Summary The major components of annual seed deposition in the rain forest at Los Tuxtlas, Veracruz, México are seeds of the pioneer tree species Cecropia obtusifolia and those of some species of Ficus. Cecropia obtusifolia Bertol. forms a relatively persistent viable soil seed bank, whereas seeds of Ficus are seldom found in the soil. Both genera require light for seed germination; however, the species differ in their germination responses to far red (FR) light under laboratory and field conditions. Seeds of C. obtusifolia did not germinate in low red/far red (R/FR) or pure FR, whereas seeds of the Ficus species did. This suggests that Ficus seeds do not become dormant under the light conditions (low R/FR ratio) beneath the leaf canopy of the rain forest. This difference may explain why the species differ in their presence in the soil seed bank.
Keywords: photoblastism, phytochrome, rain forest, seed dor-mancy, soil seed bank.
Introduction
Recently disseminated seeds may germinate soon after arrival on the ground, undergo a period of dormancy until innate or environmental conditions are appropriate for germination, or perish. In the first case, the seeds are only briefly present in the soil, whereas in the second case, a long-lasting soil reservoir of living dormant seeds persists (Thompson 1992). Many tropical forest soils are characterized by the existence of a permanent seed bank constituted mainly of seeds of canopy gap colonizers or pioneers (Whitmore 1983, Garwood 1989). Because tropical rain forest soils maintain almost continu-ously favorable conditions for seed germination, it is assumed that the presence of a persistent seed bank in these soils is asociated with either (1) abundant seed deposition, which is facilitated by efficient animal or wind dissemination (Vázquez-Yanes and Orozco-Segovia 1994), or (2) a delay in germination produced by an enforced dormancy mechanism (sensu Harper, Alistair and Ellis 1992). Seed dormancy mecha-nisms regulated by light quality through the pigment phyto-chrome have been described for several of the common pioneer plants that form seed banks in tropical forests (Vázquez-Yanes
and Orozco-Segovia 1994). The relative importance of seed deposition versus seed dormancy for seed bank persistence may be estimated by obtaining data on the amount and season-ality of seed deposition and on the physiology of dormancy and longevity of seeds in the soil seed bank (Vázquez-Yanes and Orozco-Segovia 1994).
To identify physiological differences in the regulation of seed germination among species that might partially explain the differing persistence of their seeds in the soil, we compared the germination responses of seeds of four tropical rain forest species to light quality under laboratory and field conditions.
Materials
Four tree species in two genera were selected: Cecropia obtusi-folia Bertol. and three species of Ficus. Seeds of these two genera constitute the most abundant and constant seed deposi-tion (seed rain) in this forest. Soto-Castro (1992) reported that mean annual seed deposition is roughly 1200 seeds m−2 for
C. obtusifolia and 105 seeds m−2 for Ficus spp.; however, Guevara and Laborde (1993) found that 21.7% of the total number of seeds of all the species found in seed traps placed in open places near the forest belonged to C. obtusifolia and 38.1% to Ficus species.
Cecropia obtusifolia (mean seed weight of 0.99 ± 0.04 mg) is a fast-growing, short-lived pioneer tree that colonizes large gaps. Its fleshy infructescences are produced throughout the year and the seeds are dispersed by several species of birds, bats and other mammals (Estrada et al. 1984). Once in the soil, the seeds show enforced dormancy imposed by light quality (Vázquez-Yanes and Orozco-Segovia 1990). Studies at Los Tuxtlas and at two other tropical rain forests in Mexico indicate that C. obtusifolia is the most abundant species found in the soil seed bank (Salmerón 1984, Guevara 1986, Alvarez-Buylla and Martínez-Ramos 1990). There is large variation in the number of germinating C. obtusifolia seeds per m2 of forest soil----between four and 543 seeds m−2 at Los Tuxtlas (Alvarez-Buylla and Martínez-Ramos 1990). Seeds of other species of Cecropia are also common in soil seed banks throughout tropical America (e.g., Valio and Joly 1979, Holthuijzen and
Comparison of light-regulated seed germination in
Ficus
spp. and
Cecropia obtusifolia
: ecological implications
CARLOS VÁZQUEZ-YANES, MARIANA ROJAS-ARÉCHIGA, MARIA ESTHER
SÁNCHEZ-CORONADO and ALMA OROZCO-SEGOVIA
Centro de Ecología UNAM, Apartado postal 70-275, Ciudad Universitaria, 04510 México, D.F., México
Received December 12, 1995
Boerboom 1982, Putz 1983, Ulh and Clark 1983, Williams-Linera 1990).
Several species of the pantropical genus Ficus are part of the rain forest community at Los Tuxtlas. Ficus yoponensis Desv. (mean seed weight 1.96 ± 0.15 mg) seeds are the most abun-dant among the Ficus seeds found in the seed rain both within the forest and in nearby open places (Guevara and Laborde 1993). The two other species selected for study, F. insipida Willd. (mean seed weight 1.54 ± 0.14 mg), and F.petenensis Stand. (mean seed weight 0.31 ± 0.04 mg), are also relatively common non-hemiepiphytic canopy tree species at Los Tuxtlas. In all three Ficus species, fruiting is asynchronous, with year-round fruit production. A given tree may fruit two or more times annually. Ficus seeds are dispersed by a variety of animals, among which fruit bats play a significant role (e.g., Orozco-Segovia et al. 1985).
Little is known about seed germination of Ficus species, although a species of hemiepiphytic strangling Ficus is re-ported to be photoblastic (Titus et al. 1990). The seeds of Ficus species are seldom found in the soil seed bank at Los Tuxtlas, Veracruz, México (Salmerón 1984, Guevara 1986); however, seeds of Ficus species have been found in small numbers in the soil seed bank of some tropical rain forests (e.g., Hall and Swaine 1980, Hopkins and Graham 1984, Enright 1985).
Methods
Seed collection and field experiments were carried out in the Los Tuxtlas tropical rain forest region in the southeast of the state of Veracruz, México (between 18°30′--18°40′ N and 95°03′--95°10′ W) (Bongers et al. 1988).
General procedures
Freshly collected seeds of C. obtusifolia, F. insipida, F. pe-tenensis and F. yoponensis taken from more than ten individual trees of each species were used for the germination tests. Endozoochorous dissemination was simulated by taking seeds from ripe fruits and rinsing them in acid solution (1% HCl in distilled water) followed by a wash in running water (Orozco-Segovia et al. 1985).
To determine the characteristics of light-controlled dor-mancy, 50 seeds were placed in each petri dish containing 1% agar in distilled water. Dishes were individually enclosed in polyethylene bags to avoid desiccation or flooding (Bartley and Frankland 1984). These procedures were performed in a dark room beneath a green safe light.
Germination experiments were conducted in incubators (455 Lab-line instrument, Inc., Melrose Park, Illinois) at 25 °C, in a 12-h photoperiod provided by 25 W incandescent lamps (General Electric, USA), and 20 W cool white lamps (Syl-vania, USA). Light measurements in the laboratory were made with an LI-1800 portable spectroradiometer (Li-Cor, Inc., Lin-coln, NE, USA). White light (WL) photon fluxes were meas-ured from 400 to 750 nm. Light measurements in the field were made with a radiometer SKR-100 (Skye Instruments,
Scot-land). Red (R) light was measured from 640 to 670 nm, peak at 660 nm, and far red (FR) light was measured from 690 to 748 nm, peak at 730 nm. All germination data were arcsine transformed before being subjected to a two-way ANOVA using the Statgraphics Statistical package (Statistical Graphics Corporation Graphic Software System, Inc. Rockville, MD, ).
Laboratory experiments
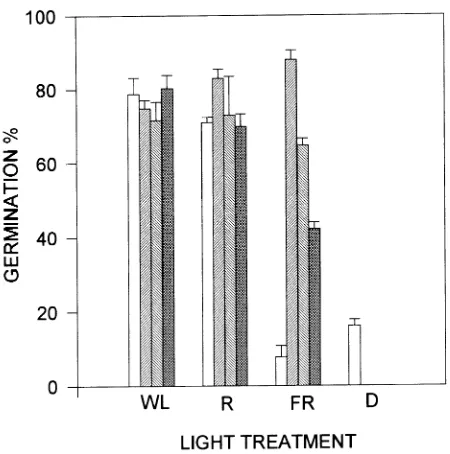
For each species, four dishes of seed were placed in each of four light treatments: WL (R/FR = 1.73, PPFD = 33.21 µmol m−2 s−1), R (R/FR = 4.4, PPFD = 9.297 µmol m−2 s−1), FR
(R/FR = 0.01, PPFD = 9.742 µmol m−2 s−1), and darkness (D),
to give a total of 16 treatment combinations. Germination was recorded after 20 days. To determine seed viability after the 20-day experiment, the remaining ungerminated seeds were placed in WL.
For the R treatment, petri dishes were placed in Plexiglas boxes (34 × 44 × 10 cm) made from two sheets of red Plexiglas (Series No. 2424, Röhm and Hass, México, D. F.) (filter trans-mittance from 590 to 700 nm, peak at 620 nm). For the FR treatment, petri dishes were placed in boxes made of one sheet of red and one sheet of blue Plexiglas (series No. 2423, Röhm and Hass, México, D. F.) (filter transmittance > 670 nm). The boxes were placed in the incubator chambers and illuminated by two fluorescent lamps for the R treatment, by two incandes-cent lamps for the FR treatment, and by both light sources for the WL treatment.
Field experiments
Petri dishes were prepared, seeded and transported to the forest in a dark container. For each species, three petri dishes per treatment were placed on the ground at each of 10 sites along a light gradient from dense forest to a recently formed light gap. The two control series consisted of (1) three petri dishes per species wrapped with aluminum foil to keep them in darkness and (2) three uncovered petri dishes per species placed outside the forest beneath a neutral shade (R/FR = 1.2) to avoid temperature fluctuation. Because each site had a particular light condition, there was a total of 12 light treat-ments per species, including controls. Seed germination was measured after 15 days. Light quality (R/FR) at each site was measured hourly from 0700 to 1700 h, to determine daily changes in R/FR ratio at each site.
Results
Laboratory experiments
There were significant differences in seed response to the light treatments among species (F3,63 = 343.7, P = 0.0001). Fewer
the germination of seeds of F.petenensis and F.yoponensis; however, the FR treatment partially but significantly inhibited the germination of F.insipida seeds (Figure 1). Ungerminated F.petenensis and F. yoponensis seeds placed in WL after the end of the light quality experiment did not germinate, whereas all of the inhibited seeds of C. obtusifolia and F. insipida germinated in WL.
Field experiments
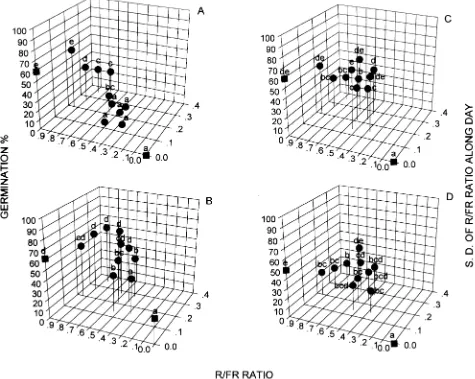
Field results closely paralleled those obtained in the labora-tory. Light quality conditions in the forest inhibited germina-tion of C. obtusifolia seeds (F11,35 = 33.4, P = 0.0001) (Figure
2A). Irrespective of the variability in R/FR ratio during the day, C. obtusifolia seeds did not germinate if the mean R/FR was below 0.6. Germination increased with increasing R/FR ratio. Germination of Ficus yoponensis seeds was inhibited when the R/FR ratio was less than 0.55 and the diurnal vari-ation in R/FR was low (Figure 2B). High R/FR ratios did not inhibit germination (F11,35 = 31.5, P = 0.0001). Ficus
petenen-sis (Figure 2C) and F.insipida (Figure 2D) germinated at low R/FR ratios and showed partial germination inhibition at high R/FR ratios under the highly variable light conditions of the relatively open forest sites. Germination at high R/FR ratios was greater when variation in light conditions was small than when it was large (F11,35 = 49.9, P = 0.0001; and F11,35 = 16.5,
P = 0.0001, respectively). Diurnal change in R/FR is indicated by the standard deviation axis (Figure 2).
Discussion
Most tree species in the mature forest produce large and fleshy seeds that germinate quickly after falling to the ground. Seed-lings then remain in a condition of arrested development or grow slowly beneath continuous forest canopy (Vázquez-Yanes and Orozco-Segovia 1993). In contrast, the species we studied produce enormous numbers of small seeds throughout the year. These small seeds have very efficient dispersal mechanisms, which might indicate that suitable places for the establishmnent of these plants are scarce or that the probability of survival is low.
Seed persistence in the soil was much greater for C. obtusi-folia than for the Ficus species (Salmerón 1984, Guevara 1986). Both genera have positive photoblastic seeds (i.e., the seeds do not germinate in darkness) that lack endogenous dormancy. The germination of C. obtusifolia was strongly inhibited by the light in the canopy shade, which is charac-terized by a low R/FR ratio (Pons 1992). Cecropia obtusifolia seeds germinated when the R/FR ratio increased as a conse-quence of canopy opening or litter disturbance (Vázquez-Yanes et al. 1990). Seeds of Ficus spp. reached their response threshold for germination at a low R/FR ratio. Although ger-mination of F.insipida was inhibited by FR in the laboratory, this was likely because the R/FR ratio used in the experiment was lower than the R/FR ratio encountered under field condi-tions (Vázquez-Yanes et al. 1990). We conclude that seeds of Ficus spp. will not exhibit long-lasting light-imposed dor-mancy when lying beneath a green canopy. Although a thin cover of litter or soil transmits light of low R/FR ratio, germi-nation of Ficus seeds will occur as has been observed in some Piper spp. (Orozco-Segovia and Vázquez-Yanes 1989) and in other species of Ficus (Raich and Gong 1990). Thus, pho-toblastism might not have any ecological significance in spe-cies that do not form seed banks.
Large gaps represent discontinuous safe sites for the estab-lishment of C. obtusifolia. Because gaps are suitable for colo-nization by heliophile plants only for a short time after their formation, the colonization of gaps requires both an abundant seed rain and the presence of seed in the soil. Seeds in the soil seed bank may be better suited physiologically to successful establishment than fresh seeds arriving through dispersal (Pons 1989).
Seedlings of Ficus spp. are seldom found in this forest, probably because they are small and easily covered by leaves. Seeds of two of the Ficus spp.showed a significant reduction in germination in the field experiment. This reduction probably resulted from the high temperatures and high R/FR ratios in the field. This response may reflect an adaptation to ensure that germination takes place beneath the canopy where environ-mental variables are more stable. Based on the germination rates of seeds of Ficus species in FR light, we estimated that the turnover rate of Ficus seeds in the soil was less than two months. Therefore, we conclude that, in the absence of seed dormancy, a species cannot form a permanent seed bank. Figure 1. Germination of seeds of (bars, left to right) Cecropia
Acknowledgments
The research was funded by CONACYT, México (400362-5-3380n). This support is deeply appreciated.
References
Alistair, J.M and R.H. Ellis. 1992. Longevity, viability and dormancy.
In Seeds, the Ecology of Regeneration in Plant Communities. Ed.
M. Fenner. C.A.B. International, Wallingford, pp 193--229. Alvarez-Buylla, E.R. and M. Martínez-Ramos. 1990. Seed bank
ver-sus seed rain in the regeneration of a tropical pioneer tree. Oecolo-gia 84:314--325.
Bartley M.R. and B. Frankland. 1984. Phytochrome intermediates and action spectra for light perception by dry seeds. Plant Physiol. 74:601--604.
Bongers, F., J. Popma , J. Meave del Castillo and J. Carabias. 1988. Structure and floristic composition of the lowland rain forest of Los Tuxtlas, Mexico. Vegetatio 74:55--80.
Enright, N. 1985. Existence of a soil seed bank under rain forest in New Guinea. Aust. J. Ecol 10:67--71.
Estrada, A., R. Coates-Estrada and C. Vázquez-Yanes. 1984. Observa-tions on fruiting and dispersers of Cecropia obtusifolia at Los
Tuxtlas, México. Biotropica 16:315--318.
Garwood, N.C. 1989. Tropical soil seed banks: A review. In Ecology
of Soil Seed Banks. Eds. A.M. Leck, V.T. Parker and R.L. Simpson. Academic Press, San Diego, pp 149--209.
Guevara, S. 1986. Plant species availability and regeneration in tropi-cal rain forest. Ph.D. Diss., Faculty of Sciences, Uppsala University, Uppsala, Sweden, 98 p.
Guevara, S. and J. Laborde. 1993. Monitoring seed dispersal at iso-lated standing trees in tropical pastures: consequences for local species availability. Vegetatio 107/108:319--338.
Hall, J.B. and M.D. Swaine. 1980. Seed stocks in Ghanaian forest soils. Biotropica 12:256--263.
Holthuijzen, A.M.A. and J.H.A. Boerboom. 1982. The Cecropia seed
Hopkins, M.S. and A.W. Graham. 1984. Viable soil seed banks in disturbed lowland tropical rain forest sites in North Queensland. Aust. J. Ecol. 9:71--79.
Orozco-Segovia, A. and C. Vázquez-Yanes. 1989. Light effect on seed germination in Piper L. Acta Oecol. Oecol. Plant. 10:123--146.
Orozco-Segovia, A., C. Vázquez-Yanes, M.A. Armella and N.A. Co-rrea. 1985. Interacciones entre una población de murciélagos de la especie Artibeus jamaicensis y la vegetación del área circundante
en la región de Los Tuxtlas, Veracruz. In Investigaciones sobre la
regeneración de selvas altas en Veracruz, México II. Eds. A. Gómez-Pompa and S. Del Amo. Alhambra Mexicana, México D.F., pp 365--377
Pons, T.L. 1989. Dormancy and germination of Calluna vulgaris (L.)
Hull and Erica tetralix L. seeds. Acta Oecol. Oecol. Plant. 10:35--43.
Pons, T.L. 1992. Seed responses to light. In Seeds: the Ecology of
Regeneration in Plant Communities. Ed. M. Fenner. C.A.B. Inter-national, Wallingford, UK, pp 259--284.
Putz, F.E. 1983. Treefall pits and mounds, buried seeds, and the importance of soil disturbance to pioneer trees on Barro Colorado Islands, Panama. Ecology 64:1070--1074.
Raich, J.W. and W.K. Gong. 1990. Effects of canopy openings on tree seed germination in a Malaysian dipterocarp forest. J. Trop. Ecol. 6:203--217.
Salmerón, R. 1984. Germinación de semillas acumuladas en el suelo de una selva húmeda tropical, los Tuxtlas, Veracruz, México. Tesis Profesional, Facultad de Ciencias UNAM, México, 79 p. Soto-Castro, A. 1992. Patrones especiales y temporales de la lluvia de
semillas de grupos de árboles en una selva húmeda de México. Tesis Profesional, Facultad de Ciencias UNAM, México, D. F., 66 p.
Titus, J.H., N.M. Holbrook and F.E. Putz. 1990. Seed germination and seedling distribution of Ficus pertusa and F. tuerckheimii: are strangler figs autotoxic? Biotropica 22:425--428.
Thompson, K. 1992. The functional ecology of seed banks. In Seeds:
the Ecology of Regeneration in Plant Communities. Ed. M. Fenner. C.A.B. International, Wallingford, UK, pp. 231--258
Ulh, C. and K. Clark. 1983. Seed ecology of selected Amazon basin successional species. Botan. Gaz. 141:419--425.
Valio, I.F.M. and C.A. Joly. 1979. Light sensitivity of the seeds on the distribution of Cecropia glaziovi Smethlage (Moraceae). Z.
Pflan-zenphysiol. 91:371--376.
Vázquez-Yanes, C. and A. Orozco-Segovia. 1990. Ecological signifi-cance of light controlled seed germination in two contrasting tropi-cal habitats. Oecologia 83:171--175.
Vázquez-Yanes, C. and A. Orozco-Segovia. 1993. Patterns of seed longevity and germination in the tropical rainforest. Annu. Rev. Ecol. Syst. 24:69--87.
Vázquez-Yanes, C. and A. Orozco-Segovia. 1994. Signals for seeds to sense and respond to gaps. In Exploitation of Environmental Het-erogeneity by Plants: Ecophysiological Processes Above and Below Ground. Ed. M. Caldwell and R. Pearcy. Academic Press, New York, pp. 261--318.
Vázquez-Yanes C., A. Orozco-Segovia, E. Rincón, M.E. Sánchez-Coronado, P. Huante, J.R. Toledo and V.L. Barradas. 1990. Light beneath the litter in a tropical forest: Effect on seed germination. Ecology 71:1952--1958.
Whitmore, T.C. 1983. Secondary succession from seed in tropical rain forest. Forest. Abst. 44:767--779.