量子力学
Quantum mechanics
School of Physics and Information Technology
CHAPTER 7
The variational principle
7.1 Theory
Suppose you want to calculate the ground-state energy
for a system described by the Hamiltonian , but you are
unable to solve the (time-independent) equation
g
E
..
schr o dinger
H
7.1 Theory
Theorem:
Theorem:
H
H
E
g≤
Ψ
Ψ
≡
is any normalized function
That is, the expectation value of in the (presumably
in correct) state is certain to overestimate the ground-state
energy. Of course, if just happens to be one of the excited
states, then obviously exceeds .
H
Ψ
H
E
gProof
Proof
Since the (unknown) eigenfunctions of form a complete
set ,we can express as a linear combination of them:
H
Ψ
, with
n n n n n
n
c H E
Ψ = Ψ Ψ = Ψ
And is normalized,
Ψ
2 *
1
m m n n
m n
m n m n n
m n n
c c
c c c
= Ψ Ψ = Ψ Ψ
2 *
m m n n
m n
m n n m n n n
m n n
H
c
H
c
c E c
E c
=
Ψ
Ψ
=
Ψ Ψ
=
Meanwhile,
Since
E
g≤
E
n ,we getg n
n
g
c
E
E
H
≥
2=
To find the ground-state energy
Aim
Processes
Examples
Examples
Processes
Step 1. Select a trial wave function
Step 2. Calculate in this state
Step 3. Minimize the
Step 4. Take as the appropriate ground-state energy
H
H
Ψ
min
Example 1. 2 2 2 2 2 1 d
H = − + m
ω
xTo find the ground-state energy for the one-dimensional
harmonic oscillator: ω 2 1 = g E
Of course, we already know the exact answer (see chapter 2):
2
2 2
H m x
m dx
ω
= − +
Pick a Gaussian function as our trial state
2
)
(
x
=
Ae
−bxΨ
where is a constant and is determined by normalization:b A
b A dx e A bx 2 1= 2 ∞ 2 2 = 2
π
The mean value of is
H
( )
2 2 2
2 2
2 2 2 2 2
2 2 2 1 2 2 = 2 8
bx d bx bx
H A e e dx m A e x dx
m dx b m m b
ω
ω
∞ ∞ − − − −∞ −∞ = − + +We can get the tightest bound through minimizing withH
respect to :
b
0 8 2 2 2 2 = − = b m m H db d
ω
2ω
m b =Putting this back into ,we findH
ω
2 1
min =
Example 2.
To look for the ground state energy of the delta-function potential:
2 2
2
( )
2
d
H
x
m dx
αδ
= −
−
Again ,we already know the exact answer (see chapter 2):
2
g
b
E α
π
= −
2
m dx
The mean value of is
H
We also Pick a Gaussian function as our trial state:
2
)
(
x
=
Ae
−bx2
0
d
a
H
=
−
=
2 2
2
m
α
b
=
Minimizing it,
(
)
2 2 2
2 2
2 2 2
2 2 ( ) 2 2 = 2
bx d bx bx
H A e e dx A e x dx
m dx b b m
α
δ
α
π
∞ ∞ − − − −∞ −∞ = − − −0
2
2
d
a
H
db
=
m
−
π
b
=
42
π
α
m
b
=
2 2 min m Hα
π
= −which is indeed somewhat higher than
E
g ,since π > 2Example 3.
To find an upper bound on the ground-state energy of
the one-dimension infinite square well, using the “triangular”
trial wave function (figure 7.1):
Figure 7.1: “triangular” trial wave function
for the infinite square well 2
a a x
( )x
3
2 2 2 2 2
0
2
1 ( )
12
a a
a
a
A x dx a x dx A
= + − =
A
2
3
a
a
=
Where is determined by normalization:A
In this case
The derivative of this step function is a delta function (see
problem 2.24b)
)
(
)
2
(
2
)
(
2
A
x
A
x
a
A
x
a
dx
d
−
+
−
−
=
Ψ
δ
δ
2 2 2 2 2
12 (0) 2 ( ) ( )
2
A a A
a
= − Ψ − Ψ + Ψ = =
and hence
2
( ) 2 ( ) ( ) ( )
2 2
A a
H x x x a x dx
m
δ
δ
δ
= − − − + − Ψ
2
(0) 2 ( ) ( ) 2
2m a 2m 2ma
= − Ψ − Ψ + Ψ = =
The exact ground state is ( see chapter 2), so
the theorem works ( )
2 2 2
2
g
E
ma
π
=
2
write down a trial wave function
The variational principle is very powerful and easy to use
Advantages
Conclusions
Calculate
tweak the parameters to get the lowest possible value
H
Even if has no relation to the true wave function, one
often gets miraculously accurate values for
Ψ
g
Limitations
It applies only to the ground state
You never know for sure how close you are to the target You never know for sure how close you are to the target
and all you can certain of is that you have got an upper
7.2
The ground state of helium
Our task
:
To calculate the ground–state energy by using the
Variational Principle
(experimental)
ev 975 .
78
− =
g
E
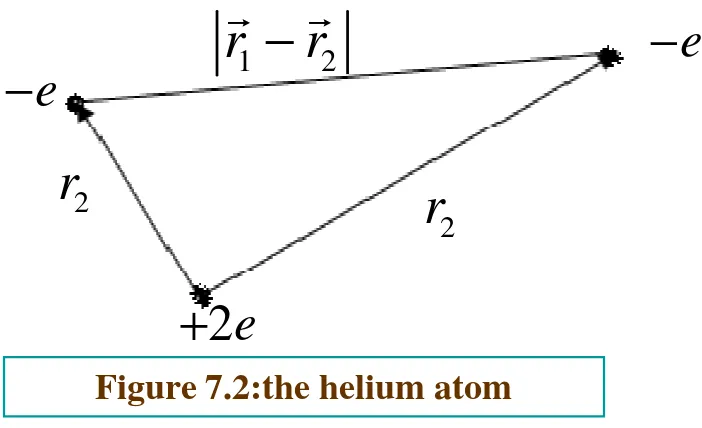
Figure 7.2:the helium atom
2
r
1 2
r
−
r
e
−
e
−
2
r
2
e
+
The Hamiltonian for the helium atom system (ignoring
fine structure and small correction) is
(
)
2 2
2 2
1 2
0 1 2 1 2
2 2 1
2 4
e H
m
πε
r r r r= − ∇ + ∇ − + −
−
2
0 1 2
1 4
e e
e V
r r
π ε
=
−
( )
( )
( )
2( 1 2)0 1 2 100 1 100 2 3 8
, r r a
r r r r e
a
π
− +
Ψ ≡ Ψ Ψ =
Consequently ,the energy that goes with this simplified If we ignore the electron-electron repulsion is, the
ground-state wave function is just
ee
V
where is hydrogen-like wave function with . Ψ100 Z = 2
picture is (see Chapter 5 ).8E1 = −109 ev
(
)
0
8
1 ee 0H
Ψ =
E
+
V
Ψ
In the following we will apply the variational principle ,
using the as the trial wave function. The eigenfunction of
Thus
1
8 ee
H = E + V where
( 1 2)
2 4 2
3 3 1 2 3
0 1 2
8
4
r r a
ee
e
e
V
d r d r
a
r
r
πε
π
− +
=
−
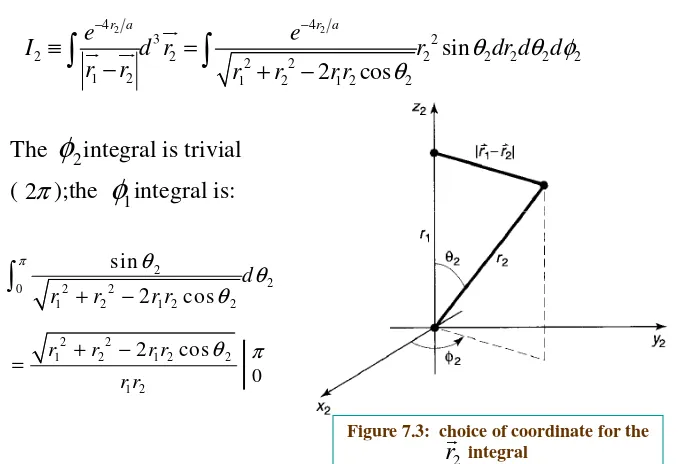
To get the above integral value conveniently, we do the
2
r
integral first and orient the coordinate system so that the
polar axis lies along (see Figure 7.3).
1
r
2
r
By the law of cosines,
2 2
1 2 1 2
2
1 2cos
2and hence
2 2
4 4
3 2
2 2 2 2 2 2 2 2 2
1 2 1 2 1 2 2
sin 2 cos
r a r a
e e
I d r r dr d d
r r r r r r
θ θ φ
θ
− −
≡ =
− + −
The integral is trivial
( );the integral is:
[image:21.842.88.766.86.550.2]2
φ
1φ
π 2Figure 7.3: choice of coordinate for the integral 2
r
2 2 2 2 01 2 1 2 2
sin
2 cos
d
r r r r
π θ
θ θ
+ −
2 2
1 2 1 2 2
1 2
2 cos
r r r r
(
)
(
)
2 2 2 2
1 2 1 2 1 2 1 2
1 2
1 2 1 2 1 2
1
2 2
1
r r rr r r rr
rr
r r r r rr
= + + − + −
= + − −
{
1 2 12 2 1
2 , if 2 , if
r r r
r r r
< >
=
Thus 1 2 2 14 2 4
2 2 2 2 2
0 1 3
1
4 r r a r a
r
I e r dr e r dr
r
π
π
∞ − − = + 1 3 4 1 1 2 1 1 8 r a r a e r aπ
− = − +It follows that is equal to
V
ee1 1
2
4 4
1
1 1 1 1 1 3
0
2 8
1 1 sin
4
r a r a
r e
e e r dr d d
a a
θ
θ φ
πε
π
− −
2 2
4 8
0
2
5
128
r a
r
r aa
re
r
e
dr
a
∞
− −
−
+
=
2
1 0
5
5
34
4
4
2
ee
e
V
E
ev
a
πε
=
= −
=
Finally , then,
The angular integals are easy( ),and the integal becomes
4
π
r
10
4
a
4
πε
2
109
34
75
H
= −
ev
+
ev
= −
ev
And therefore
Can we think of a more realistic trial function
than
0
Ψ
We try the product function
1 2 3
( ) 1
( , )
1 2 3Z r r a
Z
r r
e
a
π
− +
Ψ
≡
and treat Z as a variable rather than setting it equal to 2.
The idea is that as each electron shields the nuclear charge
seen by the other ,the effective Z is less than 2.
In the following, we will treat Z as a variational parameter,
Rewrite in the following form:
H
(
)
(
) (
)
2 2 2 2 2
1 2
0 1 2 0 1 2 1 2
2 2 1
2 4 4
Z Z
e Z Z e
H
m πε r r πε r r r r
− −
= − ∇ + ∇ − + + + +
−
The expectation value of is evidently
H
2
1
e
(
)
2 2
1
0
1
2 2 2
4 ee
e
H Z E Z V
r
πε
= + − +
r
1 1
r
Here is the expectation value of in the (one-particle)
And according to Chapter 6, we know
1 a
r = Z
The expection value of is the same as before ,except
that instead of ,we now want arbitrary Z—so we
multiply by :
2
=
Z
Z
a
ee
V
multiply by :
a
Z 22
1 0
5 5 8 4 4
ee
Z e Z
V E
a πε
= = −
Putting all this together, we find
(
)(
)
(
)
2 2
1 1
2 4 2 5 4 2 27 4
The lowest upper bound occurs when is minimized:
H
(
)
14 27 4 0
d
H Z E
dZ = − + =
from which it follows that
2 7
1 .6 9
Z = = 1 .6 9 1 6
Z = =
Putting in this value for Z, we find
6 1
1 3
77.5 2 2
H = E = − ev
7.3
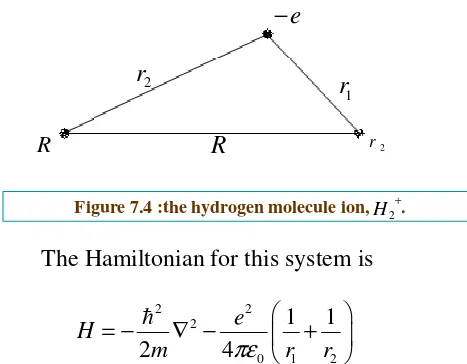
The hydrogen molecule ion
R R r 2
e
−
2
r
1
r
The Hamiltonian for this system is
2 2
2
0 1 2
1 1
2 4
e H
m πε r r
[image:29.842.166.633.130.494.2]= − ∇ − +
Figure 7.4 :the hydrogen molecule ion, .+
2
To construct the trial wave function , imagine that the ion is
formed by taking a hydrogen atom in its ground state
a r
g
e
a
r
=
−Ψ
3
1
)
(
π
and then bringing in proton from far away and nailing it down a
distance R away. If R is substantially greatly than the Bohr radius distance R away. If R is substantially greatly than the Bohr radius
a, the electron’s wave function probably isn’t changed very much.
But we would like to treat the two protons equally ,so that the electron
has the same probability of being associated with either one. So we
consider a trial function of the form
( )
1( )
2g g
A r r
Normalize this trial function:
( )
( )
2( )
( )
2 3 2 2 3 3 3
1 2 1 2
1= Ψ d r = A Ψg r d r + Ψg r d r + 2 Ψg r Ψg r d r
( )
( )
2 3
1 2
2 A 1 g r g r d r
= + Ψ Ψ
( )
1 − r +r
Let
( )
( )
( 1 2) 31 2 3
1 r r
a
g g
I r r e d r
a
π
− +
≡ Ψ Ψ =
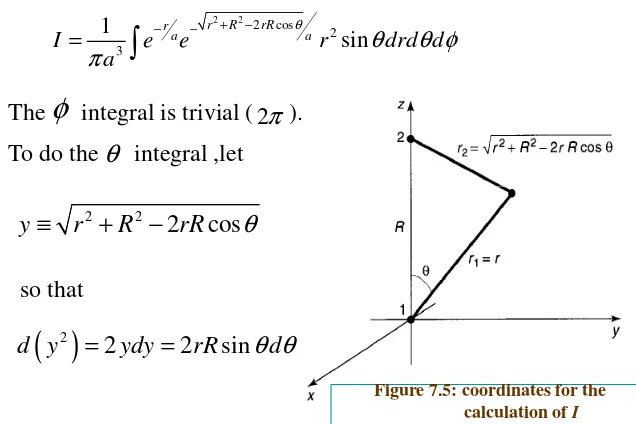
Picking coordinates so that the pronton 1 is at the origin and
proton 2 is on the z-axis at the point R (Figure 7.5),we have
1
r = r 2 2
2 2 cos
and therefore
2 2
2 cos
2 3
1
sin
r R rR r
a a
I e e r drd d
a
θ
θ θ φ π
+ − − −
=
The integral is trivial ( ).
To do the integral ,let
φ
θ
π
[image:32.842.90.726.111.535.2]2
Figure 7.5: coordinates for the calculation of I
2 2
2 cos
y ≡ r + R − rR
θ
( )
22 2 sin
d y = ydy = rR
θ θ
dThen ( )
(
)
(
)
2 2 2 cos 0 1sin r R
r R rR a y a
r R
r R a r R a
e d e ydy
rR a
e r R a e r R a
rR π θ θ θ + − + − − − − − − + = = − + + − − +
The integral is now straightforward:
r
2 ∞ R
(
)
(
)
(
)
2
2 0 0 2
2
R R a r a R a
R a r a R
I e r R a e rdr e R r a rdr a R
e r R a e rdr
∞ − − − ∞ − = − + + + − + + − +
Evaluating the integrals, we find
2
1
1 ( ) (
3
)
R a R R
I e
a a
−
In the terms of , the normalization factor is
I
2 1 2(1 ) A I = +Next we must calculate the expectation value of in the
trial state . Noting that
H
Ψ
( )
( )
2 2 2
1 1 1 0 1
1
2 4 g g
e
r E r
m πε r
− ∇ − Ψ = Ψ
Where is the ground-state energy of atomic
hydrogen and the same with in place of ,we have ev
6 . 13
1 = −
E 1
r
2r
( )
( )
( )
( )
2 2 2 1 20 1 2
2
1 1 2
0 2 1
1 1 2 4 1 1 4 g g g g e
H A r r
m r r
e
E A r r
r r
πε
πε
Ψ = − ∇ − + Ψ + Ψ
It follows that
( )
( )
( )
( )
2 2
1 1 1 1 2
0 2 1
1 1
2
4 g g g g
e
H E A r r r r
r r
πε
= − Ψ Ψ + Ψ Ψ
Calculate the two remaining quantities ,the so-called direct integral,
( )
1( )
1 21
g g
D a r r
r
≡ Ψ Ψ
2
1 R a
a a
D e
R R
−
= − +
1 a R a
X e
R
−
= +
and the exchange integral,
The results are
( )
1( )
21
1
g g
X a r r
r
Putting all this together, and recalling that
we conclude that
( )
0 1 0 1 4 2 e E a πε = − 1 ( ) 1 2 (1 ) D X H E I + = + +This is only the electron’s energy----there is also potential energy
associated with the proton-proton repulsion:
Thus the total energy of the system, in units of
and expressed as a function of ,is less than
1 E − R x a ≡ 2 2 2
2 (1 (2 / 3)
)
(1
)
( )
1
1 (1
(1/ 3)
)
x x
x
x e
x e
F x
x
x
x e
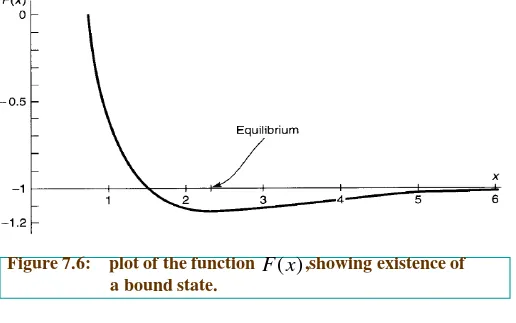
Figure 7.6: plot of the function ,showing existence of a bound state.
) (x F
Evidently bonding does occur, for there exists a region in which the graph
goes below -1,indicating that the energy is less than that of a neutral atom plus
a free proton (to wit, ).The Equilibrium separation of the protons is
about 2.4 Bohr radii, or .
ev 6 . 13
−
A