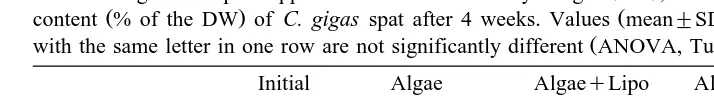www.elsevier.nlrlocateraqua-online
Incorporation of different fatty acids, supplied as
emulsions or liposomes, in the polar and neutral
lipids of Crassostrea gigas spat
M. Caers
), P. Coutteau, P. Sorgeloos
Laboratory of Aquaculture and Artemia Reference Center, UniÕersity of Ghent, Rozier 44,
B-9000 Gent, Belgium
Received 12 June 1999; received in revised form 18 November 1999; accepted 18 November 1999
Abstract
Ž . Ž .
Pacific oysters spat Crassostrea gigas were fed an algal diet Tetraselmis suecica whether or not supplemented with emulsions or liposomes rich in 18:1ny9, 18:2 ny6 and 22:6 ny3. The preferential accumulation and partitioning of the latter fatty acids between the polar and neutral lipids of C. gigas spat were followed. Additionally, the efficiency of emulsions and liposomes as fatty acid carriers for C. gigas spat were compared. The incorporation of dietary fatty acids was
Ž .
found to vary substantially according to the fatty acid carrier emulsions vs. liposomes , the
Ž . Ž
particular fatty acid 18:1ny9, 18:2 ny6 or 22:6 ny3 , and the lipid fraction neutral vs. polar
. Ž . Ž
lipids . A comparison of the percentage % of the total fatty acids and absolute concentration mg
y1 .
g dry weight of 18:1ny9, 18:2 ny6 and 22:6 ny3 in the total lipids of C. gigas fed solely T. suecica or lipid-supplemented diets, suggest the following order of preferential accumulation: 22:6 ny3)18:1ny9)18:2 ny6. Unlike 22:6 ny3 which was accumulated in both the neutral and polar lipid fraction of C. gigas, 18:2 ny6 and 18:1ny9 were mainly deposited in the neutral lipids. High dietary supplies of ny6 andror ny3 PUFA seriously altered the ny3rny6 PUFA ratio in the neutral as well as polar lipids of spat. The fatty acid profile of emulsion-fed spat indicated a rather limited ability to elongate 18:2 ny6 to 20:2 ny6 whereas no clear evidence for desaturation to 20:4 ny6 was observed. Irrespective of the diet, nonmethylene-interrupted-dienes
Ž .
and plasmalogens detected as dimethylacetals were abundant and located nearly exclusively in
Ž .
the polar lipids. The ash free dry weight AFDW of emulsion-fed spat was significantly higher than the AFDW of liposomes-fed spat which was in turn significantly higher than the AFDW of oysters fed solely algae. The growth and fatty acid composition of liposomes-fed oysters illustrated their inferior efficiency as compared with emulsions to supplement algal diets with fatty
)Corresponding author. Tel.:q32-9-2643754; fax:q32-9-2644193.
Ž .
E-mail address: [email protected] M. Caers .
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
acids. This could have been related to the small size of the liposomes vs. an emulsion.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Fatty acid; Lipid; Emulsion; Liposomes; Oyster; Crassostrea gigas
1. Introduction
Unlike in fish and shrimp lipid nutrition studies, where the use of formulated diets allows the preparation of diets with a specific amount of virtually all important fatty acids, bivalve studies are mainly performed by feeding different algal diets selected on the basis of their species-dependent fatty acid composition. Although fatty acid composi-tions of algae can be modified by altering culture condicomposi-tions, nutritional studies are
Ž
compromised by this approach relative to formulated diets Enright et al., 1986;
.
Thompson and Harrison, 1992; Thompson et al., 1993 . Bivalve nutrition studies have
Ž .
mainly focussed on ny3 polyunsaturated fatty acids PUFA whereas the ny6 PUFA have received very little attention. Moreover, with a few exceptions, they only report the impact of the diet on the fatty acid composition of the total lipids. However, considering
Ž . Ž
the different function of the polar mainly structural phospholipids and neutral mainly
.
storage triglycerides lipids, it is important to consider both lipid fractions.
Compared to emulsions, liposomes have the advantage that they can be used as an encapsulation technique for lipid as well as water-soluble components. We opted to use the pro-liposome technique since pro-liposomes are a stable product that can easily be transported and the conversion into discrete liposomes and the loading with active
Ž .
ingredients can be carried out locally using simple equipment Arnaud, 1993 . There-fore, the pro-liposome technique holds promise for application in laboratory as well as commercial hatcheries. Although pro-liposomes have been successfully used to enrich
Ž .
Artemia with vitamin C Merchie, 1995 , they have not been used for bivalves.
Ž .
In the present study, lipid emulsions were used to supply ny6 18:2 ny6 and
Ž . Ž .
ny3 PUFA 22:6 ny3 to an algal diet Tetraselmis suecica containing only 2.6% 18:2 ny6 and no 22:6 ny3. The fatty acids 18:2 ny6 and 22:6 ny3 were supplied as liposomes or emulsions and the partitioning of these fatty acids between the polar and
Ž .
neutral lipids of Crassostrea gigas Thunberg spat were followed. Additionally, the efficiency of emulsions and liposomes as fatty acid carriers for C. gigas spat were
Ž
compared. Previous studies indicated that ethyl esters of docosahexaenoic acid DHA,
.
22:6 ny3 can be used to illustrate the digestion and assimilation of lipid supplements since 22:6 ny3, supplied as ethyl esters, was clearly accumulated by larvae, spat and
Ž
adults of various bivalve species Coutteau et al., 1994, 1996; Caers et al., 1998,
. Ž .
1999a,c . Therefore, pro-liposomes based on soybean phosphatidylcholine and
emul-Ž .
sions based on soybean triglycerides were enriched with 22:6 ny3 ethyl esters.
2. Materials and methods
2.1. Diets
T. suecica was cultured in 4-l bottles in 0.22-mm filtered seawater enriched with
Ž
.
Caers et al., 1998 . They were harvested daily in the exponential growth phase and counted with a haemocytometer. Weekly, samples were removed for lipid analysis.
Ž
The experimental ICES emulsions Em1 and Em2 ICES, International Council for the
.
Exploration of the Sea prepared by INVE Technologies, Baasrode, Belgium, contained
Ž .
50% lipid soybean oil and 22:6 ny3 ethyl esters, Itochu-Chemicals, Japan on a wet
Ž . Ž
weight WW basis, water, emulsifiers, antioxidants, preservatives and vitamin E 0.1%
. Ž .
of the total lipids ICES, 1997 . The size distribution of the lipid emulsions was determined with a Malvern Mastersizer S long bed with an automated sample dispersion
Ž . Ž .
unit MSX-17 , 300 RF lens, optic model olive oil in water, 3NAD : Particle R.I.s Ž1.4564, 0.0000 , Dispersant R.I.. s1.3300. The particle size of both emulsions was log-normally distributed with 50% between 1 and 10 mm.
Ž .
Pro-liposomes Lucas Meyer France, Chelles Cedex, France mainly consisted of a mixture of phospholipid, principally unsaturated soybean phosphatidylcholine and small quantities of charged lipids, dispersed in aqueous ethanol. A mixture of 22:6 ny3 ethyl esters, vitamin E and ethoxyquin were mixed with the pro-liposome gel. The conversion into a fully loaded concentrated liposome mixture was done by gradually adding a 3% aqueous NaCl solution. This resulted in liposomes with a lipid content of 9.8% and a vitamin E and ethoxyquin concentration of 0.1% and 0.88% of the total lipids,
Ž .
respectively. The fatty acid composition of T. suecica ns4 and the lipid supplements
Žns3 are given in Table 1..
2.2. Culture conditions and experimental design
Ž .
Pacific oyster spat C. gigas were obtained from Guernsey Sea Farms, UK in February 1996. The system for rearing spat consisted of 12 aquaria placed in a water bath at 218C. Each 5-l aquarium contained one down-welling silo and one point aeration to keep the food in suspension. The spat were fed twice a day for 4 weeks, the water
Ž .
was renewed three times per week Caers et al., 1998 . T. suecica was fed at a
Ž . Ž Ž . Ž . .
weight-specific feeding ration FR % dry weight DW per wet weight WW per day
y1 y1 Ž .
of 1% DW WW day . The algal diet was fed alone Algae or supplemented with
Ž . Ž . Ž .
liposomes AlgaeqLipo , emulsion 1 AlgaeqEm1 or emulsion 2 AlgaeqEm2 at a
Ž .
concentration of 50% lipid expressed as percentage of total algal DW . All treatments were run in triplicate aquaria. The spat were stocked at an initial concentration of 0.75 g WW per silo and every week were rinsed with tap water, blotted dry with paper towel,
Ž .
weighed and restocked at 0.75 g per silo WW . The amount of food was adjusted dailyi
Ž .
to keep the FR constant as previously described in Caers et al. 1999c .
2.3. Biochemical analysis
Before sampling, spat were starved for 24 h to empty their guts and the number of oysters in each silo was counted. The total weight of the freeze-dried oysters of each silo was determined after which the oysters were pulverised in a mortar. For each silo, three
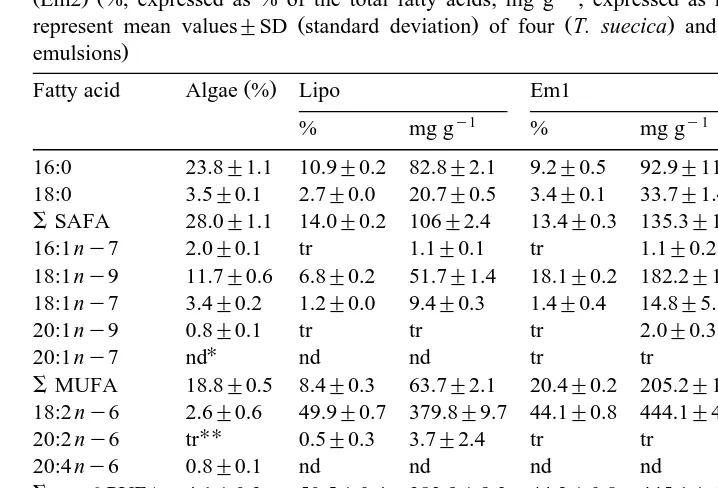
Ž . Ž
Table 1
Ž . Ž . Ž .
Fatty acid composition Tetraselmis suecica Algae , liposomes Lipo and emulsions 1 Em1 and emulsion 2
ŽEm2. Ž%, expressed as % of the total fatty acids; mg gy1, expressed as mg fatty acids per g lipid . Data.
Ž . Ž . Ž
represent mean values"SD standard deviation of four T. suecica and three replicates liposomes and
.
emulsions
Ž .
Fatty acid Algae % Lipo Em1 Em2
y1 y1 y1
at 4508C determinations, the rest of the powder was used for lipid extraction. The ash
Ž .
free dry weight AFDW defines the organic matter content of the spat and was calculated as the difference between DW and ash and expressed as percentage of the DW.
Total lipids of freeze-dried and pulverised samples of the diets and oysters were
Ž .
extracted with chloroform and methanol 2:1, vrv and determined gravimetrically.
Ž . Ž .
Fatty acid methyl esters FAME and dimethyl acetals DMA of total lipid were
Ž .
prepared by transmethylation with a mixture of sulfuric acid and methanol 1:100, vrv for 16 h at 508C, using 22:4 ny6 as the internal standard. Neutral and polar lipids were
Ž .
separated on a silicic acid column according to the method of Marty et al. 1992 . The
Ž
fatty acid and DMA compositions were determined as previously described Caers et al.,
.
1998, 1999a,c .
2.4. Statistical analysis
Ž .
Statistical analysis included one way analysis of variance ANOVA and Tukey’s
Ž .
-Ž .
TICA Microsoft, Statsoft . The homogeneity of the variances of means was checked by Bartlett’s x2
-test. Arcsine 6 transformation was used prior to statistical analyses of
Ž .
percentage data Sokal and Rohlf, 1995 .
3. Results
3.1. Fatty acid composition of the diets
Ž .
The major saturated fatty acids SAFA of T. suecica were 16:0, 18:0, the
monoun-Ž .
saturated fatty acids MUFA were dominated by 18:1ny9 whereas 18:3ny3, 18:4 n
Ž .
y3 and 20:5ny3 were the major PUFA Table 1 . The predominant fatty acids in both
Ž .
emulsions were 18:1ny9, 18:2 ny6 and 22:6 ny3 Table 1 . Em2 was prepared to
Ž
contain similar amounts of 18:2 ny6 and 22:6 ny3 34% of the total fatty acids or 318 y1.
mg g lipid whereas the 22:6 ny3 content of Em1 was similar to the 22:6 ny3
Ž y1 .
content of the liposomes 158 and 153 mg g lipid , respectively . The fatty acid profile of the liposomes was dominated by 22:6 ny3 and especially 18:2 ny6 which compro-mised 20% and 50%, respectively, of the total fatty acids. Compared to the emulsions, the percentage of 18:1ny9 was approximately three times lower. Except for 22:6 ny3, which was present as ethyl esters in the liposomes and the emulsions, fatty acids of the liposomes were supplied as phosphatidylcholine whereas fatty acids of the emulsion were supplied as triglycerides.
The total amount of lipid supplemented to the algal diet via the liposomes or
Ž .
emulsions was similar 50% of the dry weight of the algal diet . However, transesterifi-cation of 1 g of triglycerides or ethyl esters yields a higher amount of FAME than transesterification of 1 g of phosphatidylcholine. Therefore, the total amount of fatty
Ž . Ž .
acids mg supplied in via the liposome diet AlgaeqLipo was much lower than the
Ž
total amount of fatty acids supplied via the emulsion diets AlgaeqEm1 and Algaeq
. Ž .
Em2 Table 1 .
3.2. Fatty acid composition of spat fed solely T. suecica
The predominant fatty acids in the neutral lipids of spat fed solely T. suecica were
Ž .
16:0, 18:1ny9, 18:3ny3 and 20:5ny3 Tables 2 and 3 . In the polar lipids, the
Ž . Ž .
abundance of the nonmethylene-interrupted dienes NMID and DMA mainly 18:0 was compensated by a decrease of the percentage of total MUFA, mainly 18:1ny9. The percentage of 22:6 ny3 was twice as high in the polar lipids than in the neutral lipids
ŽTables 2 and 3 ..
3.3. Fatty acid composition of emulsion-fed spat
The percentage of 18:1ny9, 18:2 ny6 and 22:6 ny3 was significantly higher in
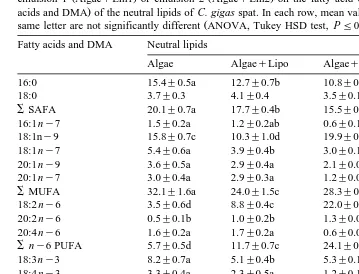
Ž .
Table 2
Ž . Ž .
Effect of feeding 1% T. suecica Algae , whether or not supplemented with liposomes AlgaeqLipo ,
Ž . Ž . Ž
emulsion 1 AlgaeqEm1 or emulsion 2 AlgaeqEm2 on the fatty acid composition % of the total fatty
. Ž .
acids and DMA of the neutral lipids of C. gigas spat. In each row, mean values ns3"SD followed by the
Ž .
same letter are not significantly different ANOVA, Tukey HSD test, PF0.05 Fatty acids and DMA Neutral lipids
gigas fed solely T. suecica Algae . The greatest accumulations of 18:1ny9, 18:2 ny6 and 22:6 ny3 were observed in the neutral lipids of spat fed the emulsion with the
Ž
highest concentration of these fatty acids Em1 for 18:1ny9 and 18:2 ny6, Em2 for
.
22:6 ny3 . The same situation was observed in the percentage and absolute
concentra-Ž y1.
tion mg g DW of 18:1ny9 and 18:2 ny6 and 22:6 ny3 in the total lipids of C.
Ž .
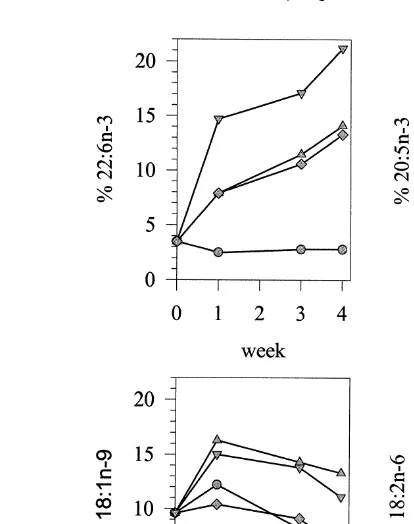
gigas Tables 2–4; Fig. 1A, B, C and D . From the first week onwards, Em 1 and Em2
supplements resulted in a drastic elevation of the percentage of 22:6 ny3 in the total
Ž .
lipids of C. gigas and this remained the case until the end of the experiment Fig. 1A . For treatments consisting of either emulsion, a 50% reduction of 20:5ny3 was observed in the total lipids of C. gigas during the first week after which it remained
Ž .
quite constant at approximately 8% Fig. 1B . As with 22:6 ny3, a profound increase in 18:1ny9 and 18:2 ny6 occurred in the first week. However, a parallel decrease in the
Ž
proportions of both fatty acids took place during the last 3 weeks of the experiment Fig.
.
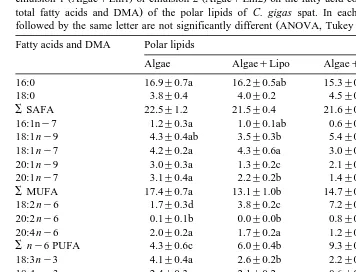
Table 3
Ž . Ž .
Effect of feeding 1% T. suecica Algae , whether or not supplemented with liposomes AlgaeqLipo ,
Ž . Ž . Ž
emulsion 1 AlgaeqEm1 or emulsion 2 AlgaeqEm2 on the fatty acid composition expressed as % of the
. Ž .
total fatty acids and DMA of the polar lipids of C. gigas spat. In each row, mean values"SD ns3
Ž .
followed by the same letter are not significantly different ANOVA, Tukey HSD test, PF0.05 Fatty acids and DMA Polar lipids 18:0DMA 11.4"0.4b 13.1"0.4a 13.4"0.4a 12.6"0.9ab
SDMA 13.5"0.5a 15.5"0.5b 15.2"0.5b 14.7"0.6ab
Ž . Ž . Ž
22:6 ny3 Em2 , a similar increase 15% of both fatty acids compared to spat fed
.
solely T. suecica was observed in the neutral lipids of C. gigas whereas a clear
Ž .
preferential incorporation 17% vs. 3% of 22:6 ny3 compared to 18:2 ny6 occurred
Ž .
in the polar lipid fraction Tables 2 and 3 . Supplementing similar amounts of 18:1ny9
Ž18% and 22:6 n. y3 16%Ž . ŽEm1 resulted in a significant increase the percentage of. Ž
22:6 ny3 in the polar and neutral lipids of AlgaeqEm1-fed spat compared to spat fed
.
solely T. suecica while a significant augmentation of the percentage of 18:1ny9 only
Ž .
took place in the neutral lipid fraction Tables 2 and 3 .
When the fatty acid composition is expressed as percentage of the total fatty acids
Ž
and DMA, a strong increase of one fatty acid such as the percentage of 18:1ny9,
.
18:2 ny6 and especially 22:6 ny3 in emulsion-fed oysters inevitably results in a reduction of the percentage of other fatty acids. This explains why, e.g., the percentage
Ž
of 16:0 in total lipids of emulsion-fed oysters decreased 15.4%, 12.4% and 12.1% in
.
Algae, AlgaeqEm1 and AlgaeqEm2-fed oysters, respectively even though the
Ž y1. Ž .
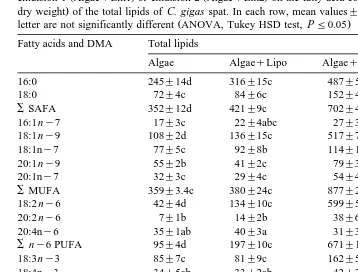
Table 4
Ž . Ž .
Effect of feeding 1% T. suecica Algae , whether or not supplemented with liposomes AlgaeqLipo ,
Ž . Ž . Ž
emulsion 1 AlgaeqEm1 or emulsion 2 AlgaeqEm2 on the fatty acid composition expressed asmg per g
. Ž .
dry weight of the total lipids of C. gigas spat. In each row, mean values"SD ns3 followed by the same
Ž .
letter are not significantly different ANOVA, Tukey HSD test, PF0.05 Fatty acids and DMA Total lipids
20:5ny3 260"19b 267"20b 315"20a 298"20ab 22:6 ny3 44"5d 283"23c 552"43b 726"18a
or Em2 also significantly reduced the percentage of 20:4 ny6 from 2.2% to 0.8% and
. Ž .
1.0%, respectively and 20:5ny3 from 16.3% to 8.1% and 8.7%, respectively in the total lipids of C. gigas while the concentration of the latter two fatty acids was not
Ž .
affected Table 4 . Similarly, the five-fold increase of the 20:2 ny6 tissue concentration
Ž .
in emulsion-fed oysters Table 4 was limited to a two-fold increase in the percentage of
Ž .
20:2 ny6 from 0.5% to 1.0% and 1.2%, respectively .
3.4. Fatty acid composition of liposome-fed spat
Although the concentration of 22:6 ny3 was similar in Em1 and the liposomes
Ž152.6 and 157.8 mg gy1., the 22:6 ny3 concentration was twice as high in Algaeq
Ž y1. Ž y1.
Em1-fed oysters 552 mg g than in AlgaeqLipo-fed oysters 283 mg g . The supplementation of liposomes caused a significant increase of the percentage of 18:2 ny
Ž . Ž . Ž .
Fig. 1. Evolution of the percentage % of the total fatty acids and DMA of 22:6 ny3 A , 20:5ny3 B ,
Ž . Ž . Ž .
18:1ny9 C and 18:2 ny6 D in the total lipids of C. gigas spat fed T. suecica Algae , whether or not
Ž . Ž . Ž .
supplemented with liposomes AlgaeqLipo , emulsion 1 AlgaeqEm1 or emulsion 2 AlgaeqEm2 . Data represent the mean of three replicates, SD never exceeded 10% of the mean and were omitted for clarity.
did not clearly reflect the abundance of 18:2 ny6 in the liposomes. Feeding Em1
Ž44.1% 18:2 ny6 , resulted in a much higher percentage of 18:2 n. y6 in the polar Ž7.2% and neutral 22.0% lipids of C. gigas than feeding liposomes 49.9% 18:2 n. Ž . Ž y6. Ž3.8% and 8.8% 18:2 ny6 in the polar and neutral lipids, respectively . Similarly,.
Ž y1.
despite the higher 18:2 ny6 concentration in the liposomes 380 mg g than in Em2
Ž317 mg gy1., spat fed the latter supplement contained 599 mg gy1 of 18:2 n
y6
compared to 134mg gy1 in Algae
qLipo-fed oysters.
3.5. Growth and surÕiÕal
No mortality was observed during the experimental period of 4 weeks. The supple-mentation of liposomes caused a significant increase in AFDW per oyster but DW and
Ž .
Table 5
Ž . Ž .
Effect of algal and lipid supplemented diets on the dry weight DW , ash free dry weight AFDW and ash
Ž . Ž .
content % of the DW of C. gigas spat after 4 weeks. Values mean"SD from three replicates indicated
Ž .
with the same letter in one row are not significantly different ANOVA, Tukey HSD test, PF0.05
Initial Algae AlgaeqLipo AlgaeqEm1 AlgaeqEm2
Ž .
DWroyster mg 4.3"0.2 11.9"0.9b 12.7"0.9b 18.2"1.8a 17.2"1.0a
Ž .
AFDWroyster mg 265"10 694"22c 851"56b 1362"54a 1285"83a
Ž .
AshrDW % 94.6"0.2 94.5"0.3a 93.9"0.1a 92.9"0.5b 93.0"0.1b
significantly lower than in spat fed T. suecica whether or not supplemented with
Ž .
liposomes Table 5 .
4. Discussion
It is well established that nonmethylene-interrupted-dienes are abundant and located
Ž .
nearly exclusively in the polar lipid fraction of many bivalve species Joseph, 1989 . Their precise role is unknown but the association with membrane lipids and the selective retention in starved animals suggests a structuralrmetabolic function andror a strong
Ž
resistance against degradation Klingensmith, 1982; Joseph, 1989; Thompson and
.
Harrison, 1992; Fang et al., 1993 . The abundance of plasmalogen in bivalve polar lipids has been reported but information concerning their function, metabolism and fatty acid
Ž .
chain composition is still very limited Joseph, 1989; Jeong et al., 1999 . In agreement
Ž
with recent information on the alkenyl chain composition of C. gigas Koizumi et al.,
. Ž . Ž
1990 , Argopecten purpuratus Caers et al., 1999a,b , Patinopecten yessoensis Jeong
. Ž .
et al., 1999 and Tapes philippinarum Caers et al., 1999c , the quantitatively most important alkenyl chain in the polar lipids of C. gigas was 18:0 but the biological significance of these observations remains to be elucidated.
The present study showed a fatty acid-specific partitioning of dietary lipid between the polar and neutral lipid fractions of spat. The preferential accumulation of 22:6 ny3
Ž .
in the polar lipids was also observed in clam spat Caers et al., 1999c . As for NMID, our data support the idea of an important structuralrmetabolic function for 22:6 ny3 in the membrane-associated lipids. The importance of 22:6 ny3 is further strengthened by
Ž
its selective retention in starved animals and those fed 22:6 ny3-deficient diets Cout-teau et al., 1996; Knauer and Southgate, 1997a,b; Caers et al., 1998, 1999c and present
.
study . Information related to diet-induced changes in the fatty acid composition of spat tissue during the experimental feeding period is limited to the work of Albentosa et al.
Ž1994 with Venerupis pullastra. In the present study, acclimatisation for 1 week on T.. suecica was sufficient to obtain a stable percentage of 22:6 ny3 throughout the next 4 weeks of culture. After feeding T. suecica for 4 weeks, the final percentage of
Ž . Ž .
22:6 ny3 in C. gigas 3% was higher than in V. pullastra spat 0.27% but confirms
Ž . Ž
data obtained with T. philippinarum 2.7% cultured in similar conditions Caers et al.,
. Ž
1999c . Consistent with other studies Langdon and Waldock, 1981; Thompson et al.,
.
diet was shown to directly affect the percentage of 22:6 ny3 in the total lipids of larvae and spat of bivalves. However, the percentage of 22:6 ny3 in gelatin acacia
microcap-Ž .
sules GAM which varied from 0.0% to 78.5%, was not correlated with its level in
Ž .
GAM-fed C. gigas spat Knauer and Southgate, 1997a,b . Unlike 22:6 ny3, both 18:2 ny6 and 18:1ny9 were mainly deposited in the neutral lipids where they can serve as an energy substrate or be reused for the synthesis of new fatty acids. The limited impact of 18:2 ny6 and 18:1ny9 supplementation on the polar compared to the neutral lipids confirms the widely observed conservative nature of phospholipids in
Ž .
marine organisms Beninger and Stephan, 1985; Joseph, 1989; Sargent et al., 1993 . A clear preferential incorporation of 22:6 ny3 compared to 18:2 ny6 in the total lipids was demonstrated in emulsion-fed and especially liposomes-fed C. gigas. As shown in Fig. 1, the gradual decrease of the percentage of 18:2 ny6 and 18:1ny9 was accompanied by a gradual increase of the percentage of 22:6 ny3 in spat fed lipid supplemented diets. When equal amounts of emulsified 22:6 ny3 and 18:2 ny6 were
Ž .
supplied and only half as much 18:1ny9 AlgaeqEm1 , spat accumulated twice as much 22:6 ny3 than 18:2 ny6, whereas the final 18:2 ny6 and 18:1ny9
concentra-Ž .
tions were similar Table 4 . Our results thus suggest the following order of preferential accumulation: 22:6 ny3)18:1ny9)18:2 ny6. At the contrary, Knauer and
South-Ž .
gate 1997a,b did not observe a preferential 22:6 ny3 accumulation in C. gigas spat fed 22:6 ny3-rich GAM.
Information on the ny6 PUFA requirements of bivalves is limited. Trider and
Ž .
Castell 1980 were the first to mention that oysters have a requirement for both ny3 and ny6 PUFA, with the former probably being more important. Recently, Mai et al.
Ž1996 suggested that juvenile abalone Haliotis tuberculata and H. discus hannai have.
a requirement for both ny3 and ny6 PUFA, including 18:2 ny6. On the other hand,
Ž .
Enright et al. 1986 suggested that the ny3rny6 ratio of algal diets should be higher
Ž .
than 2 to obtain optimal growth for Ostrea edulis spat. Numaguchi and Nell 1991 and
Ž .
Knauer and Southgate 1997a,b obtained better growth rates in oyster larvae and spat when the algal diet was supplemented with ny3 PUFA-rich than with ny6 PUFA-rich lipids. In the present experiment, it is not known whether the improved growth rate of
Ž .
emulsion-fed C. gigas as compared to C. gigas fed solely T. suecica is related to the
Ž . Ž .
quantity extra supply of energy andror quality fatty acid composition of dietary lipids. Doubling the dietary 22:6 ny3 supply from 16% or 158 mg gy1 in Em1 to 34% or 318 mg gy1 in Em2 did not further enhance growth of C. gigas spat. It supports the hypothesis of the existence of a threshold level beyond which more essential fatty acids
Ž
such as 22:6 ny3 will not improve growth nor survival Thompson and Harrison, 1992;
.
Caers et al., 1999c .
Fish fed 18:2 ny6-rich diets could be more susceptible to stress, diseases and
Ž .
pollutants Sargent et al., 1993; Thompson et al., 1996 and recent studies reported that
Ž .
increased amounts of ny6 PUFA in the polar lipids of oyster C. gigas and O. edulis
( .
and clam T. philippinarum and T. decussatus spat appeared to indicate a response to
Ž .
stress factors Laing and Child, 1996; Child and Laing, 1998 . However, the reduction of the ny3rny6 ratio in the polar lipids of C. gigas from 7.5 to 3.4 or 4.9
Žrespectively by supplementation of Em1 or Em2 did not have an adverse impact on.
reflected in the fatty acid composition of spat fed these lipid supplements, the growth rate was similar in both treatments. Similarly, supplementing algal diets with 18:2 ny
6-Ž .
rich GAM based on soybean or corn oil did not reduce growth or survival of oyster
Ž . Ž
larvae Saccostrea commercialis, Numaguchi and Nell, 1991 or spat C. gigas, Knauer
.
and Southgate, 1997a,b . Nevertheless, an excessive dietary 18:2 ny6 supply may affect bivalve health under stressful conditions or when evaluated over a longer period of time.
Ž . Ž
The present study supports the findings of Waldock and Holland 1984 juvenile C.
. Ž . Ž .
gigas , Chu and Greaves 1991 adult C. Õirginica and Knauer and Southgate
Ž1997a,b. ŽC. gigas spat which indicated that oysters have a rather limited ability to.
elongate 18:2 ny6 to 20:2 ny6 whereas no clear evidence for desaturation to 20:4 ny6 was observed. Although an exceptionally high conversion of 18:2 ny6 to 20:2 ny6
Ž .
was recorded by deMoreno et al. 1976 for the adult clam Mesoderma mactroides, desaturation to 20:4 ny6 was absent.
Contrary to expectation, a two-fold increase of the 16:0 and 18:0 concentration was observed in the total lipids of AlgaeqEm1-fed spat although 18:0 was only a minor
Ž .
component in the lipid supplement 3.4% . Besides the impact of environmental parameters, the physiological condition of the animal and the concentration and formula-tion in the diet, several other factors affect the final whole-tissue concentraformula-tion of a
Ž . Ž .
particular fatty acid such as the extend of 1 de novo synthesis from acetate, 2
Ž .
elongation and desaturation of precursor fatty acids, 3 partial breakdown or complete
Ž .
oxidation to CO and 4 the degree of preferential accumulation compared to other fatty2 acids. Radiolabeled 16:0 and 18:0 in animals fed 14C-labeled 18:2 ny6 provided evidence that de novo synthesis occurred with reutilization of14C-acyl groups derived
Ž
from ß-oxidation in adult oysters and clams deMoreno et al., 1976, 1977; Chu and
.
Greaves, 1991 . Possibly, this process has contributed to the relative high 16:0 and 18:0 concentrations found in lipid-supplemented C. gigas in the present study.
The significantly higher absolute concentrations of 18:2 ny6 and 22:6 ny3 in
Ž
liposome-fed spat than in spat fed only T. suecica indicates that the lipids ethyl esters
.
and phosphatidylcholine supplied via the liposomes could at least partially be digested
Ž .
and assimilated by the spat. This confirms the results of Parker and Selivonchick 1986 who demonstrated the digestion and assimilation of 14C and fluorescence labelled
Ž . Ž .
liposomes by juvenile C. gigas 15–25 mm . Vaskovsky and Suppes 1972 showed phospholipase A activity for a phosphatidylcholine substrate in the hepatopancreas of 12
Ž . Ž 14 .
bivalves, including C. gigas. Later, Parker and Selivonchick 1986 found that 1- C dipalmitoyl phosphatidylcholine, supplied as liposomes, was redistributed into the oyster triglycerides and phospholipids. On the other hand, our data clearly showed that when equal amounts of 18:2 ny6 or 22:6 ny3 were supplied via liposomes and emulsions, the accumulation of 18:2 ny6 or 22:6 ny3 was much lower in liposome-fed oysters than in emulsion-fed oysters. The liposomes used in the present experiment were
Ž
normally distributed with 50% between 225 and 250 nm Lucas Meyer, France, personal
.
communication which is small compared to the liposomes prepared by Parker and
Ž . Ž . Ž .
Selivonchick 1986 0.4–8.0 mm , the lipid emulsions 50% between 1 and 10mm or
Ž .
carriers. Finally, the results confirm that oyster spat are not an exception to earlier observations that emulsions are an acceptable lipid supplement for various live stages
Ž .
and species of bivalves Coutteau et al., 1994, 1996; Caers et al, 1998, 1999a,c .
Acknowledgements
Ž .
This work was supported by a grant from the Flemish government IWT to Marrit
Ž
Caers. The help of Mohamed Abdul Hasanat MSc student at the ARC, Oct. 1994–Sept.
.
1996 for culturing algae and oysters is appreciated. We would like to thank Dr. P. Van
Ž
der Meeren and ir. P. Spanoghe Particle and Interfacial Technology Group, Faculty
.
Agricultural and Applied Biological Sciences, University of Ghent for the determina-tion of the particle size of the emulsions.
References
Albentosa, M., Labarta, U., Perez-Camacho, A., Fernandez-Reiriz, M.J., Beiras, R., 1994. Fatty acid´ ´ composition of Venerupis pullastra spat fed on different microalgae diets. Comp. Biochem. Physiol. 108A, 639–648.
Arnaud, J.-P., 1993. In: Liposomes. The Pro-Liposome Approach Vol. 14 Lucas Meyer, Hamburg, pp. 1–29. Beninger, P.G., Stephan, G., 1985. Seasonal variations in the fatty acids of the triacylglycerols and
Ž .
phospholipids of two populations of adult clam Tapes decussatus L. and T. philippinarum reared in a common habitat. Comp. Biochem. Physiol. 81B, 591–601.
Caers, M., Coutteau, P., Cure, K., Morales, V., Gajardo, G., Sorgeloos, P., 1999a. The Chilean scallop
Ž .
Argopecten purpuratus Lamarck, 1819 : I. Fatty acid composition and lipid content of six organs. Comp.
Biochem. Physiol. 123B, 89–96.
Caers, M., Coutteau, P., Cure, K., Morales, V., Gajardo, G., Sorgeloos, P., 1999b. The Chilean scallop
Ž .
Argopecten purpuratus Lamarck, 1819 : II. Manipulation of the fatty acid composition and lipid content
of the eggs via lipid supplementation of the broodstock diet. Comp. Biochem. Physiol. 123B, 97–103. Caers, M., Coutteau, P., Lombeida, P., Sorgeloos, P., 1998. The effect of lipid supplementation on the growth
Ž .
and fatty acid composition of Tapes philippinarum L. spat. Aquaculture 162, 287–299.
Caers, M., Coutteau, P., Sorgeloos, P., 1999c. Dietary impact of algal and artificial diets, fed at different
Ž .
feeding rations, on the growth and fatty acid composition of Tapes philippinarum L. spat. Aquaculture 170, 307–322.
Child, A.R., Laing, I., 1998. Comparative low temperature tolerance of small juvenile European, Ostrea edulis L., and Pacific oysters, Crassostrea gigas Thunberg. Aquacult. Res. 29, 102–113.
Chu, F.E., Greaves, J., 1991. Metabolism of palmitic, linoleic and linolenic acids in adult oysters, Crassostrea Õirginica. Mar. Biol. 110, 229–236.
Ž .
Coutteau, P., 1996. Micro-algae. In: Lavens, P., Sorgeloos, P. Eds. , Manual on the Production and Use of Live Food for Aquaculture. FAO, Rome, pp. 7–48, FAO Fisheries Technical Paper. No. 361.
Coutteau, P., Caers, M., Mallet, A., Moore, W., Manzi, J.J., Sorgeloos, P., 1994. Effect of lipid
supplementa-Ž
tion on growth, survival and fatty acid composition of bivalve larvae Ostrea edulis L. and Mercenaria
. Ž .
mercenaria L. . In: Kestemont, P., Muir, J., Sevilla, F., Williot, P. Eds. , Proceedings of Bordeaux
Aquaculture ’94. CEMAGREF, France, pp. 213–218.
Coutteau, P., Castell, J.D., Ackman, R.G., Sorgeloos, P., 1996. The use of lipid emulsions as carriers for essential fatty acids in bivalves: a test case with juvenile Placopecten magellanicus. J. Shellfish Res. 15, 259–264.
deMoreno, J.E.A., Moreno, V.J., Brenner, R.R., 1976. Lipid metabolism of the yellow clam, Mesoderma
deMoreno, J.E.A., Moreno, V.J., Brenner, R.R., 1977. Lipid metabolism of the yellow clam, Mesoderma
mactroides: 3. Saturated fatty acids and acetate metabolism. Lipids 12, 804–808.
Enright, C.T., Newkirk, G.F., Craigie, J.S., Castell, J.D., 1986. Growth of juvenile Ostrea edulis L. fed
Chaetoceros gracilis Schutt of varied chemical composition. J. Exp. Mar. Biol. Ecol. 96, 15–26.
Fang, J., Comet, P.A., Brooks, J.M., Wade, T.L., 1993. Nonmethylene-interrupted fatty acids of hydrocarbon seep mussels: occurrence and significance. Comp. Biochem. Physiol. 104B, 287–291.
ICES, 1997. Report of the Working Group on Marine Fish Culture for the International Council for the Exploration of the Sea. Murcia, Spain, 23–26 June 1997. ICES, CM 1997rF:7.
Jeong, B.Y., Ohshima, T., Koizumi, C., 1999. Changes in the fatty chain compositions of lipid classes during
Ž .
frozen storage of the adductor muscle of giant ezo scallop Patinopecten yessoensis . Comp. Biochem. Physiol. 122B, 415–422.
Ž .
Joseph, J.D., 1989. Marine invertebrates. In: Ackman, R.G. Ed. , Marine Biogenic Lipids, Fats, and Oils. CRC Press, Boca Raton, FL, pp. 49–143.
Klingensmith, J.S., 1982. Distribution of methylene and nonmethylene-interrupted dienoic fatty acids in polar
Ž .
lipids and triacylglycerols of selected tissues of the hard clam Mercenaria mercenaria . Lipids 17,
976–981.
Ž .
Knauer, J., Southgate, P.C., 1997a. Growth and fatty acid composition of Pacific oyster Crassostrea gigas
Ž .
spat fed a spray-dried freshwater microalga Spongiococcum excentricum and microencapsulated lipids. Aquaculture 154, 293–303.
Ž .
Knauer, J., Southgate, P.C., 1997b. Growth and fatty acid composition of Pacific oyster Crassostrea gigas spat fed a microalga and microcapsules containing varying amounts of eicosapentaenoic and docosahex-aenoic acid. J. Shellfish Res. 16, 447–453.
Koizumi, C., Jeong, B.Y., Ohshima, T., 1990. Fatty chain composition of ether and ester glycerophopholipids
Ž .
in the Japanese oyster Crassostrea gigas Thunberg . Lipids 25, 363–370.
Ž .
Laing, I., Child, A.R., 1996. Comparative tolerance of small juvenile palourdes Tapes decussatus L. and
Ž .
Manila clams Tapes philippinarum Adams and Reeve to low temperature. J. Exp. Mar. Biol. Ecol. 195, 267–285.
Langdon, C.J., Waldock, M.J., 1981. The effect of algal and artificial diets on growth and fatty acid composition of Crassostrea gigas spat. J. Mar. Biol. Assoc. U.K. 61, 431–448.
Mai, K., Mercer, J.P., Donlon, J., 1996. Comparative studies on the nutrition of two species of abalone,
Haliotis tuberculata L. and Haliotis discus hannai Ino: V. The role of polyunsaturated fatty acids of
macroalgae in abalone nutrition. Aquaculture 139, 77–89.
Marty, J., Delaunay, F., Moal, J., Samain, J.F., 1992. Changes in the fatty acid composition of Pecten
Ž .
maximus L. during larval development. J. Exp. Mar. Biol. Ecol. 163, 221–234.
Merchie, G., 1995. Nutritional effect of vitamin C on the growth and physiological condition of the larvae of aquaculture organisms. PhD Thesis, University of Ghent, Ghent, Belgium.
Numaguchi, K., Nell, J.A., 1991. Effect of gelatin–acacia microcapsule and algal meal supplementation of
Ž .
algal diets on growth rates of Sydney rock oyster, Saccostrea commercialis Iredale and Roughley , larvae. Aquaculture 94, 65–78.
Parker, R.S., Selivonchick, D.P., 1986. Uptake and metabolism of lipid vesicles from seawater by juvenile
Crassostrea gigas. Aquaculture 53, 215–228.
Sargent, J.R., Bell, J.G., Bell, M.V., Henderson, R.J., Tocher, D.R., 1993. The metabolism of phospholipid and polyunsaturated fatty acids in fish. In: Aquaculture: Fundamental and Applied Research. Lahlou, B.,
Ž .
Vitiello, P. Eds. , Coastal and Estuarine Studies 43 American Geophysical Union, Washington, pp. 103–124.
Sokal, R.R., Rohlf, F.J., 1995. In: Biometry. Principles and Practice of Statistics in Biological Research. W.H. Freeman, San Francisco, p. 887.
Thompson, P.A., Guo, M.X., Harrison, P.J., 1993. The influence of irradiance on the biochemical composition
Ž
of three phytoplancton species and their nutritional value for larvae of the Pacific oysters Crassostrea
.
gigas . Mar. Biol. 117, 259–268.
Thompson, P.A., Harrison, P.J., 1992. Effect of monospecific algal diets of varying biochemical composition
Ž .
on the growth and survival of Pacific oyster Crassostrea gigas larvae. Mar. Biol. 113, 645–654.
Ž . Ž .
Trider, D.J., Castell, J.D., 1980. Effect of dietary lipids on growth, tissue composition and metabolism of the
Ž .
oyster CrassostreaÕirginica . J. Nutr. 110, 1303–1309.
Vaskovsky, V.E., Suppes, Z.S., 1972. Phospholipases of marine invertebrates: I. Distribution of phospholipase A. Comp. Biochem. Physiol. 43B, 601–609.