Summary We studied the in vitro responses of cambial tissue and dormant vegetative buds obtained from top and epicormic branches of three mature black locust (Robinia pseudoaca-cia L.) trees. Cambial tissues isolated from epicormic branches produced more callus than cambial tissues isolated from top branches, whereas in vitro shoot cultures derived from buds excised from top branches grew faster than those derived from buds excised from epicormic branches. There were no signifi-cant differences between the two branch sources in in vitro bud break or shoot multiplication from bud explants or cambial-de-rived callus tissue, respectively. Furthermore, the top branches, generally considered to be the most mature in a tree, were not recalcitrant in terms of morphogenic capacity compared to epicormic branches.
Keywords: black locust, cambial tissue culture, juvenility/ma-turity, shoot regeneration.
Introduction
Clonal forestry has gained increasing recognition as an alter-native to conventional practices (Zobel and Talbert 1984). Planting genetically superior clones in place of seedlings of varying phenotypic performance may increase forest produc-tivity. Furthermore, tissue culture of forest tree species may provide a superior alternative to traditional cloning techniques. Micropropagation has been applied to many tree species (Bonga and von Aderkas 1992, Iriondo et al. 1995). The micro-propagated trees exhibit juvenile characteristics including rapid vegetative growth (Jones 1994). Birch (Betula pendula Roth) trees produced by micropropagation are more uniform in height and trunk girth, show less bark fissuring (a mature characteristic), and tend to flower earlier than seedling-derived trees (Jones et al. 1996).
In combination with current genetic engineering techniques, tissue culture can serve as a tool to accelerate tree improve-ment programs and exploit maximum genetic gain (Haissig et al. 1987). However, in many tree species the use of tissue
culture techniques has been confined mainly to juvenile mate-rial. This limits the application of tissue culture as an alterna-tive to traditional methods because most economically important traits are expressed only in mature trees, and juve-nile--mature phenotypic correlations are often low. It may be possible to solve this problem by using explants for in vitro culture obtained from those parts of a mature tree that retain juvenile characteristics, such as epicormic shoots or shoots arising from the lower part of the trunk (Chalupa 1984, Vieitez et al. 1985).
Both juvenile and mature phases may occur on the same mature tree (Kramer and Kozlowski 1979, Hackett 1985, Bonga 1987). The upper parts of a tree exhibiting determinate growth are chronologically younger and often exhibit mature characteristics, whereas the lower, ontogenetically older parts may retain juvenile attributes (Hackett 1985). Many studies have shown that juvenile tissue can usually be more easily cultured in vitro than mature tissue explants (e.g., Bonga 1987, Sanchez and Vieitez 1991).
Several attempts have been made to understand the relation-ship between tissue culture reactivity and physiological gradi-ents in maturity by comparing the in vitro behavior of explants from mature trees with that of seedling explants (Chevre et al. 1983, Favre and Juncker 1987, Welander 1988). Unfortunately, the seedling material used in these studies was not the same genotype as the mature material, hence the results are con-founded by genotype. Sanchez and Vietez (1991) were able to compare the in vitro reactivity of explants from crown branches with that of explants from basal sprouts of the same mature chestnut (Castanea spp.) tree. They concluded that cultures from basal shoots were more responsive than cultures from crown branches.
We have chosen epicormic and top branches of mature black locust (Robinia pseudoacacia L.) trees to study the relation-ship between in vitro performance and phase difference (juve-nile versus mature). Black locust can serve as a model species for such studies because direct comparison of juvenile and mature materials within the same genotype is possible. In
Tissue culture responses of explants taken from branch sources with
different degrees of juvenility in mature black locust (
Robinia
pseudoacacia
) trees
KYUNG-HWAN HAN,
1,4DONG-ILL SHIN
2and DANIEL E. KEATHLEY
31
Department of Forest Science, Oregon State University, Corvallis, OR 97331, USA
2 Department of Plant Breeding, College of Natural Resources, Hyusung Catholic University, Hayang, Kyungsan-gun, Kyungpook, Korea 3 Department of Forestry, Michigan State University, East Lansing, MI 48824, USA
4
Present address: Kumho Life and Environmental Science Laboratories, 572 Ssangamdong, Kwangsangu, Kwangju, Korea
Received September 9, 1996
black locust, the lower branches possess juvenile charac-teristics, such as large spines and rapid growth, whereas the spineless and flowering upper branches represent the mature phase (Trippi 1963, Kramer and Kozlowski 1979). In addition, both tissue culture (Davis and Keathley 1987, Han et al. 1993a, Arrillaga et al. 1994) and genetic transformation systems are well developed for the species (Davis and Keathley 1989, Han et al. 1993b). Specifically, we tested the hypothesis that ex-plants from epicormic branches would exhibit greater callus induction and growth, shoot regeneration and multiplication, and rooting capacities than explants from top branches of mature black locust.
Materials and methods
Plant materials
In January, newly grown branches measuring 0.5--1.0 cm in diameter were collected from top and epicormic branches of 15- (Trees 2 and 3; Daegu, Korea, 35.8° N 128.5° E) and 25-year-old (Tree 1; Davis and Keathley 1987) black locust trees and stored at 4 °C. All plant cultures were incubated in a growth chamber at 25 °C in a 16-h photoperiod (50--75 µmol m−2 s−1).
In vitro bud culture
Dormant vegetative buds were isolated and cultured as pre-viously described (Davis and Keathley 1987). Shoots derived from the in vitro bud cultures were maintained by subculturing at 4-week intervals in 25 × 150 mm culture tubes containing MS (Murashige and Skoog 1962) medium supplemented with 1 µM 6-benzylaminopurine (BAP).
Cambial tissue culture
For callus induction and spontaneous shoot organogenesis, cambial tissue explants were surgically removed (0.2--0.5 mm thick and 4 × 5 mm in size) from the excised branches with a scalpel and placed in a 15 × 100 mm petri-dish (10 explants per dish) containing MS medium supplemented with 10 µM α -naphthaleneacetic acid (NAA) and 5 µM BAP (Han et al. 1993a). After 4 weeks of culture, the diameter of each primary callus growth was measured.
To study the effects of exogenously supplied phytohor-mones on primary cambial callus growth, cambial tissue ex-plants taken from the epicormic and top branches of Tree 1 (20 explants per treatment, 10 explants per dish) were cultured on MS medium supplemented with either 5 µM BAP and different concentrations of NAA (0, 0.1, 0.32, 1.0, 3.2, 10.0, or 32 µM) or 10 µM NAA and different concentrations of BAP (0, 1.3, 2.5, 5, 10, and 20 µM). After 4 weeks of culture, callus growth was recorded as fresh weight.
Shoot regeneration
Calli (5--10 mm in diameter), obtained from cambial tissue explants cultured on MS with 10 µM NAA and 5 µM BAP, were transferred to a 15 × 100 mm petri-dish (five explants per dish) containing MS medium augmented with 25 µM BAP (cf.
Han et al. 1990, 1993a). Shoot regeneration from primary callus tissue was scored after 4 weeks of culture.
Rooting
Shoots regenerated from primary callus tissue were transferred to a rooting medium consisting of half-strength MS medium supplemented with 1 µM IBA. After 3 weeks of culture in rooting medium, rooting was assessed as the percent of shoots producing roots.
Statistical analysis
For the comparison of spines, internodal length, shoot growth, and shoot multiplication from bud culture, a two-sample t-test was performed. Chi-square values from 2 × 2 contingency tables were calculated for the comparison of branch source effects on ex vitro and in vitro bud break, callus induction, shoot morphogenesis, and rooting by means of the normal approximation (Steele and Torrie 1980). The data for callus growth were analyzed by a general linear models analysis of variance.
Results and discussion
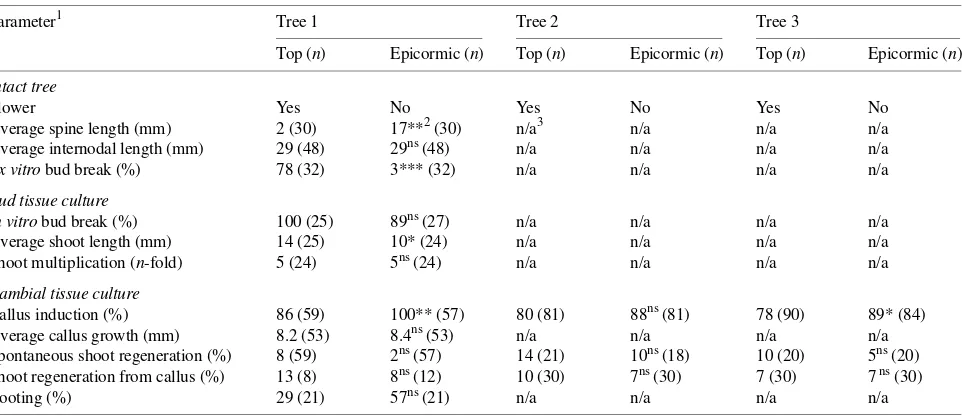
Morphological observations of the epicormic and top branches confirmed the characteristics of juvenile and mature phases as described previously (Trippi 1963, Kramer and Kozlowski 1979, Bonga and von Aderkas 1992). Epicormic branches had large spines and no flowers, whereas top branches had small or negligible spines and flowers (Table 1). Epicormic branches showed more rapid growth than top branches even though internodal length was not significantly different between the two branch sources. When excised branches were placed in water, significantly more dormant vegetative buds flushed within 10 days on the top branches than on the epicormic branches (78 versus 3% of total buds on the branches). How-ever, there was no significant difference between the branch sources with respect to in vitro bud break (Table 1), suggesting that the physiological differences between the two branch sources may be small at the explant level.
Use of isolated dormant vegetative buds to initiate in vitro shoot cultures allowed us to compare the buds themselves in the absence of any influences from the surrounding tissues. Shoots derived from the in vitro culture of buds obtained from top branches showed more rapid growth than shoots derived from the in vitro culture of buds obtained from epicormic branches; however, shoot multiplication during the first sub-culture period was not significantly different between the two branch sources.
(Vieitez et al. 1985). Shoot-tip explants showed poorer prolif-eration than nodal or basal explants, perhaps reflecting differ-ent physiological states of the buds at the differdiffer-ent stem positions. However, Vieitez et al. (1985) found that the source of explants (embryonic axes, seedlings or mature tree) did not affect shoot multiplication in pedunculate oak. Similarly, Han-nus and Rohr (1987) achieved comparable shoot multiplica-tion from both juvenile material and stump sprouts on a MS basal medium. These findings may indicate either that the endogenous substances necessary for organogenesis were pre-sent in all of the explants studied or that the effects of explant source are species specific. However, Iriondo et al. (1995) observed no significant differences in shoot multiplication among five generations of subcultures of shoots derived from mature trees of oleaster (Elaeagnus angustifolia L.), whereas Economou and Spanoudaki (1988) reported increased shoot
multiplication after the first and second subculture from a mature tree of the same species.
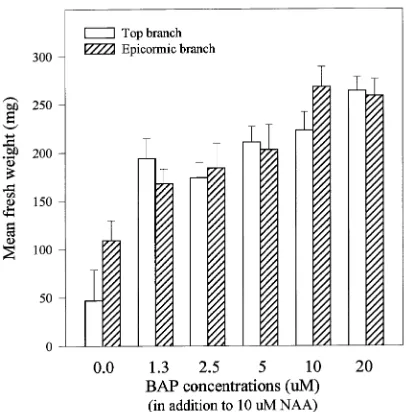
Callus cultures were easily induced from cambial tissue isolated from both top and epicormic branches. Although a higher percentage of callus cultures were obtained from ex-plants from epicormic branches compared with top branches (Table 1), there was no effect of branch source on primary callus growth (Table 2). There was a highly significant interac-tion between branch source and NAA concentrainterac-tion (P < 0.001). As the concentration of NAA in the culture me-dium increased, primary callus increased in fresh weight for the top branch source but decreased for the epicormic branch source (Figure 1). The interaction between branch source and BAP concentration was not significant (Table 2). With increas-ing concentration of BAP, primary callus growth was stimu-lated (Pearson’s correlation r = 0.53, P < 0.01) regardless of Table 1. Comparison of in vitro shoot induction from dormant vegetative buds and cambial tissues obtained from top and epicormic branches of a mature Robinia pseudoacacia tree (n = number of explants for each measurement).
Parameter1 Tree 1 Tree 2 Tree 3
Top (n) Epicormic (n) Top (n) Epicormic (n) Top (n) Epicormic (n)
Intact tree
Flower Yes No Yes No Yes No
Average spine length (mm) 2 (30) 17**2 (30) n/a3 n/a n/a n/a
Average internodal length (mm) 29 (48) 29ns (48) n/a n/a n/a n/a
Ex vitro bud break (%) 78 (32) 3*** (32) n/a n/a n/a n/a
Bud tissue culture
In vitro bud break (%) 100 (25) 89ns (27) n/a n/a n/a n/a
Average shoot length (mm) 14 (25) 10* (24) n/a n/a n/a n/a
Shoot multiplication (n-fold) 5 (24) 5ns (24) n/a n/a n/a n/a
Cambial tissue culture
Callus induction (%) 86 (59) 100** (57) 80 (81) 88ns (81) 78 (90) 89* (84)
Average callus growth (mm) 8.2 (53) 8.4ns (53) n/a n/a n/a n/a
Spontaneous shoot regeneration (%) 8 (59) 2ns (57) 14 (21) 10ns (18) 10 (20) 5ns (20) Shoot regeneration from callus (%) 13 (8) 8ns (12) 10 (30) 7ns (30) 7 (30) 7 ns (30)
Rooting (%) 29 (21) 57ns (21) n/a n/a n/a n/a
1 Ex vitro bud break = percent of dormant buds flushing 10 days after three branches bearing a total of 32 dormant buds were cut from
top/epicormic branches and placed in water at room temperature; average shoot length was measured after 4 weeks of in vitro bud culture; shoot multiplication = average number of shoots multiplied from one shoot after 4 weeks of culture; average callus growth = diameter of primary calli after 4 weeks of culture; spontaneous shoot regeneration = adventitious shoot production from cambial tissues on callus induction medium (MS + 10 µM NAA + 5 µM BAP); shoot regeneration from primary callus tissue was scored after 4 weeks of culture on MS supplemented with 25 µM BAP; rooting = percent of shoots producing roots after 3 weeks of culture on 1/2 MS supplemented with 1 µM IBA.
2 * = Significantly different at the 0.05 probability level; ** = significantly different at the 0.01 probability level; ns = not significant. 3 n/a = Not available.
Table 2. Analysis of variance summary for the effects of phytohormones on primary cambium callus growth (ns = not significant; *** = significant
F-value at the 0.001 level).
Source BAP NAA
DF MS F-value DF MS F-value
Branch source 1 6267 1.2ns 1 51 0.01ns
Phytohormone concentration 5 87948 16.9*** 6 19966 5.14***
Branch × Phytohormone concentration 5 6312 1.2ns 6 39978 10.30***
branch source (Figure 2). We were unable to determine whether the differential responses of branch sources mani-fested in callus growth were a result of differential concentra-tions of endogenous auxin or differential sensitivity to exogenously provided NAA. Although we did not find any significant effect of branch source alone on primary callus growth, Polito and Alliata (1981) reported that the growth index of juvenile-phase callus was 3.5 times higher than that of mature-phase callus in English ivy (Hedera helix L.); how-ever, their comparison was confounded by the use of different genotypes.
During the callus induction period, spontaneous shoot or-ganogenesis occurred at similar rates in cambial tissues from both branch sources (Table 1). Shoot regeneration was readily induced when cambium-derived callus from both branch sources was transferred to regeneration medium. The use of cambial tissues from epicormic and top branches to compare the effects of explants from different phases enabled us to compare directly the biochemical status of the source materials with respect to in vitro morphogenic potential. The disadvan-tages of our system include the possibility that excision of cambial tissues containing high concentrations of endogenous growth regulators may improve in vitro performance of the explants (Durzan 1984), and the possibility that physiological shock (caused by dehydration, wounding, and oxidation) dur-ing the excision of cambial tissues biased the results.
There was no noticeable difference in in vitro rootability between explants from the top and epicormic branches (Ta-ble 1). This finding contrasts with published reports that the position of the explant source material influences rooting abil-ity. For example, shoot cultures from basal sprouts of a mature oak tree had higher rooting rates than crown-derived cultures (Sanchez et al. 1996). Geneve et al. (1991) studied adventitious rooting in English ivy by means of reciprocal grafts involving leaf petioles and laminas of the juvenile and mature phases and concluded that root initiation was mainly a function of the potential of cells in the petiole to respond in a specific morpho-genic pattern.
In summary, previous studies (Chauvin and Salesses 1988, Welander 1988, Sanchez and Vieitez 1991) have shown that the lower parts of mature trees are less recalcitrant to in vitro culture than the upper parts. In contrast, we found that top branches were not recalcitrant compared to epicormic branches. Furthermore, shoot cultures derived from top branches grew more rapidly than shoot cultures derived from epicormic branches. Although physiological differences be-tween the two branch sources were demonstrated by differen-tial responses to a range of exogenous auxin concentrations, these differences did not affect the overall in vitro culture capacity of the tissues. Our findings have practical applications for the initiation of in vitro culture from selected mature genotypes of black locust.
Acknowledgments
This work was supported in part by a Korean Government Scholarship to K.-H. Han. We thank Rick Meilan for critical reading of the manuscript.
References
Arrillaga, I., J.J. Tobolski and S.A. Merkle. 1994. Advances in somatic embryogenesis and plant production of black locust (Robinia pseudoacacia L.). Plant Cell Rep. 13:171--175.
Bonga, J.M. 1987. Clonal propagation of mature trees: problems and possible solutions. In Cell and Tissue Culture in Forestry. Eds. J.M. Bonga and D.J. Durzan. Martinus Nijhoff, Dordrecht, The Nether-lands, pp 249--271.
Figure 1. Effect of NAA concentration on the growth of primary cambium callus from Tree 1. Bars show one standard error of the mean.
Bonga, J.M. and P. von Aderkas. 1992. In vitro culture of trees. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 72--125. Chalupa, V. 1984. In vitro propagation of oak (Quercus robur L.) and
linden (Tilia cordata Mill.) Biol. Plant. 26:374--377.
Chauvin, J.E. and G. Salesses. 1988. Advances in chestnut micro-propagation (Castanea spp.). Acta Hortic. 227:340--345.
Chevre, A.M., S.S. Gill, A. Mouras and G. Salesses. 1983. In vitro
vegetative multiplication of chestnut. J. Hortic. Sci. 58:23--29. Davis, J.M. and D.E. Keathley. 1987. Differential responses to in vitro
bud culture in mature Robinia pseudoacacia L. (black locust). Plant Cell Rep. 6:431--434.
Davis, J.M. and D.E. Keathley. 1989. Detection and analysis of T--DNA in crown gall tumors and kanamycin-resistant callus of Robinia pseudoacacia. Can. J. For. Res. 19:1118--1123.
Durzan, D.J. 1984. Special problems: adults vs. juvenile explants. In
Handbook of Plant Cell Culture. Vol. II. Eds. W.R. Sharp, D.A. Evans, P.V. Ammirato and Y. Yamada. Collier Macmillan, London, pp 471--503.
Economou, A.S. and M.J. Spanoudaki. 1988. Regeneration in vitro of oleaster (Elaeagnusangustifolia L.) from shoot tips of mature trees. Acta Hortic. 227:363--368.
Favre, J.M. and B. Juncker. 1987. In vitro growth of buds taken from seedlings and adult plant material in Quercus robur L. Plant Cell Tissue Organ Cult. 8:49--60.
Geneve, R.L., M. Mokhtari and W.P. Hackett. 1991. Adventitious root initiation in reciprocally grafted leaf cuttings from the juvenile and mature phase of Hedera helix L. J. Exp. Bot. 42:65--69.
Hackett, W.P. 1985. Juvenility, maturation, and rejuvenation in woody plants. Hortic. Rev. 7:109--155.
Haissig, B.E., N.D. Nelson and G.H. Kidd. 1987. Trends in the use of tissue culture in forest improvement. Bio-Technology 5:52--59. Han, K.-H., J.M. Davis and D.E. Keathley. 1990. Differential
re-sponses persist in shoot explants regenerated from callus of two mature black locust trees. Tree Physiol. 6:235--240.
Han, K.-H., D.E. Keathley and M.P. Gordon. 1993a. Cambial tissue culture and subsequent shoot regeneration from mature black locust (Robinia pseudoacacia L.). Plant Cell Rep. 12:185--188. Han, K.-H., D.E. Keathley, J.M. Davis and M.P. Gordon. 1993b.
Regeneration of a transgenic woody legume (Robinia pseudoaca-cia L., black locust) and morphological alterations induced by
Agrobacterium rhizogenes-mediated transformation. Plant Sci. 88:149--157.
Hanus, D. and R. Rohr. 1987. In vitro plantlet regeneration from juvenile and mature sycamore maple. Acta Hortic. 212:77--82.
Iriondo, J.M., M.D. Iglesia and C. Perez. 1995. Micropropagation of
Elaeagnus angustifolia from mature trees. Tree Physiol. 15:691--693.
Jones, O.P. 1994. Physiological change and apparent rejuvenation of temperate fruit trees from micropropagation. In Physiology, Growth and Development of Plants in Culture. Eds. P.J. Lumsden, J.R. Nicholas and W.J. Davies. Kluwer Academic Publishers, Dor-drecht, The Netherlands, pp 323--331.
Jones, O.P., M. Welander, B.J. Waller and M.S. Ridout. 1996. Micro-propagation of adult birch trees: production and field performance. Tree Physiol. 16:521--525.
Kramer, P.J. and T.T. Kozlowski. 1979. Internal factors affecting growth. In Physiology of Woody Plants. Eds. P.J. Kramer and T.T. Kozlowski. Academic Press, San Francisco, pp 546--627. Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth
and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473--497.
Polito, V.S. and V. Alliata. 1981. Growth of callus derived from shoot apical meristems of adult and juvenile English ivy (Hedera he-lix L.). Plant Sci. Lett. 22:387--393
Sanchez, M.C. and A.M. Vieitez. 1991. In vitro morphogenic compe-tence of basal sprouts and crown branches of mature chestnut. Tree Physiol. 8:59--70.
Sanchez, M.C., M.C. San-Jose, A. Ballester and A.M. Vieitez. 1996. Requirements for invitro rooting of Quercus robur and Q. rubra
shoots derived from mature trees. Tree Physiol. 16:673--680. Steele, R.G.D. and J.H. Torrie. 1980. Principles and procedures of
statistics. A biomedical approach. McGraw-Hill Book Co., New York, pp 495--520.
Trippi, V.S. 1963. Studies on ontogeny and senility in plants. I. Changes in growth vigor during the juvenile and adult phases of ontogeny in Tilia parviflora and growth in juvenile and adult zones of Tilia, Ilex aquifolium and Robinia pseudoacacia. Phyton 20:137--145.
Vieitez, A.M., M.C. San-Jose and E. Vieitez. 1985. In vitro plantlet regeneration from juvenile and mature Quercus robur L. J. Hortic. Sci. 60:99--106.
Welander, M. 1988. Biochemical and anatomical studies of birch (Betula pendula Roth) buds exposed to different climatic conditions in relation to growth in vitro. In Genetic Manipulation of Woody Plants. Eds. J.W. Hanover and D.E. Keathley. Plenum Press, New York, pp 79--99.