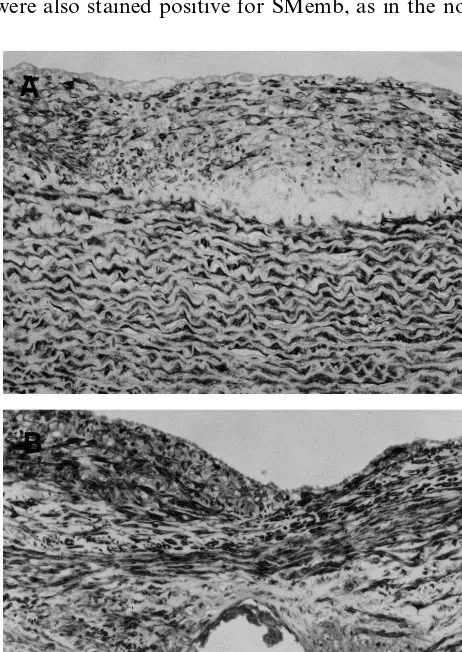
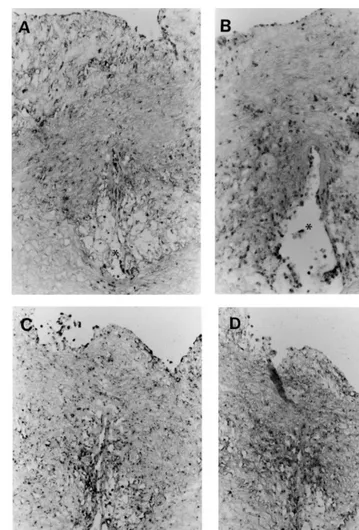
The immunostaining for MHC isoforms in the frozen sections of thoracic aorta without stent implantation showed that medial SMCs were stained positive for SM1 and SM2, and negative for SMemb except for weak staining in the superficial media, while the intimal SMCs were SMemb-positive and SM2-negative, indi-cating that embryonic phenotype SMCs predominate in the fibrofatty lesions (Fig. 3A and B). Endothelial cells were also stained positive for SMemb, as in the normal
aorta [4], whereas, in the atherosclerotic thoracic aorta 56 days after implantation, the intimal SMCs remained SMemb-positive and SM2-negative throughout the ex-perimental period (Fig. 3C and D).
3.2. Chronological changes in extracellular matrix —
immunohistochemistry and mRNA in situ hybridization
3.2.1. Control group without stent implantation
By immunohistochemistry, the media were not stained for decorin, while the adventitia were strongly stained. With in situ hybridization, neither biglycan nor decorin mRNAs were detected in the intima or media of the control animals. Detection of mRNA signals in the adventitia was not possible because of a nonspecific reaction (data not shown).
3.2.2. Control group after stent implantation
By immunohistochemistry, decorin was not stained in the neointima or media throughout the experimental period; it showed strong and constant staining only in the adventitia (Fig. 4).
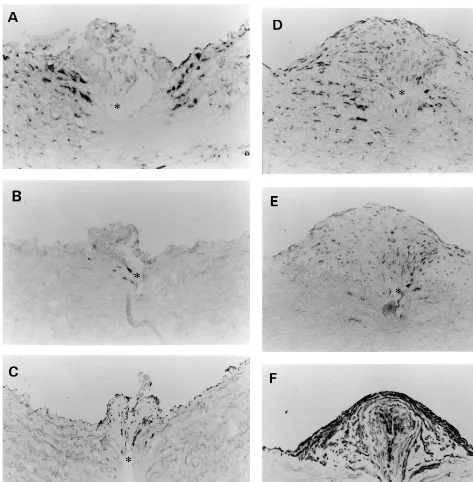
In an mRNA in situ hybridization study, biglycan and TGF-b mRNA were found to be maximally ex-pressed in the media around the sites injured by the stent wires on day 7 (Fig. 5A and B), whereas they were expressed slightly on day 7 and maximally on day 14 in the fibrocellular neointima (Fig. 5D and E). These expressions were transient and had almost disappeared on day 56 (Fig. 5G and H). These mRNA expressions were closely associated with the distribution of SMemb-positive embryonic type SMCs (Fig. 5C, F and I).[4] The gene expressions of decorin and IL-1b were unde-tectable in the intima and the media throughout the experimental period.
3.2.3. Atherosclerotic group without stent implantation
In the atherosclerotic group without stent implanta-tion, decorin immunostaining was faintly positive in the intima, in addition to strong staining in the adventitia, as observed in the control animals. With in situ hy-bridization, however, biglycan and decorin mRNAs were undetectable in the intima and media (data not shown).
3.2.4. Atherosclerotic group after stent implantation
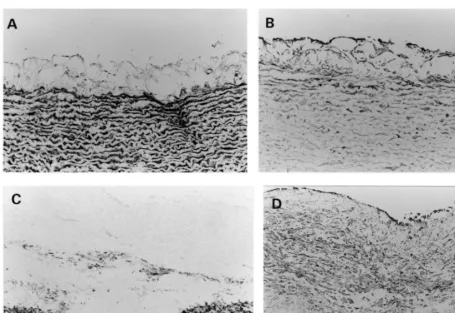
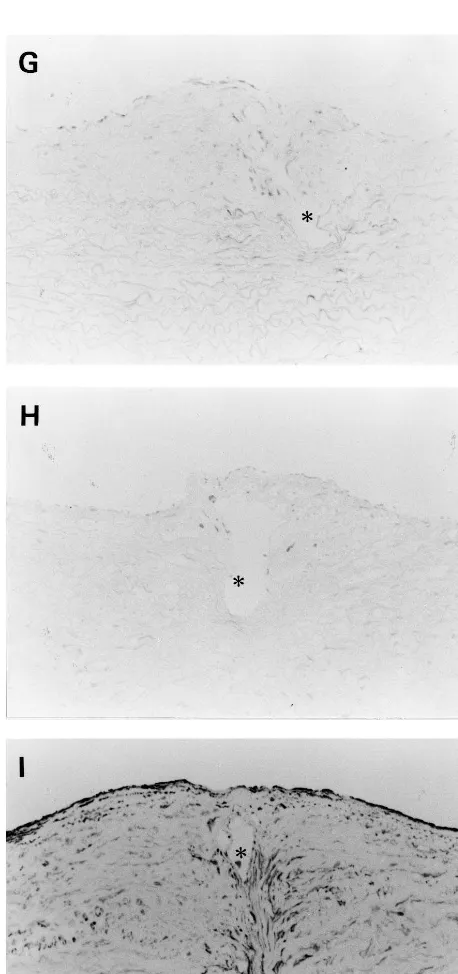
As described above, stent implantation induced accel-erated and extensive neointima formation. Decorin was not recognized in the newly-formed intima until day 14, but it was deposited in such a manner that it concealed the macrophages that accumulated around the stent wire on day 56 (Fig. 6A and B).
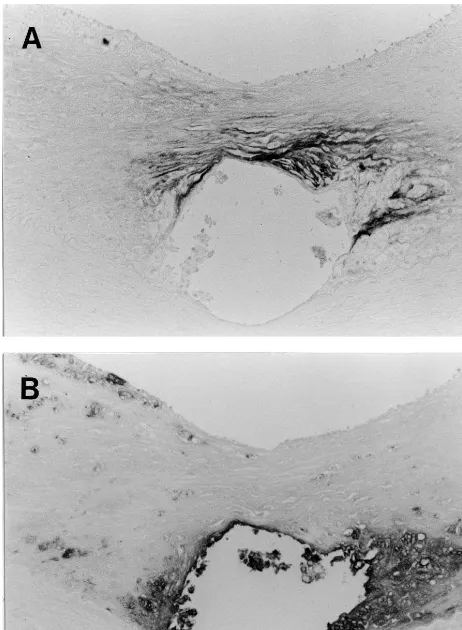
The in situ hybridization showed that both biglycan and TGF-b mRNAs were expressed in the cells around the injured area of media on day 4, and newly formed intima around the stent wire was seen on day 7 (Fig. 7A
Fig. 3. Immunohistochemical stainings for the myosin heavy chain isoforms SM2 (A and C) and SMemb (B and D) in frozen sections of thoracic aortas. Aortic tissues were dissected from animals sacrificed without stent implantation (A and B) and on day 56 after stent implantation (C and D). Bar in panel B, 20mm, and applies to panels A and B. Bar in panel D, 40mm, and applies to panels C and D. Asterisks in C and D indicate vacuities due to stent wires.
and B) similar to normal aorta. The expression of the IL-1b gene was also detected in the neointima around the stent wire on day 7 (Fig. 7C) unlike the result in the control group. Biglycan and TGF-bmRNA expressions continued to be widely present in the neointima throughout the experimental period (Fig. 8A and B). The IL-1bexpression was detected strongly around the stent wire, where macrophages were observed (Fig. 8C). Decorin mRNA was not detected until days 7 and 14 (data not shown). On day 56, its expression was ob-served in SMCs which surrounded the macrophages accumulating near the stent wires (Fig. 8D).
4. Discussion
The present study aimed to extend our previous study in normal rabbit aortas to investigate the effects of stent implantation on atherosclerotic aortas, and demonstrated three major observations about the changes in ECM formation after stent implantation. (1) The transcription of TGF-band biglycan was activated in correspondence with the chronology and localization of embryonic-type vascular SMCs in normal aortas,
which initially appeared in the media around the area injured by stenting and subsequently in the formed neointima, and disappeared within 56 days after the stent implantation. (2) In atherosclerotic aortas, the transcriptional activity of TGF-b and biglycan was sustained even 56 days after the operation, which was in accord with the prolonged expression of embryonic-type vascular SMCs. (3) Decorin, which does not exist
Fig. 5. Biglycan (A, D and G) and TGF-b(B, E and H) mRNA in situ hybridization in the control group compared with SMemb immunostaining (C, F and I). Digoxigenin-labeled antisense cRNA probes were hybridized on frozen tissues dissected from animals sacrificed on day 7 (A – C), 14 (D – F), and 56 (G – I). Bar, 20mm, and applies to all panels. Asterisks in all panels indicate vacuities due to stent wires.
in normal aortas, nor in the neointima formed on normal vessels, appeared in the atherosclerotic aortas in the confined area of vascular SMCs surrounding the macrophages around the stent wire.
In our previous study dealing with stenting in normal rabbit aortas, we were most intrigued by the phenotypic modulations observed both in medial and neointimal SMCs by stent implantation: the medial cells around the site injured by the struts of the stent wire were
experimental period, which is in contrast to the tran-sient appearance of embryonic-type SMCs in the nor-mal aorta after stent implantation. Nagai et al. reported the appearance of embryonic phenotype SMCs in both mechanically injured and high choles-terol-fed rabbit aortas [19] and in human coronary arteries [20]. Our studies and these reports suggest that the strong response to arterial injury in atherosclerotic lesions may be related to the pre-exis-tence of embryonic type SMCs, possessing the poten-tial for producing and secreting various kinds of ECM.
Both decorin and biglycan have a homologous 45 kD core protein and contain chondroitin sulfate or dermatan sulfate chain; decorin has one chain and biglycan has two chains. Decorin and biglycan bind to TGF-b, the most potent inducer of ECM synthe-sis. Decorin, in particular, neutralizes the effect of TGF-b activities in vitro and in vivo [14,15]. TGF-b has various effects on endothelial cells and SMCs, and thus it is thought to be involved in vascular re-modeling. TGF-b1 expression is increased in human
restenotic tissue in primary atheromatous and
nonatheromatous tissues [21]. Majesky et al. reported that TGF-b1 mRNA expression was upregulated in the neointima formed by a balloon injury in a rat carotid artery [22]. In addition, Nabel et al. demon-strated that the transfection of the TGF-b1 gene into arteries induced the production of collagen and inti-mal hyperplasia [23]. In light of these findings, it is possible that TGF-b is one of the inducers of intimal hyperplasia in atherosclerosis and restenosis after vas-cular intervention. It was thus suggested that decorin is a component of a feedback system neutralizing the biological activity of TGF-b [15].
Several animal studies have investigated the proteo-glycan accumulation in neointima induced by vascular injury [24,25]. Nikkari et al. reported the chronology of the gene expression of a proteoglycan subtype after balloon injury [26]. However, these reports provided little information about the spatial correlation of pro-teoglycan distribution and cellular components. In ad-dition, the distribution of decorin has not been reported. Riessen et al. described the distribution of decorin and biglycan in atherosclerotic and restenotic human coronary arteries [11]. They demonstrated that atherosclerotic lesions were strongly stained for decorin and biglycan, but only biglycan was seen in fibroproliferative tissue in human coronary arteries. Lin et al. reported that a small amount of biglycan was only seen in the intima and was absent in the media and adventitia in human atherosclerotic arter-ies; decorin was detected at high levels in the intima and adventitia [27].
In the present study, we used immunohistochemical and in situ hybridization methods to investigate the gene expressions of biglycan and decorin in compari-son with those of TGF-b and IL-1b in stent-im-planted rabbit aortas. We observed differences in the distribution of biglycan and decorin despite their similar structures; the biglycan gene was expressed mainly in proliferating SMCs and decreased after SMC proliferation ceased. Decorin accumulation in the neointima was found in the late stage, when the accumulation of macrophages occurred around the stent wire, and the distribution of decorin and macrophages were adjacent to each other. This
result suggests that the regulation of
Fig. 6. Immunohistochemical staining for decorin (A), and macrophages (B) on day 56 after stent implantation in the atheroscle-rotic group. Bar in panel B, 30mm, and applies to all panels.
because the cholesterol-loaded rabbit aorta presented circumferential intimal thickening similar to that of adult humans. Using this model, we found that atherosclerotic vessels presented a strong response to injury, probably because of the presence of embryonic-type SMCs in the pre-existing atherosclerotic lesions. We also found that there were differences between biglycan and decorin in their spatial and temporal distributions and relationships to the cytokines TGF-b and IL-1b, and phenotypical expression of SMCs.
Fig. 7. In situ hybridization of sections from animals in the atherosclerotic group sacrificed on day 7. Probes for biglycan (A), TGF-b(B), and IL-1b(C) were used. Bar, 20mm, and applies to all panels. Asterisks in all panels indicate vacuities due to stent wires.
decorin expression is associated with macrophages. Sev-eral animal studies have investigated the regulation of the production of these proteoglycans in vitro. Edwards et al. reported that macrophage-conditioned media se-lectively stimulated decorin production by arterial SMCs, and this effect was abrogated by an antibody to IL-1 [28]. IL-1 was reported to upregulate decorin gene expression by fibroblasts and SMCs [29,30]. TGF-b increases mainly the production of biglycan, not decorin, by fibroblasts [31 – 34] and arterial SMCs [35]. In the present study, we observed that IL-1b mRNA expressed mainly on macrophages accumulating around the stent wire, and decorin mRNA was detected on the SMCs adjacent to the macrophages accumulating around the stent wire. These results are in close agree-ment with those described in several reports stating that IL-1bupregulates decorin gene expression in vitro [28 – 30]. Thus, macrophages accumulating in such lesions may have a pivotal role in the regulation of ECM accumulation during neointima formation, through the production of cytokines and phenotypic modulation of SMCs.
Fig. 8. In situ hybridization of the sections from animals in the atherosclerotic group sacrificed on day 56. Probes for biglycan (A), TGF-b(B), IL-1b(C) and decorin (D) were used. Bar, 30mm, and applies to all panels. Asterisks in all panels indicate vacuities due to stent wires.
from the Ministry of Health and Welfare of Japan, and the Takeda Medical Research Foundation. We thank Dr Ryozo Nagai for generously donating antibodies for MHC isoforms, and Gunpei Kikuchi and the late Kimio Yoshizawa for producing the stents used in this experi-ment.
Acknowledgements
References
[1] Schatz RA, Baim DS, Leon M, Ellis SG, Goldberg S, Hirshfeld JW, Cleman MW, Cabin HS, Walker C, Stagg J, et al. Clinical experience with the Palmaz – Schatz coronary stent: initial results of multicenter study. Circulation 1991;83:148 – 61.
[2] Serruys PW, Jaegere P, Kiemeneij F, Maacaya C, Rutsch W, Heyndrckx G, Emanuelsson H, Marco J, Legrand V, Materne, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery dis-ease. N Engl J Med 1994;441:489 – 95.
[3] Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn L, Detre K, Veltri L, Ricci D, Nobuyoshi M, Cleman M, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med 1994;331:496 – 501.
[4] Bai H, Masuda J, Sawa Y, Nakano S, Shirakura R, Shimazaki Y, Ogata J, Matsuda H. Neointima formation after vascular stent implantation: spatial and chronological distribution of smooth muscle cell proliferation and phenotypic modulation. Arterioscler Thromb 1994;14:1846 – 53.
[5] Stary HC, Blankenhorn DH, Chandler AB, Glagpv S, Insull W, Richardson M, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wag-ner WD, Wissler RW. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the committee on vascular lesions of the council on arteriosclero-sis, American Heart Association. Arterioscler Thromb 1992;12:120 – 34.
[6] Madri JA, Kocher O, Merwin JR, Bell L, Tucker A, Basson CT. Interactions of vascular cells with transforming growth factors-b. Ann New York Acad Sci 1990;593:243 – 58.
[7] Thyberg J, Hedin U, Sjo¨und M, Palmberg L, Bottger BA. Regulation of differentiated properties and proliferation of arte-rial smooth muscle cells. Arteriosclerosis 1990;10:966 – 90. [8] Ruoslahti E, Yamaguchi Y. Proteoglycans as modulators of
growth factor activities. Cell 1991;64:867 – 9.
[9] Krull NB, Zimmermann T, Gressner AM. Spatial and temporal patterns of gene expression for the proteoglycans biglycan and decorin and transforming growth factor-b1 revealed by in situ hybridization during experimentally induced liver fibrosis in the rat. Hepatology 1993;18:581 – 9.
[10] Westergren-Thorsson G, Hernna¨s J, Sa¨rnstrand B, Oldberg A,, Heinega˚rd D, Malmstro¨m A. Altered expression of small proteo-glycans, collagen, and transforming growth factor-b1 in develop-ing bleomycin-induced pulmonary fibrosis in rats. J Clin Invest 1993;92:632 – 7.
[11] Riessen R, Isner JM, Blessing E, Loushin C, Nikol S, Wight TN. Regional differences in the distribution of the proteoglycans biglycan and decorin in the extracellular matrix of atheroscle-rotic and restenotic human coronary arteries. Am J Pathol 1994;144:962 – 74.
[12] Sporn MB, Roberts AB. Transforming growth factorb: multiple actions and potential clinical applications. J Am Med Assoc 1989;262:938 – 48.
[13] Roberts AB, Heine UI, Flanders KC, Sporn MB. Transforming growth factor-b: major role in regulation of extracellular matrix. Ann New York Acad Sci 1990;580:225 – 32.
[14] Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-bby the proteoglycan decorin. Na-ture 1990;346:281 – 4.
[15] Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. Natural inhibitor of trans-forming growth factor-bprotects against scarring in experimen-tal kidney disease. Nature 1992;360:361 – 4.
[16] Edwards IJ, Xu H, Wright MJ, Wagner WD. Intrerleukin-1 upregulates decorin production by arterial smooth muscle cells.
Arterioscler Thromb 1994;14:1032 – 9.
[17] Hirota S, Ito A, Mori E, Wanaka A, Tohyama M, Kitamura Y, Nomura S. Localization of mRNA for c-kit receptor and its ligand in the brain of adult rats: an analysis using in situ hybridization. Mol Brain Res 1992;15:47 – 54.
[18] Masuda J, Ross R. Atherogenesis during low level hypercholes-terolemia in the nonhuman primate. I. Fatty streak formation. Arteriosclerosis 1990;10:164 – 77.
[19] Kuro-o M, Nagai R, Nakahara K, Katoh H, Tsai RC, Tsuchi-mori H, Yazaki Y, Ohkubo A, Takaku F. cDNA cloning of a myosin heavy chain isoform in embryonic smooth muscle and its expression during vascular development and in arteriosclerosis. J Biol Chem 1991;266:3768 – 73.
[20] Aikawa M, Sivam PN, Kuro-o M, Kimura K, Nakahara K, Takeuchi S, Ueda M, Yamaguchi H, Yazaki Y, Periasamy M, Nagai R. Human smooth muscle myosin heavy chain isoforms as molecular markers for vascular development and atheroscle-rosis. Circ Res 1993;73:1000 – 12.
[21] Nikol S, Isner JM, Pickering JG, Kearney M, Lecierc G, Weir L. Expression of transforming growth factor-b1 is increased in human vascular restenosis lesions. J Clin Invest 1992;90:1582 – 92.
[22] Majesky MW, Lindner V, Twardzik DR, Schwartz SM, Reidy MA. Production of transforming growth factorb1 during repair of arterial injury. J Clin Invest 1991;88:904 – 10.
[23] Nabel EG, Shum L, Pompili VJ, Yang Z, San H, Shu HB, Liptay S, Gold L, Gordon D, Derynck R, Nabel GJ. Direct transfer of transforming growth factor b1 gene into arteries stimulates fibrocellular hyperplasia. Proc Natl Acad Sci USA 1993;90:10759 – 63.
[24] Wight TN, Curwen KD, Litrenta MM, Alonso DR, Minick CR. Effect of endothelium on glycosaminoglycan accumulation in injured rabbit aorta. Am J Pathol 1983;113:156 – 64.
[25] Merrilees MJ, Scott LJ. Effects of endothelial removal and regeneration on smooth muscle glycosaminoglycan synthesis and growth in rat carotid artery in organ culture. Lab Invest 1985;52:409 – 19.
[26] Nikkari ST, Ja¨rvela¨inen HT, Wight TN, Ferguson M, Clowes AW. Smooth muscle cell expression of extracellular matrix genes after arterial injury. Am J Pathol 1994;144:1348 – 56.
[27] Lin H, Wilson JE, Roberts CR, Horley KJ, Winters GL, Costanzo MR, McManus BM. Biglycan, decorin and versican protein expression patterns in coronary arteriopathy of human cardiac allografts: distinctness as compared with native atherosclerosis. J Heart Lung Trans 1996;15:1233 – 47.
[28] Edwards IJ, Wagner WD, Owens RT. Macrophage secretory products selectively stimulate dermatan sulfate proteoglycan pro-duction in cultured arterial smooth muscle cells. Am J Pathol 1990;136:609 – 21.
[29] Heino J, Ka¨ha¨ri VM, Mauviel A, Krusius T. Human recombi-nant interleukin-1 regulates cellular mRNA levels of dermatan sulfate proteoglycan core protein. Biochem J 1988;252:309 – 12. [30] Pulkkinen L, Kainulainen K, Krusius T, Mkinen P, Schollin J,
Gustavsson KH, Peltonen. Deficient expression of the gene coding for decorin in a lethal form of Marfan syndrome. J Biol Chem 1990;265:17780 – 5.
[31] Ka¨ha¨ri VM, Larjava H, Uitto J. Differential regulation of extracellular matrix proteoglycan (PG) gene expression: trans-forming growth factorb1 up-regulates biglycan (PGI) and versi-can (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J Biol Chem 1991;266:10608 – 15.
[33] Westergren-Thorsson G, Schmidtchen A, Sa¨rnstrand B, Fransson L, Malmstro¨m A. Transforming growth factor-b in-duces selective increase of proteoglycan production and changes in copolymeric structure of dermatan sulphate in human skin fibroblasts. Eur J Biochem 1992;205:277 – 86.
[34] Mauviel A, Santra M, Chen YQ, Uitto J, Iozzo RV. Transcrip-tional regulation of decorin gene expression. Induction by
quies-cence and repression by tumor necrosis factor-alpha. J Biol Chem 1995;20:11692 – 700.