Combining yeasts or a bacterial biocontrol agent and heat
treatment to reduce postharvest decay of ‘Gala’ apples
Britta Leverentz
a,*, Wojciech J. Janisiewicz
b, William S. Conway
a,
Robert A. Saftner
a, Yoram Fuchs
c, Carl E. Sams
d, Mary J. Camp
eaU.S.Department of Agriculture,Belts6ille Agricultural Research Center,Agricultural Research Ser6ice, Horticultural Crops Quality Laboratory,Bldg.002,Rm117,BARC-West,10300Baltimore A6enue,Belts6ille,
MD20705-2350,USA
bU.S.Department of Agriculture,Agricultural Research Ser6ice,Appalachian Fruit Research Station,45Wiltshire Road, Kearneys6ille,WV 25430,USA
cDepartment of Posthar6est Science of Fresh Produce,ARO,The Volcani Center,Bet Dagan50250Israel dDepartment of Plant and Soil Sciences,The Uni
6ersity of Tennessee,Knox6ille,TN37901,USA eU.S.Department of Agriculture,Biometrical Consulting Ser
6ice,Belts6ille Agricultural Research Center, Agricultural Research Ser6ice,Belts6ille,MD20705,USA
Received 1 May 2000; accepted 17 July 2000
Abstract
‘Gala’ apples were treated after harvest with heat (38°C for 4 days), and then wound-inoculated with the pathogen Penicillium expansum and the antagonist Pseudomanas syringae, or one of two yeast antagonists, to reduce postharvest decay. After storage for 7 days at 20°C or 3 months at 1°C, the least decay was found on fruit where wounds had been allowed to cure by heat treatment (38°C) or cold storage (1°C) for 4 days before inoculation with the pathogen. Addition of any of the antagonists before or after heat treatment further reduced the number and size of the lesions. The highest lesion incidence occurred on apples wounded after heat treatment followed by inoculation with the pathogen. Addition of the yeast antagonists to these fresh wounds reduced the fruit decay as well. While the heat treatment is phytosanitary in that it significantly reduces the pathogen population on the apple surface, it provides little residual protection. The residual protection from the antagonists adds to the control provided by the heat treatment. Published by Elsevier Science B.V.
Keywords:Biological control; Fresh-cut; Microbes; Competition
www.elsevier.com/locate/postharvbio
1. Introduction
Attempts to find alternatives to chemical con-trol to reduce losses from postharvest decays have been ongoing for some time. Many fungi are becoming more resistant to commonly used fungi-* Corresponding author. Tel.: +1-301-5046982; fax: +
1-301-5045107.
E-mail address:[email protected] (B. Leverentz).
cides and there is an increasing demand by con-sumers to reduce chemical residues on produce due to health and environmental concerns. Alter-natives to chemical control, when used alone, are generally less effective than fungicides.
There has been an increasing interest in the use of prestorage heat treatments to control insect pests, prevent fungal decay, and to modify the ripening of commodities (Lurie, 1998). Heat treat-ment provides a non-damaging physical treattreat-ment as a substitute for chemical control. It has shown potential as a method to reduce postharvest decay (Lurie, 1998). Exposing ‘Golden Delicious’ apples to 38°C for 4 days reduced decay caused by
Penicillium expansum Link in fruit inoculated af-ter 6 months of storage at 0°C (Sams et al., 1993). Decay caused by Botrytis cinerea Pers.:Fr. or P.
expansumwas nearly eliminated in stored ‘Golden Delicious’ apples when the fruit were heat-treated at 38°C after inoculation with the fungus (Fallik et al., 1995; Klein et al., 1997).
Another promising alternative to fungicide-based control is biological control of postharvest pathogens (Janisiewicz, 1988; Wilson and Wis-niewski, 1989; Korsten et al., 1994). Decay caused by B. cinerea and P. expansum has been con-trolled on pome fruits by bacterial and yeast antagonists in laboratory and pilot storage tests (Roberts, 1990; Janisiewicz and Marchi, 1992; Janisiewicz et al., 1994; Chand-Goyal and Spotts, 1996). In some cases, commercially acceptable levels of control have been achieved (Roberts, 1990; Janisiewicz and Marchi, 1992). For exam-ple, Pseudomonas syringae van Hall (strain ESC-11) is being used to control postharvest decay on pome fruits caused byB.cinerea,Mucor piriformis
Fischer, andP.expansum(Janisiewicz and Jeffers, 1997; Stack, 1998).
Since the alternatives to chemical control are not as broad spectrum and are often not as effective as fungicides, a combination of alterna-tive methods may be more effecalterna-tive than any one alone. A bacterial isolate related to Pseudomonas glatheiZolg and Ottow gave moderate control of green mold caused by Penicillium digitatum Sacc. on citrus (Huang et al., 1995). The biocontrol by this isolate was significantly improved when the inoculated fruits were incubated at 30°C for 24 h
before being moved to 25°C. The incubation con-ditions prevented the fungal spores from germi-nating, but the bacterial populations increased greatly in the wound sites. In more recent work (Conway et al., 1999), ‘Gala’ apples were heated after harvest (38°C for 4 days), pressure-infiltrated with a 2% solution of CaCl2, or treated with the antagonist P. syringae, alone or in combinations, to reduce postharvest decay caused by P. expan
-sum. After 6 months in storage at 1°C, no decay lesions developed on the fruit that were heated after inoculation with P. expansum. Prior heat treatment alone did not influence lesion size in parallel lots of fruit that were inoculated after 6 months in storage at 1°C. The least decay oc-curred on apples treated with a combination of calcium plus the antagonist, or calcium plus the antagonist and heat.
The alternatives discussed above all have limita-tions. While heat treatment virtually eliminates decay if fruit are inoculated prior to heating, it has little effect when inoculation occurs after heating (Klein et al., 1997). The effect of external factors on biological control is generally greater than on fungicides. For example, as fruit mature, higher concentrations of the antagonist must be used to achieve the same level of control as on immature fruit (unpublished data). Combining heat treatment with an antagonist would provide the sanitary effect of the heat treatment with the protection of the biocontrol agent. In addition, a more heat resistant antagonist would be more effectively established in the wound site, since the populations of the pathogen would be reduced by the heat treatment. In the research described above (Conway et al., 1999), heat treatment after inoculation of the wounded fruit with P.syringae
reduced the population of this antagonist, making it less effective. Control ofP.expansumby theP.
syringae antagonist was greater when applied af-ter heat treatment rather than before.
2. Materials and methods
2.1. Fruit
‘Gala’ apples that had been treated with Retain (aminoethoxyvinylglycine, AVG; Abbott Labora-tories, Abbott Park County, IL) were harvested at the beginning of the climacteric stage from a commercial orchard in Pennsylvania and random-ized on the same day. The physiological charac-teristics one day after harvest were calculated as an average of 6 measurements for the respiration rates and ethylene production, an average of 14 or 15 measurements for firmness, titratable acidity, headspace volatiles and starch values. The respira-tion rate was at 4992 nmol kg−1 s−1 of CO
2 using a CO2 analyzer (CD-3A; Ametek, Pitts-burgh, PA) and ethylene production rate was 6.191.0 pmol kg−1 s−1 using a gas chro-matograph (GC, 5890a Series II; Hewlett Pack-ard, Rockville, MD) equipped with a flame ionization detector (FID). Apple volatiles were separated and identified using the GC and GC/
mass spectrometer (MS) procedure as previously described (Saftner et al., 1998). The sum total of the quality-associated volatiles in the headspace of apple extracts was relatively low at 495918 FID area response units. The firmness of the fruit was 10097 N and was measured using a manually controlled digital penetrometer (EPT-1 with an 11.1-mm-tip; Lake City Technical Products, Kelowna, B.C., Canada) set in the Magness – Tay-lor mode as described previously (Saftner et al., 1998). The titratable acidity as % malic acid equivalents was 0.5090.02 and the starch value based on Cornell University’s 8-point scale was 3.991.1. The application of the treatments began one day after harvest.
2.2. Heat treatment
Wounded and non-wounded fruit were placed on fruit pack trays, five trays per box lined with perforated polyethylene bags. They were heated to 38°C for 4 days in a thermostatically controlled (91°C) walk-in chamber. The relative humidity in the chamber was maintained at \85%. Storage conditions were monitored with a
hygrother-mograph (Belfort Instrument Co., Baltimore). The non-heated fruit were placed at 1°C for the same time period. All fruit were removed from heat or cold storage after 4 days, allowed to equilibrate to room temperature overnight and inoculated according to the different treatments.
2.3. Pathogen
The pathogen Penicillium expansum (MD-8) was an aggressive isolate from our collection and was isolated from a decayed apple in storage. It was maintained on potato – dextrose – agar (PDA) and continued virulence was assured by periodic transfers through apple. The conidial suspension for fruit inoculations was prepared as previously described (Janisiewicz and Marchi, 1992).
2.4. Antagonists
Three antagonists, one previously used Pseu
-domonas syringaestrain (Conway et al., 1999) and two yeasts, were tested for their effectiveness to prevent apple decay by P. expansumfor different lengths of time under different conditions. The antagonists were grown in 50 ml of nutrient yeast – dextrose – broth (NYDB) medium in 250-ml Erlenmeyer flasks on a rotary shaker at 150 rpm and 26°C for 24 h. The cells were harvested by centrifugation at 7000× g for 10 min and resus-pended in sterile distilled water. The concentra-tions of the P. syringaeisolate and the two yeast isolates were adjusted to 85 and 75% transmit-tance respectively with a spectrophotometer. This resulted in a concentration of 107 CFU/ml for
P. syringae and 106 CFU/ml for the two yeast strains. The two yeast antagonists A2 and A3 were originally isolated from apple surfaces and selected for heat resistance at 38°C.
2.5. Fruit inoculation and lesion measurement
into five different sections as follows: (I) wH+
AP, wounding, heating for 4 days, inoculation with P. expansumand the antagonists; (II) w*+
AP, wounding, storage at 1°C for 4 days, inocula-tion with P. expansum and the antagonist; (III) wAP, wounding and immediate inoculation with
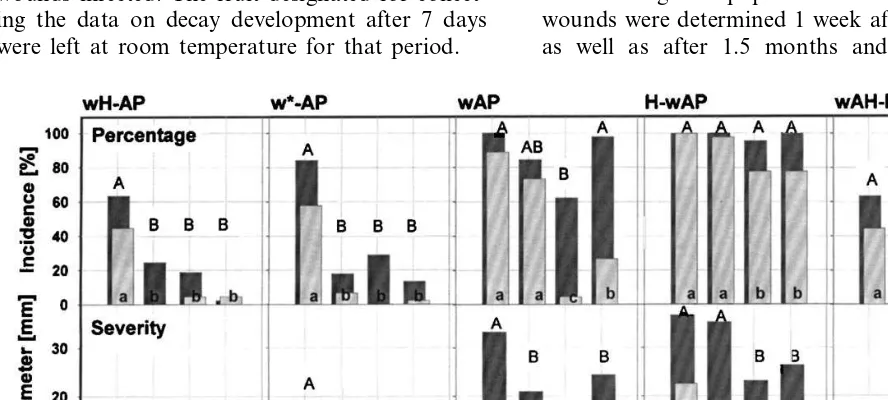
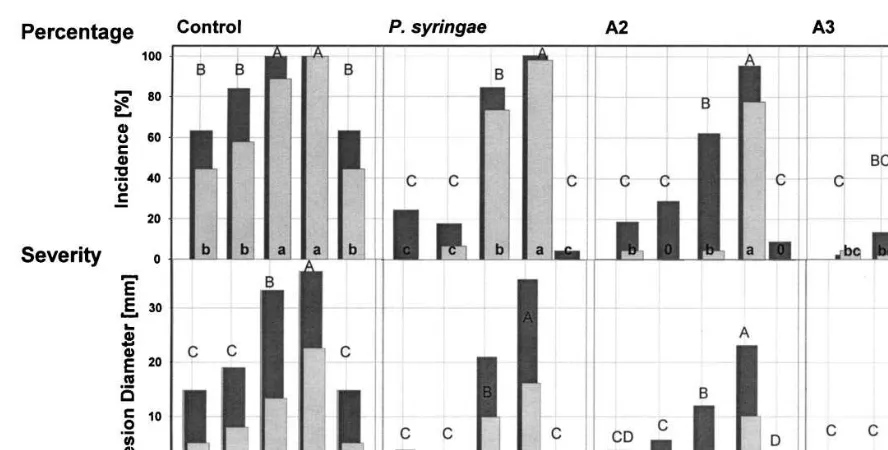
P. expansum and the antagonist; (IV) H-wAP, heating, wounding and immediate inoculation withP.expansumand the antagonist; (V) wAH-P, wounding and antagonist application, heating for 4 days and inoculation withP.expansum and the antagonist. The control treatment for group I was identical to group V and it is listed for reasons of comparison in both groups (Fig. 1 and Fig. 2). Following the inoculation (top and bottom), severity of decay was evaluated by measuring the lesion diameter after 1 week and after 3 months. At the same time, the incidence of decay was determined by the presence or absence of infec-tion at the wound site and reported as percent of wounds infected. The fruit designated for collect-ing the data on decay development after 7 days were left at room temperature for that period.
Each apple was wounded twice on the same side (top and bottom) with a standardized tool that had two 16-penny nails (4 mm diameter) placed 2 cm apart and protruding 3 mm from a wooden block. One wound was 10 mm above the equator and the other was 10 mm below the equator directly under the first wound. The con-trol wounds were inoculated with 25 ml of the conidial suspension of the pathogen (5×104 conidia/ml). The treatments were inoculated with 25 ml of a suspension containing P. expansum
(5×104
conidia/ml; final concentration) and one of the antagonists (106
CFU/ml; final concen-tration). There were 15 fruit per replicate and three replicates per treatment. The fruit were ar-ranged in a randomized block design.
2.6. Antagonist reco6ery
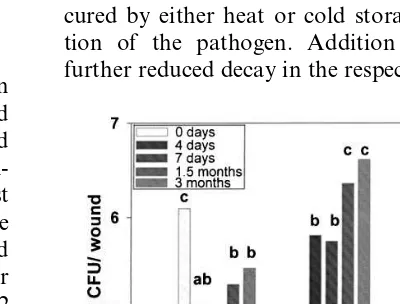
The antagonist populations established in the wounds were determined 1 week after inoculation as well as after 1.5 months and 3 months in
Fig. 2. Lesion incidence (top) and lesion severity (bottom) of wounded apples treated with different combinations of heat treatment, storage duration, time of wounding and inoculation of the antagonists and/orP.expansum. The data are plotted and analyzed by organisms. The lesion incidence (percentage) was analyzed separately for each set and time. Numbers with different letters are different at the experiment-wise significance level 0.05. The lesion severity (diameter) means and means comparisons were analyzed within each set by time. Numbers with different letters are different for the experiment-wise error (0.05). Treatments without lesions are labeled with 0 instead of a letter. The treatments were labeled with the following letters:w, wounding;H, heating for 4 days; w*, storage at 1°C for 4 days after wounding;P,Penicillium expansum;P.s.,Pseudomonas syringae; A2, antagonist 2, yeast; A3, antagonist 3, yeast. Lower case letters indicate statistical differences after 1 week incubation and capital letters indicate differences after 3 months of storage.
storage. The antagonist was recovered from the top wounds of four apples per treatment. The incubation and counting of the colonies was per-formed according to the procedure described pre-viously (Conway et al., 2000).
2.7. Statistical analyses
The absence of decay (no-lesion pattern) was similar, but not identical, for the top and bottom wounds. As there were more treatments where the top lesions developed decay, the top values were chosen as the worst case scenario and used for the statistical analysis.
The percentage of apples with lesions is given in the top part of Fig. 1 and Fig. 2. Comparisons of the treatments were made using Fisher’s exact test in StatXact (Cytel Software Corp., 1996) for sam-ples from two independent binomial populations.
The Sidak adjustment was used so that the exper-iment-wise error rate was 0.05.
The apple top lesion diameter values plotted in the bottom part of Fig. 1 and Fig. 2 were ana-lyzed as a one-factor general linear model using PROC MIXED (SAS Institute Inc., 1997) with treatment as the factor. The four treatments with no lesions on the top wound were not included in the analysis. The assumptions of the general linear model were tested. To correct variance hetero-geneity, the values were natural log plus one (ln(x+1)) transformed and treatments were grouped into similar variance groups for the anal-ysis. Results from the analysis of variance showed that there are significant differences between treat-ments (df=50, F=977.86, P50.0001).
The means were compared (Fig. 1 and Fig. 2) using pair-wise comparisons with Sidak adjusted
comparison category was 0.05. The means are in the original lesion diameter measurement scale.
Data means collected from the recovery of the antagonists were separated using the Waller – Duncan multiple range test. Each treatment was analyzed separately over time.
3. Results
3.1. Effect of treatments on lesion incidence and size
3.1.1. Cured wounds
On wounds cured for 4 days by either heat or cold prior to inoculation, the addition of any of the three antagonists reduced lesion size and inci-dence compared to the control which was inocu-lated only with P. expansum (Fig. 1). This was true regardless of whether the antagonists had been added before or after heat treatment and wound curing (Fig. 1). However, addition of the antagonists before heat treatment further reduced the size of the lesions compared to the addition after heat treatment, while the incidence of decay was the same (Fig. 2).
3.1.2. Fresh wounds
Incidence and size of lesions were greater when fresh wounds were inoculated compared to cured wounds (Fig. 1 and Fig. 2). Heat treatment did not affect the incidence of the decay but it in-creased lesion size (Fig. 1 and Fig. 2). The least reduction of decay severity and incidence by the antagonists was on heat-treated apples (Fig. 1 and Fig. 2). Similar results occurred the previous year (data unpublished). Addition of antagonists A2 and A3 to fresh wounds consistently reduced the lesion incidence and severity after 7 days of incu-bation and the lesion severity after 3 months (Fig. 1 and Fig. 2). After 7 days, the lesion incidence and severity in these treatments were not different from that of cured wounds (Fig. 1 and Fig. 2). After 3 months storage, fewer and smaller lesions occurred on non-heated than on heat-treated ap-ples, when fresh wounds were protected with the
P. syringae and A2 antagonists, while there was no difference on A3-treated fruit (Fig. 1 and Fig.
2). Antagonists did not reduce the severity of decay on heat-treated apples evaluated after 3 months in storage (Fig. 1).
All three antagonists reduced lesion severity after 7 days of incubation in all treatments except for P. syringae on non-heated apples inoculated immediately after wounding. In this instance, the average lesion size was not reduced (Fig. 2).
3.2. Antagonist reco6ery
The populations of both yeast antagonists in apple wounds increased during heat treatment and continued to increase during cold storage. After 3 months in storage the population in-creases were almost two logs for both yeasts (Fig. 3). Heat treatment decreased populations of P.
syringaeuntil 3 days after heat treatment, but the populations appeared to have recovered after 1.5 months in storage (Fig. 3).
4. Discussion
The least decay developed on wounds that were cured by either heat or cold storage before addi-tion of the pathogen. Addiaddi-tion of antagonists further reduced decay in the respective treatments.
The protection established by the wound curing process at 38°C was similar to that at 1°C after 4 days when comparing lesion diameters. Allowing the wounds to cure after addition of the antago-nist, but before inoculation of the pathogen, fur-ther reduced the decay. This sequence may allow the antagonist to establish itself better in the wounds, resulting in greater protection. Previous work indicated that almost no decay developed on fruit that were wounded before heating, but inoc-ulated at the end of the heat treatment (Lurie et al., 1998). Similarly, in our research, lesion sizes around wounds cured at 1°C for 4 days before inoculation were comparable to lesion sizes around wounds cured at 38°C for 4 days. A previous study found that wounds in harvested mature, preclimacteric ‘Golden Delicious’ and ‘Granny Smith’ apples were resistant to infection by B. cinerea and P. expansum within 4 days at 5°C following wounding (Lakshiminarayana et al., 1987). Factors other than wound healing may have been involved in this resistance. Enhanced production of phenolic substances near wounded tissues is known to be a common response to wounding. These phenolics may be incorporated into lignified cell walls during wound healing or may be directly toxic to the pathogen (Lakshimi-narayana et al., 1987). On ‘d’Anjou’ pears stored for 4 days at −1°C after wounding, compounds such as callose, gums, pectic substances, starch, suberin and phenolics increased in the wound tissue (Spotts and Chen, 1987). The accumulation of these compounds appears to precede or coin-cide with the increase in resistance to decay. The same wound healing response is accelerated and occurs after 1 day, when the fruit are heated at 28°C (Spotts et al., 1998).
Heat treatment is effective in sanitizing the fruit and enhancing the wound curing process (Lurie et al., 1998; Spotts et al., 1998). It has the added benefit of improving fruit color but does not lead to softening, since it inhibits the synthesis of cell wall hydrolytic enzymes in the apple fruit (Lurie et al., 1998).
In our current research, heat treatment prior to wounding and inoculation resulted in higher inci-dence and severity of decay. This may be due to the heat-related destruction of enzymes that are
otherwise used for defense against pathogen inva-sion. Previous work has shown that there is only a small residual effect of heat treatment on decay when ‘Gala’ fruit were inoculated with P. expan
-sum3 and 6 months after storage (Conway et al., 1999). Heat had very little effect on ‘Golden Delicious’ fruit subsequently inoculated with B.
cinerea (Klein et al., 1997). Prestorage heat treat-ment of pears prior to wounding and inoculation with various postharvest pathogens, including B.
cinerea and P. expansum, also did not control decay (Spotts and Chen, 1987).
The addition of the antagonists reduced the lesion size, although in inoculations of fresh wounds, the two yeasts reduced decay more effec-tively than P. syringae after 7 days and after 3 months of storage. Added antagonists may provide a beneficial effect on wounded fruit in addition to the wound curing process, since the best overall decay control resulted from the com-bination of wound curing and antagonist applica-tion. An increase in populations of both yeast antagonists in apple wounds during the heat treat-ment, and excellent decay control by these antag-onists indicates that antagantag-onists selected for resistance to 38°C heat can be applied prior to heat treatment. This gives greater flexibility as to the timing of the antagonist application. It can also be advantageous for decay control because it allows heat treatment to kill pathogens that were already on the fruit. This allows the establishment of the antagonists in wounds to protect against future infection after heat treatment.
Acknowledgements
We thank Michele Auldridge, George A. Brown, and Michael Ball for valuable technical assistance.
References
Conway, W.S., Janisiewicz, W.J., Klein, J.D., Sams, C.E., 1999. Strategy for combining heat treatment, calcium infiltration, and biocontrol to reduce postharvest decay of ‘Gala’ apples. HortScience 34, 700 – 704.
Conway, W.S., Leverentz, B., Saftner, R.A., Janisiewicz, W.J., Sams, C.E., Leblanc, E., 2000. Survival and growth of Listeria monocytogenes on fresh-cut apple slices and its interaction with Glomerella cingulataand Penicillium ex -pansum. Plant Dis. 84, 177 – 181.
Cytel Software Corp., 1996.StatXact3for Windows. pp. 795. Cambridge, MA: Cytel Software Corp.
Fallik, E., Grinberg, S., Gambourg, M., Lurie, S., 1995. Prestorage heat treatment reduces pathogenicity ofPenicil -lium expansumin apple fruit. Plant Path. 45, 92 – 97. Huang, Y., Deverall, B.J., Morris, S.C., 1995. Postharvest
control of green mould of oranges by a strain of Psue-domonas glatheiand enhancement of its biocontrol by heat treatment. Postharvest Biol. Technol. 5, 129 – 137. Janisiewicz, J., 1988. Biological control of diseases of fruits.
In: Mukerji, K.G., Garg, K.L. (Eds.), Biocontrol of Plant Diseases, vol. 2. CRC Press, Boca Raton, FL, pp. 153 – 165.
Janisiewicz, W.J., Jeffers, S.N., 1997. Efficacy of commercial formulation of two biofungicides for control of blue and gray mold of apples in cold storage. Crop Protection 16, 629 – 633.
Janisiewicz, W.J., Marchi, A., 1992. Control of storage rots on various pear cultivars with a saprophytic strain of Pseu-domonas syringae. Plant Dis. 76, 555 – 560.
Janisiewicz, W.J., Peterson, D.L., Bors, B., 1994. Control of storage decay of apples withSporobolomyces roseus. Plant Dis. 78, 466 – 470.
Klein, J.D., Conway, W.S., Whitaker, B.D., Sams, C.E., 1997. Botrytis cinereadecay in apples is inhibited by postharvest heat and calcium treatments. J. Amer. Soc. Hort. Sci. 122, 91 – 94.
Korsten, L., De Villiers, E.E., Sehner, F.C., Kotze, J.M., 1994. A review of biological control of postharvest diseases of subtropical fruits. In: B.R. Champ, E. Highly and G. L. Johnson (Eds.), 172 – 185. ACIAR Proc. 50. Postharvest
Handling of Tropical Fruits.
Lakshiminarayana, S., Sommer, N.F., Polito, V., Fortlage, R.J., 1987. Development of resistance to infection by Botrytis cinerea and Penicillium expansum in wounds of mature apple fruits. Phytopathology 77, 1674 – 1678. Lurie, S., 1998. Postharvest heat treatments. Postharvest Biol.
Technol. 14, 257 – 269.
Lurie, S., Fallik, E., Klein, J.D., Kozar, E., Kovacs, K., 1998. Postharvest heat treatment of apples to control San Jose scale (Quadraspidotiotus perniciosus Comstock) and blue mold (Penicillium expansumLink) and maintain fruit firm-ness. J. Amer. Soc. Hort. Sci. 123, 110 – 114.
Roberts, R.G., 1990. Postharvest biological control of gray mold of apple byCryptococcus laurentii. Phytopathology 80, 526 – 530.
Saftner, R.A., Conway, W.C., Sams, C.E., 1998. Effects of postharvest calcium and fruit coating treatments on postharvest life, quality maintenance, and fruit-surface in-jury in ‘Golden Delicious’ apples. J. Amer. Soc. Hort. Sci. 123, 294 – 299.
Sams, C.E., Conway, W.S., Abbott, J.A., Lewis, R.J., Ben-Shalom, N., 1993. Firmness and decay of apples following postharvest pressure infiltration of calcium and heat treat-ment. J. Amer. Soc. Hort. Sci. 118, 623 – 627.
SAS Institute Inc., 1997.SAS/STATSoftware: Changes and Enhancements through Release 6.12. pp. 1167. Cary, NC, SAS Institute Inc.
Spotts, R.A., Chen, P.M., 1987. Prestorage heat treatment for control of decay of pear fruit. Phytopathology 77, 1578 – 1582.
Spotts, R.A., Sanderson, P.G., Lennox, C.L., Sugar, D., Cer-vantes, L.A., 1998. Wounding, wound healing and staining of mature pear fruit. Postharvest Biol. Technol. 13, 27 – 36. Stack, J.P., 1998. Postharvest biological control: commercial successes and a model for public and private sector cooper-ation. 7th International Congress of Plant Pathology, Ed-inburgh, Scotland, BSPP, London.
Wilson, C.L., Wisniewski, M.E., 1989. Biological control of postharvest diseases of fruits and vegetables: An emerging technology. Annu. Rev. Phytopathol. 27, 425 – 441.