Brain Research 888 (2001) 138–149
www.elsevier.com / locate / bres
Research report
21 1
LY393615, a novel neuronal Ca
and Na
channel blocker with
neuroprotective effects in models of in vitro and in vivo cerebral
ischemia
a ,
*
a a aMichael J. O’Neill
, Caroline A. Hicks , Mark A. Ward , David J. Osborne ,
a c c c
Graham Wishart , Kusum S. Mathews , Daniel P. McLaughlin , Jonathan A. Stamford ,
b b b b
Deborah R. McCarty , Kelly E. Patrick , Carlos Roman , Jerome H. Fleisch ,
a a
Jeremy Gilmore , John R. Boot
aLilly Research Centre Ltd., Erl Wood Manor, Windlesham, Surrey GU20 6PH, UK b
Lilly Corporate Center, Indianapolis, IN 46285-0814, USA c
Neurotransmission Laboratory, Academic Department of Anaesthesia and Intensive Care, St. Bartholomew’s and the Royal London School of
Medicine and Dentistry, Whitechapel, London E1 1BB, UK
Accepted 26 September 2000
Abstract
In the present studies we have examined the effects of a new calcium channel blocker, LY393615 ((N-Butyl-[5,5-bis-(4-fluoro-phenyl)tetrahydrofuran-2-yl]methylamine hydrochloride, NCC1048) in a model of hypoxia–hypoglycaemia in vitro and in a gerbil model of global and in two rat models of focal cerebral ischaemia in vivo. Results indicated that LY393615 protected against hypoxia– hypoglycaemic insults in brain slices and also provided significant protection against ischaemia-induced hippocampal damage in gerbil
global cerebral ischaemia when dosed at 10, 12.5 (P,0.05) or 15 mg / kg i.p. (P,0.01) 30 min before and 2 h 30 min after occlusion. The
compound penetrated the brain well after a 15 mg / kg i.p. dose and had a half-life of 2.5 h. In further studies LY393615 was protective 1 h post-occlusion when administered at 15 mg / kg i.p. followed by 2 doses of 5 mg / kg i.p. 2 and 3 h later. LY393615 dosed at 15 mg / kg i.p. followed by 2 further doses of 5 mg / kg i.p. (2 and 3 h later) also produced a significant reduction in the infarct volume following
Endothelin-1 (Et-1) middle cerebral artery occlusion in the rat when administration was initiated immediately (P,0.01) or 1 h (P,0.05)
after occlusion. The compound was also evaluated in the intraluminal monofilament model of focal ischaemia. The animals had the middle cerebral artery occluded for 2 h, and 15 min after reperfusion LY393615 was administered at 15 mg / kg i.p. followed by 2 mg / kg / h i.v. infusion for 6 h. There was no reduction in infarct volume using this dosing protocol. In conclusion, in the present studies we have reported that a novel calcium channel blocker, LY393615, with good bioavailability protects against neuronal damage caused by hypoxia–hypoglycaemia in vitro and both global and focal cerebral ischaemia in vivo. The compound is neuroprotective when
administered post-occlusion and may therefore be a useful anti-ischaemic agent. 2001 Elsevier Science B.V. All rights reserved.
Theme: Disorders of nervous system
Topic: Ischaemia
Keywords: Cerebral ischemia; Neuroprotection; Neuronal calcium channel; LY393615
1. Introduction mechanism of ischaemia-induced neuronal cell death re-mains to be elucidated. It is thought that during ischaemia, Although cerebral ischaemia is of major clinical impor- the lack of energy to the brain may depolarize neurones tance in stroke and cerebrovascular disorders, the exact and result in large increases in neurotransmitters such as glutamate, aspartate, dopamine and serotonin [13,39,40]. Glutamate, through an action on N-methyl-D-aspartate
*Corresponding author. Tel.:144-1276-853-547; fax:1
44-1276-853-(NMDA) and a-amino-3-hydroxy-5-methyl-4-isoxazole 525.
E-mail address: oneill michael [email protected] (M.J. O’Neill).] ] propionate (AMPA) receptors, allows calcium to enter the
cell [10,22,51]. Glutamate can also act on metabotropic channel blocker that exhibits broad activity against neuro-receptors leading to the production of diacylglycerol nal calcium channels and at 10 mg / kg i.p. protected (DAG) and inositol triphosphate (IP3) which, lead to the against global ischaemia-induced brain damage in the release of calcium from intracellular stores [51]. Calcium gerbil when administered 30 min after occlusion [4,7]. also enters neurones through voltage-dependent calcium More recent studies have reported that SB 201823-A channels which open in response to cellular depolarization antagonises calcium currents in rat central neurones and [39,40]. The net result of these various mechanisms by reduces the infarct volume in rat and mouse models of which calcium concentrations are elevated is a calcium focal ischaemia [4] and that another neuronal calcium ‘overload’ which leads to activation of proteases, nu- antagonist, NS-649, has provided protective effects in the cleases, phospholipases, NO synthase and other degrada- mouse MCAO model [49].
tive enzymes that lead to free radical production, mito- At Eli Lilly & Co. Ltd. we ran a high throughput screen chondrial degeneration and cell death [39,40]. In addition, using HEK293 cells transfected with humana1A,a1B and recent studies have shown that apoptotic mechanisms a1E calcium channel subunits (representing N, P/ Q and contribute to cell death in vitro and damage following R-type calcium channels). The aim of this screen was to cerebral ischaemia in vivo [17]. Therefore, strategies discover small organic molecules that block neuronal aimed at inhibiting apoptosis (caspases inhibitors, etc.) calcium channels and thereby modulate neurotransmitter may also be useful interventions in ischaemic situations release in disease situations. We identified a compound that
(for review see [25,50]). the medicinal chemistry department modified to the more
In line with the above mechanisms, several studies have active compound LY393615 (NCC1048), which inhibited reported that compounds acting as antagonists of excitatory N, P and Q-type calcium channels. At a concentration of amino acid receptors have beneficial effects against is- 10 mM, LY393615 produced 75616%, 8668% and chaemic insults [11,12,22,28,36]. However, several early 9563% inhibition of calcium flux in HEK 293 cells studies also reported neuroprotection with compounds such transfected with humana1A,a1B ora1E calcium channel
21 as (S )-emopamil, which belong to the phenylalkylamine subunits. The compound was was also evaluated on Ca class of calcium channel inhibitors, in animal models of currents in HEK 293 cells transfected with humana1B or
21
cerebral ischaemia [18,23,24]. In addition, other L-type a1E Ca channel subunits and on P-type calcium chan-calcium antagonists such as nimodipine and nicardipine nels in isolated Purkinje cells using voltage patch clamp
21
have been shown to display neuroprotective effects in techniques. LY393165 inhibited peak Ca currents dose-some animal models of ceebral ischaemia [1,15,33]. dependently with IC50 values of 1.960.1mM ina1B cells, At least 5 subtypes of high threshold (HVA) denoted L, 5.261.1 mM in a1E cells and 4.061.0 mM in Purkinje N, P, Q and R and one type of low threshold (LVA), the cells. This report summarises the neuroprotective effects of T-type, voltage-dependent calcium channels have been LY393615 in an in vitro hypoxia–hypoglycemic slice described [47]. The availability of several synthetic model and in gerbil global ischaemia and two models of conopeptides has provided an opportunity to evaluate the rat focal cerebral ischaemia in vivo.
therapeutic potential of selective blockade of N-type calcium channels in a variety of pathological conditions
including cerebral ischaemia. A single bolus intravenous 2. Materials and methods
administration of v-conotoxin MVIIA (SNX-111)
pro-vided protection even when administered 24 h after the 2.1. In vitro hypoxia–hypoglycemia ischaemic insult [48]. Similar effects were observed in two
other studies [42,55]. SNX-111 has also been found to be Male Wistar rats (150650 g) were sacrificed by cervical highly effective in reducing the neocortical infarct volume dislocation. Coronal brain slices (350 mm thick) at the in rat models of focal ischaemia, both when administered level of the striatum (typically bregma60.5 mm: Paxinos during the occlusion [43] and after the ischaemic episode and Watson [30]) were incubated in chambers containing [9]. Buchan et al. [9] administered SNX-111 at 5 mg / kg 500 ml oxygenated artificial cerebrospinal fluid (aCSF) at i.v. either 6 or 24 h after 10 min of 4-vessel occlusion 348C for 30 min. Slices were then subjected to ‘ischaemia’ (4VO) and found protection in both cases [9]. The group by transfer to a chamber containing 500 ml deoxygenated, also showed protection with the same dose in a rat model hypoglycaemic aCSF for 10 min [45] followed by a further
of focal ischemia. 30 min of reincubation in oxygenated aCSF. Control slices
More recent studies have reported neuroprotective ef- were maintained in oxygenated aCSF throughout. In a fects with smaller non-peptide compounds that inhibit proportion of cases, LY393615 (10 mM) was included in neuronal calcium channels. It has been reported that NNC the aCSF throughout the pre-incubation, ischaemia and 09-0026 inhibits neuronal voltage-dependent calcium chan- post-ischaemic periods.
140 M.J. O’Neill et al. / Brain Research 888 (2001) 138 –149
formalin and transferred to coverslips. Stained slices were face mask throughout the operation. Through a midline initially scanned in true colour using an AGFA ‘Studios- cervical incision, both common carotid arteries were can’ desktop scanner at 60 mm resolution. The ensuing exposed and freed from surrounding connective tissue. In images were converted to 8-bit greyscale (0–255) for animals to be rendered ischaemic, both common carotid mathematical analysis of staining [21]. The intensity of arteries were clamped for 5 min. At the end of the striatal and cortical TTC staining was analysed by OsirisE occlusion period, blood flow was re-established. In sham-(University Hospital of Geneva, Switzerland) or operated animals, the arteries were exposed but not ScionImageE (Scion Corporation) software and expressed occluded. The wound was then sutured and the animals as a mean percentage greyscale value over the whole allowed to recover. Throughout surgery, body temperature region of interest (striatum or surrounding cortex). was maintained at 378C using a ‘K-TEMP’ temperature Statistical comparisons between groups were made by controller / heating pad (International Market Supply, Che-One Way ANOVA with posthoc application of the Stu- shire, UK). After surgery, the animals were placed in a
dent–Newman–Keuls test. four compartmental thermacage (Beta Medical and
Sci-entific, UK) which maintained the environmental
tempera-2.2. Pharmacokinetics ture at 288C and rectal temperatures were measured for a 6
h period after occlusion. LY393615 was administered at To assess exposure of LY393615, gerbils were dosed 10, 12.5 or 15 mg / kg i.p. 30 min before and 2 h 30 min with 15 mg / kg i.p. and brains harvested at 15, 30, 45 min, post-occlusion in the first experiment. LY393615 was 1, 2, 4, 6 and 8 h later. We also evaluated exposure in rats administered at 15 mg / kg i.p. 30 or 60 min post-occlusion at the same time points after 1 mg / kg i.v. In both cases followed by two further doses of 5 mg / kg i.p. 2 and 3 h brains were weighed and then homogenised with two after the initial dose in the second and third experiments. volumes of distilled water. A 20 mM aqueous solution of Five days after surgery, the animals were perfused LY393613 (N-Butyl-2-[bis-(4-fluorophenyl)methox- transcardially with 30 ml of 0.9% saline followed by 100 y]ethylamine hydrochloride) was prepared for use as an ml of 10% buffered formalin solution. The brains were internal standard. 300ml aliquots of brain homogenate and removed and placed in 10% formalin for 3 days, processed 10 ml internal standard solution were transferred to 1.5 ml and embedded in paraffin wax. Coronal sections (5 mm) eppendorf centrifuge tubes, mixed thoroughly and ex- were taken 1.5, 1.7 and 1.9 mm caudal to bregma using a tracted with 10 ml trifluoroacetic acid (TFA) and 100 ml microtome (Leitz 1400 sledge microtome). The slices were acetonitrile. Tubes were spun, in a microcentrifuge, for 5 stained with haematoxylin and eosin and the neuronal min at 13,000 r.p.m. and the supernatant transferred to density in the CA1 subfield of the hippocampus was HPLC microvials for assay. LY393615 standards of 50, 20, measured using a microscope with grid lines (0.0530.05 5, 2, 0.5 and 0.1 mM concentration were prepared in mm) as described previously [28]. The neuronal density is homogenised control rat brain and then treated in the same expressed as number of viable cells per mm CA1
hip-manner as the samples. pocampus. Statistical analysis of histological data was
Samples and standards were analysed by HPLC / MS assessed using a 2-tailed unpaired Students t-test, with P using a Hewlett Packard 1100 series autosampler and values ,0.05 being considered statistically significant. binary pump (Hewlett Packard, Bracknell, UK) coupled to
a Micromass Platform II mass spectrometer (Micromass, 2.4. Focal ischaemia Manchester, UK). Chromatography was performed on a 25
cm32.1 mm Hichrom 5 C18 column (Hichrom, Theale, 2.4.1. Endothelin-1 model. Rats were anaesthetised with UK) using acetonitrile:water (60:40) containing 5 mM inhalation anaesthetic and placed on a thermostatically ammonium formate with the pH adjusted to 2.8 with TFA. controlled heating blanket to maintain body temperature Twenty ml volumes were injected. MS analysis was within the range 37–388C. The rat was then placed in a performed in the electrospray mode with a source tempera- Kopf stereotaxic frame and the scalp incised so as to ture of 1208C and cone voltage of 25 V. Ions at m /z 346 uncover the parietal bones. A 28-guage steel cannula was (LY393615) and m /z 320 (LY393613) were monitored. then inserted stereotaxically at the following co-ordinates (from bregma: AP5 10.9 mm, L5 25.2 mm and 28.7
2.3. Global ischaemia mm below skull, Sharkey et al. [35]). Endothelin-1 (200
pmol in 3 ml) was infused over a 3 min period. The Male Mongolian gerbils (Bantin and Kingman, Hull, cannula was left in situ for a further 5 min and then UK) at least 3 months old and weighing in excess of withdrawn. The wounds (muscles and skin) were sutured 60–80 g were used. The animals were maintained in and the rat allowed to recover.
follow by 10% buffered formalin via the heart. The brains ‘ischaemia’ in both striatum (P,0.01) and cortex (P,
were removed for histology, stained with cresyl violet and 0.001).
the area of ischaemic damage at 8 stereotaxic levels was For global ischaemia studies, 5mm sections taken 1.5– measured using Optimus 5.2 software and from this an 1.9 mm caudal to the bregma in the anterior hippocampus infarct volume was calculated. Statistical analysis of were examined under a microscope with grid lines. The histological data was carried out using ANOVA followed pyramidal cell density was counted at three different by Students t-test using P,0.05 as the level of signifi- stereotaxic levels in the CA1 region of the hippocampus
cance. and the results expressed as mean6S.E.M. neuronal
den-sity per 1 mm CA1. The results indicated that there was 2.4.2. Monofilament model. In the monofilament model, a severe loss of neurones in the CA1 region of the hip-segment of 220 mm diameter nylon monofilament coated pocampus of 5 min occluded animals. The neuronal death with nail polish at the tip (370 mm), was inserted into the involved nearly all the pyramidal neurones and this internal carotid artery of each male Wistar rat (295–315 neurodegeneration was not evident in any other forebrain g), and advanced until it blocked the origin of the middle region. LY393615 provided dose-dependent neuroprotec-cerebral artery (Belayev et al. [6]; Zea Longa et al. [54]). tion against the ischaemia-induced cell death in the CA1. After 2 h, the monofilament was retracted and reperfusion Thus, LY393615 provided very good protection when occurred. LY393615 was administered at 15 mg / kg i.p. 15 administered at 15 mg / kg i.p. 30 min before and 2 h 30 min after reperfusion followed by 2 mg / kg / h i.v. infusion min after occlusion (Figs. 4 and 5). We also evaluated the for 6 h. Twenty-four hours later the brains were removed, effects of lower doses of LY393615 at 10 mg / kg or 12.5 frozen, sliced into 30 mm sections and stained with cresyl mg / kg i.p. 30 min before and 2 h 30 min post-occlusion. violet. The infarct area of each slice was measured with Results indicated that 10 mg / kg provided a small, but imaging system software ImagePro Plus, and infarct significant neuroprotection (Fig. 4). The intermediate dose volumes were calculated. Statistical analysis of histological (12.5 mg / kg i.p.) also provided a significant neuroprotec-data was carried out using ANOVA followed by Students tive effect (Fig. 4).
t-test using P,0.05 as the level of significance. We then went on to study the pharmacokinetics (at 15, 30, 45 min, 1, 2, 4, 6 and 8 h) of LY393615 in the gerbil brain after 15 mg / kg i.p. The results indicated that the
3. Results compound crossed the blood–brain barrier quickly and was detectable in the brain as early as 15 min after injection The structure of (N-Butyl-[5,5-bis-(4-fluorophenyl)tetra- (Fig. 6). Maximal levels were observed at 1–2 h after hydrofuran-2-yl]methylamine hydrochloride, LY393615) is injection and the half-life was approximately 2.5 h.
illustrated in Fig. 1. Based on the pharmacokinetic studies we postulated that
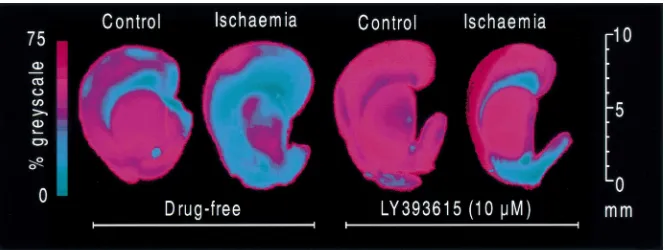
Figs. 2 and 3 show the effects of LY 393615 against a loading dose (15 mg / kg i.p.) followed by two further ischaemic insult in vitro. Control slices mostly showed ‘top-up’ doses (5 mg / kg i.p.) would give relatively uniform staining throughout the cortex and striatum fol- consistent high brain levels for several hours after in-lowing TTC. Fig. 2 consists of representative pseudo- jection. Therefore, in the next study we evaluated the coloured corticostriatal sections showing the effect of effects of 15 mg / kg i.p. 30 min after occlusion followed ischaemia and LY393615. Ischaemia (10 min) caused a by 2 further doses of 5 mg / kg i.p. at 2 h 30 min and 3 h 30 significant (P,0.05) reduction in mean TTC staining min post-occlusion. Histological results indicated that this intensity in both striatum and cerebral cortex (Fig. 3). dosing protocol also provided good (P,0.01) neuroprotec-LY393615 had no significant effect on TTC staining tion (Fig. 7). We then determined if the time window could intensity in control slices (if anything it appeared to protect be extended using the above protocol by administering the slice) but prevented the reduction in staining induced by LY393615 at 15 mg / kg 1 h post-occlusion followed by 5 mg / kg at 2 and 3 h post-occlusion. Results indicated that this dosing protocol also provided significant (P,0.05) neuroprotection (Fig. 8).
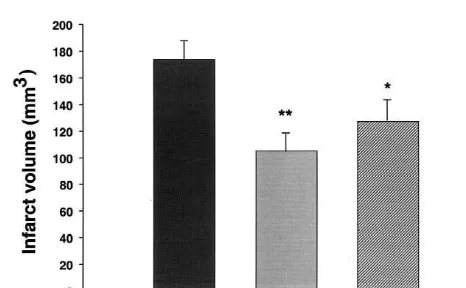
Two models of focal cerebral ischaemia were carried out; the endothelin-1 model and the monofilament model. In the endothelin-1 model LY393615 was administered at 15 mg / kg i.p. either immediately or 1 h after endothelin-1 injection onto the middle cerebral artery in the rat and followed by two further doses at 3 and 4 h after the initial
2
injection injection. The area of damage in mm was measured at 8 stereotaxic levels and the infarct volume
3
calculated in mm . LY393615 produced a reduction (P,
Fig. 1. Illustrates the structure of
142
M
.J
.
O
’Neill
et
al
.
/
Brain
Research
888
(2001
)
138
–
149
4. Discussion
The in vitro profile of LY33165 is summarised in Table 1. At a concentration of 10 mM, LY393615 produced 75616%, 8668% and 9563% inhibition of calcium flux in HEK 293 cells transfected with a1A, a1B or a1E calcium channel subunits. In vitro electrophysiological studies using patch clamp techniques indicated that
21
LY393165 inhibited peak Ca currents dose-dependently with IC50 values of 1.960.1 mM in a1B cells, 5.261.1
mM ina1E cells and 4.061.0mM in Purkinje cells. In the present studies we have evaluated the effects of LY393615 in models of in vitro and in vivo cerebral ischaemia. The results indicate that LY393615 was protective in vitro and provided excellent protection when administration was Fig. 3. Effect of LY393615 (10mM) on ischaemia-induced reduction in
initiated prior to occlusion and good protection when TTC staining (expressed as percent greyscale). All values are
means6S.E.M. (n511 / 15).*P,0.05 vs. Control, **P,0.01,***P, administered 30 min post-occlusion in global ischaemia.
0.001 vs. Ischaemia, $ P,0.001 vs. Control1LY393615. The compound also had significant protective effects when
delayed until 1 h post-occlusion in both global and focal immediately after occlusion (Fig. 9). The compound also cerebral ischaemia.
provided some protection (P,0.05) when administration
was initiated 1 h post-occlusion (Fig. 9). 4.1. In vitro ischaemia Pharamcokinetic studies in rats indicated that LY393615
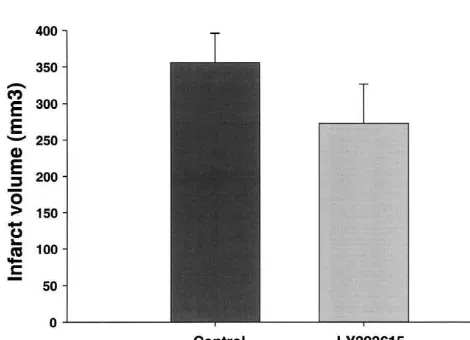
penetrated the brain rapidly after a 1 mg / kg i.v. dose (Fig. Several studies have reported that depolarization follow-10). The half-life was approximately 2 h and therefore an ing cerebral ischaemia opens voltage-gated calcium chan-infusion would give most consistent brain levels for further nels and this in turn produces massive neurotransmitter focal ischaemia studies. release. The large release in neurotransmitters is thought to LY393615 was also examined in the monofilament produce excitotoxicity, free radical production, membrane model of cerebral ischaemia. The animals had the middle degeneration and ultimately lead to cell death. We have cerebral artery occluded for 2 h. Fifteen minutes after previously show that v-conotoxin GVIA and v-agatoxin reperfusion LY393615 was administered at 15 mg / kg i.p. IVA delay neurotransmitter release in striatal slices, while followed by 2 mg / kg / h i.v. infusion for 6 h. The results in contrast nimodipine and nicardipine do not [46]. There-indicated that there was no significant reduction in infarct fore, N-and P/ Q-, but not L-type calcium channels seem to
volume (Fig. 11). control this ischaemia-induced neurotransmitter release. In
the present studies we have used a selective neuronal calcium channel blocker, LY393615, and investigated if this compound provides protection against hypoxia-hypo-glycaemia damage in vitro. LY393615 has been shown to block calcium currents in HEK293 cells transfected with
21
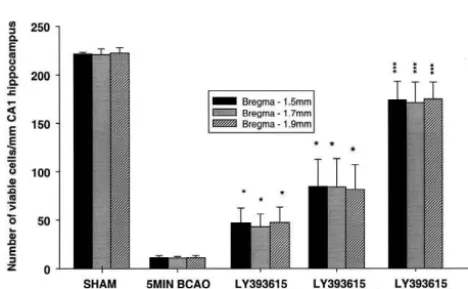
humana1A,a1B and a1E Ca channel subunits, P-type calcium channels in dissociated Purkinje cells and inhibit synaptic transmission in hippocampal slices [44]. The results of the present studies indicate that LY393615 provided significant protection against hypoxic–hypo-glycaemic insult in striatal slices using 2,3,5-triphenyltet-razolium chloride staining [21]. Other recent studies have reported that v-conotoxin (selective N-type blocker) protects against hypoxia induced neurodegeneration in organotypic hippocampal-slice cultures [32]. While further Fig. 4. The effects of LY393615 on the neuronal density in the CA1
studies have used combinations of compounds in an region of the hippocampus 5 days after 5 min bilateral carotid artery
occlusion. LY393615 was administered at 10, 12.5 or 15 mg / kg i.p. 30 attempt to identify the calcium channel subtypes contribut-min pre and 2 h 30 contribut-min post-occlusion. Results are expressed as ing to neuronal injury in rat hippocampal slices subjected mean6S.E.M. viable cells / mm CA1 hippocampus (n58 animals per to oxygen and glucose deprivation [41]. These authors group). 5 min BCAO caused a severe loss in neuronal cells in the CA1
found that nimodipine (L-type) and daurisoline (P-type) region (P,0.001). LY393615 provided significant neuroprotection
provided less than 20% protection, v-conotoxin MVIIA against the ischaemia-induced cell death. *P,0.05 vs. 5 min BCAO
144
M
.J
.
O
’Neill
et
al
.
/
Brain
Research
888
(2001
)
138
–
149
Fig. 6. Brain levels of LY393615 (15 mg / kg i.p.) at 15, 30, 45 min, 1, 2, 4, 6 and 8 h after dosing in gerbils. Results indicate that LY393615
Fig. 8. The effects of LY393615 on the neuronal density in the CA1 penetrates the brain and attains levels that are above the IC50for calcium region of the hippocampus 5 days after 5 min bilateral carotid artery channel blockade. The compound had a half-life of approximately 2.5 h.
occlusion. LY393615 was administered at 15 mg / kg i.p. 1 h post-occlusion followed by 5 mg / kg at 3 and 4 h post-post-occlusion. Results are expressed as mean6S.E.M. viable cells / mm CA1 hippocampus (n58 IVA (P1Q-type) provided 75% protection. However, v- animals per group). Five minutes BCAO caused a severe loss in neuronal cells in the CA1 region (P,0.001). LY393615 provided significant conotoxin MVIIC (P1Q1N-type) provided complete
neuroprotection against the ischaemia-induced cell death. *P,0.05 vs. 5 protection, suggesting that all three neuronal calcium
min BCAO control. Student’s t-test. channels contribute to ischaemic brain injury in the
hippocampus.
would be a better candidate for treatment of ischaemic
4.2. Global ischaemia conditions. We have demonstrated that LY393615 provided
good neuroprotection when dosed at 15 mg / kg i.p., 30 min Earlier studies had also reported neuroprotection with before occlusion in the gerbil model. This protection was
v-conotoxin GVIA (SNX-111) in models of transient dose-dependent, with the 15 mg / kg dose providing much global ischaemia in rats and gerbils [9,48]. However, these greater protection than the lower 10 and 12.5 mg / kg doses. peptides had poor blood brain barrier penetration and have Pharmacokinetic studies indicated that the compound to be administered centrally or intravenously at high doses, penetrated into the brain and that the half-life was approxi-which effected blood pressure. Therefore, a small molecu- mately 2.5 h. Based on these studies, we calculated that 15 lar calcium channel blocker with good brain penetration mg / kg i.p. followed by two further doses of 5 mg / kg 2 and 3 h later would give consistent brain levels that were several fold higher than the IC50 for LY393615 on
Fig. 7. The effects of LY393615 on the neuronal density in the CA1 region of the hippocampus 5 days after 5 min bilateral carotid artery
3
146 M.J. O’Neill et al. / Brain Research 888 (2001) 138 –149
Table 1
a The in vitro profile of LY393615
21
(A) Inhibition of Ca flux at 10 mm using Fluoroscan:
HEK 293 cells transfected with human a1A 75616 HEK 293 cells transfected with human a1B 8668 HEK 293 cells transfected with human a1E 9563
(B) Inhibition of Glutamate release at 10mm:
KC1-induced glutamate release 7666 Electrical-induced glutamate release 63 (n52) Veratradine-induced glutamate release 81 (n52)
(C) Patch Clamp IC50values (mm) for inhibition of:
HEK 293 cells transfected with human a1B 1.960.1 HEK 293 cells transfected with human a1E 5.261.1 P-type calcium channels in isolated Purkinje cells 4.061.0
a
Fig. 10. Brain levels of LY393615 (1 mg / kg i.v.) at 15, 30, 45 min, 1, 2, Effects of LY393615 ON (A) calcium flux in HEK 293 cells transfected 4, 6 and 8 h after dosing in rats. Results indicate that LY393615 with humana1A,a1B anda1E calcium channel subunits (B) inhibition penetrates the brain rapidly has a half-life of approximately 2 h. of DC1 Electrical and Veratradine-induced glutamate release in symapto-somes and (C) IC50 values for inhibition of calcium channels in transferred cell limes and isolated Purkinje cells using whole cell patch neuronal calcium channel subunits in vitro. Using this clamp electrophysiological techniques.
protocol we went on to demonstrate that LY393615 provided significant protection in this model when the first
injection was delayed until 1 h post-occlusion. tion when the compound (30 mg / kg i.p.) was administered Several other small molecules; NNC 09-0026 ((2)- once 30 min post-occlusion. NNC 09-0026 (30 mg / kg i.v.) trans-1-butyl-4-(4-dimethylaminophenyl) - 3 - [(4-trifluoro - administered slowly over a 1 h period beginning 30 min methylphenoxy)methyl]piperidine dihydro chloride); SB post-ischaemia has also been reported to reduce the infarct 201823-A (4-[2-(3,4-dichlorophenoxy)ethyl]-1-pentyl volume in a rat model of focal ischaemia [3]. CNS 1237 is
1 21
piperidine hydrochloride); NS 649 (2-amino-1-(2,5-di- a use-dependent Na and Ca channel blocker that methoxyphenyl)-5-trifluoromethyl benzimidazole); CNS produced a reduction in infarct volume when dosed as a 1237 (N-acenaphthyl-N9-4-methoxynaphth-1-yl guanidine) bolus of 3 mg / kg i.v. followed by an infusion of 0.75 have also been reported to have neuroprotective effects in mg / kg / h for 4 h [14]. It has also been reported that SB models of cerebral ischaemia. For example, Sheardown 201823-A administered at 10 mg / kg i.p. at 30 min post-and co-workers [38] have reported that NNC 09-0026 (30 occlusion attenuated ischaemia-induced hyperactivity and mg / kg i.p.) protected in the gerbil when administered 30 protected the CA1 pyramidal cells in the hippocampus min, 24 and 48 h post-occlusion [37], but no neuroprotec- following 8 min of BCAO in the gerbil [7]. Other studies have indicated that SB 201823-A reduces the infarct volume in rat and mouse models of focal ischaemia [4]. NS 649 is another compound that has been reported to have protective effects in the mouse MCAO model [49].
We have previously evaluated all of these small mole-cules as reference neuronal calcium channel antagonists in the gerbil model of cerebral ischaemia [26]. The doses selected were based on previously published work and tolerability in animals. NNC 09-0026 (30 mg / kg i.p.) provided 22% neuroprotection when administration was initiated 30 min before occlusion followed by two further doses at 24 and 48 h post-occlusion. CNS 1237 provided some neuroprotection (21%) against the ischaemia-induced cell death in the CA1 region of the hippocampus. SB 201823-A, when administered at 10 mg / kg 30 min before and 2 h 30 min after occlusion failed to provide any 3
Fig. 11. Effects of LY393615 on infarct volume (mm ) after 2 h of neuroprotection and NS 649 at 50 mg / kg i.p. (dosed 30 middle cerebral artery occlusion in the rat using the monofilament min before and again at 2 h 30 min after occlusion) also method. LY393615 was administered at a loading dose of 15 mg / kg i.p. failed to provide any protection against the ischaemia-15 min after reperfusion followed by an i.v. infusion of 2 mg / kg / h for 6
induced damage in the CA1 hippocampal region of the h and infarct volume measured at 1 day post-surgery (n512–16 animals
gerbil brain. These results clearly indicate LY393615 is far per group). LY393615 failed to reduce the infarct volume under this
channel blockers as neuroprotective agents in global of focal ischaemia [19]. More recent work has indicated cerebral ischaemia. The compound exhibited good solu- that small organic molecules such as SB 201823A are bility and penetrated the brain well and this probably protective in models of focal ischaemia [3,4]. The newer accounts for the good neuroprotection we observed. compound SB 206284A has been reported to protect when In the past 5 years we have evaluated several pharmaco- administered 4 h post-occlusion in a photochemical model logical interventions in cerebral ischaemia. As mentioned of focal ischaemia [52]. However, many of the studies to above, the level of protection observed with LY393615 in date have used short occlusion times i.e. 90 min whereas in this model is much greater than that we have previously our studies we have used the standard 2 h occlusion, which observed, using the same protocol, with other calcium is a more severe model.
channel blockers (NCC 09-0026, CNS1237 [26]).
Interest-ingly, the protection with LY393615 is also much greater 4.4. Mechanism of action of LY393615 than that provided by competitive NMDA antagonists
(CGS19755, CPP [28]; polyamine site antagonists of the As mentioned above, LY393615 blocks several types of NMDA receptor [5] and glycine site antagonists neuronal calcium channels and also inhibits sodium chan-(ACEA1021, GV150526A [16]), non-competitive AMPA nels. Therefore, the exact mechanism of neuroprotection is antagonists (GYKI52466, LY300164 [20]), nitric oxide not clear. In theory all of the activities (i.e. inhibition of inhibitors (L-NAME and 7-nitroindazole [27]) and similar N-type, P/ Q-type of calcium channels or sodium channels) to what we have seen with the competitive AMPA antago- could be neuroprotective. On reviewing the literature it is nists (LY302679; LY293558 [28]), the iGluR5 antagonist clear that selective N-type calcium channel blockers and (LY377770 [29]) and the mGluR2 / 3 agonist (LY379268 sodium channel blockers have provided consistent protec-[8]). Therefore, LY393615 has comparable neuroprotection tion in a variety of models. However, results are more to some of the best compounds we have evaluated to date variable with P/ Q-type calcium blockers. Therefore, it is in the gerbil model of cerebral ischaemia. most likely that the N-type calcium channel blockade and the sodium channel effects account for the observed
4.3. Focal ischaemia neuroprotection. It is interesting to note that there is an
increasing tendency to screen for selective molecules using In focal ischaemia studies we evaluated the neuroprotec- transfected cell lines, but there are multiple receptors tive potential of LY393615 in the endothelin-1 and mono- contributing to ischaemia-induced brain damage. The filament models of middle cerebral artery occlusion pharmacological profile of LY393615 may make it more (MCAO) in rats. The results indicated that LY393615 suitable as a neuroprotectant than a compound that only provided a significant reduction in infarct volume when blocked one receptor subtype. In the present studies we did administered immediately or 1 h post-occlusion in the not measure brain temperature and it is well established endothelin-1 model of focal cerebral ischaemia. The that hypothermia is neuroprotective. We did monitor rectal degree of protection was similar to that previously reported temperatures at 30 min intervals and this is thought to be a with MK-801 in this model [29,34]. LY393615 failed to good reflection of brain temperature. The compound did protect when administered at 15 mg / kg i.p. 15 min after 2 not alter rectal temperature at the neuroprotective doses. In h of MCAO and followed by a 6 h infusion of 2 mg / kg a similar way the protection observed could be due to i.v. for 6 h in the monofilament model. However, the effect on blood pressure. In parallel studies we found that compound was administered 2 h 15 min after ischaemia there was no changes in blood pressure using a dose of 15 and further pharmacokinetic studies indicated that although mg / kg i.p. Therefore, the neuroprotective actions of LY393615 penetrated the brain following i.v. administra- LY393615 appear to be mediated by calcium and / or tion, it has a short half-life (2.04 h) and, therefore, a higher sodium channel blockade.
infusion dose (3.5 mg / kg / h) may have been more optimal In summary, in the present studies we have reported to achieve maximal chances of protection. Other studies neuroprotective effects with LY393615 a model of in vitro have reported that the conotoxin SNX-111, which blocks ischaemia in striatal slices and in models of global and N-type calcium channels is effective at reducing infarct focal cerebral ischaemia in vivo. The compound had good volume in rat models of focal cerebral ischaemia when solubility and pharmacokinetic studies demonstrated that administered during [43] or after the occlusion (Buchan et the compound had good brain penetration with a half-life al. [9]; Yenari et al. [53]). The compound was also of 2–2.5 h. LY393615 was active post-occlusion and may reported to have neuroprotective effects in a rabbit model therefore be useful as an anti-ischaemic agent.
of focal ischaemia [31]. Other recent studies have demon-strated that i.c.v. administration of v-agatoxin IVA, which
blocks P/ Q-type calcium channels, protects against focal References ischaemia in rats [2]. However, other investigators have
148 M.J. O’Neill et al. / Brain Research 888 (2001) 138 –149
¨
[2] K. Asakura, Y. Matsuo, T. Kanemusa, M. Ninomiya, P/ Q-type [19] K. Lingenhohl, D.L. Small, R. Monette, A.M. Buchan, P. Morley, Ca21 channel blocker v-conotoxin IVA protects against brain P.R. Allegrini, W. Frostl, D. Sauer, M. Schmutz, T. Knopfel,¨ injury after focal ischemia in rats, Brain Res. 776 (1997) 140–145. Exploration of P-type Ca21 channels as drug targets for the [3] F.C. Barone, W.J. Price, P. Jakobsen, M.J. Sheardown, G. Feuerstein, treatment of epilepsy or ischemic stroke, Neuropharmacology 36
Pharmacological profile of a novel neuronal calcium channel blocker (1997) 107–113.
includes reduced cerebral damage and neurological deficits in rat [20] D. Lodge, A. Bond, M.J. O’Neill, C.A. Hicks, M.G. Jones, focal ischemia, Pharmacol. Biochem. Behav. 48 (1994) 77–85. Stereoselective effects of 2,3-benzodiazepines in vivo: elec-[4] F.C. Barone, P.G. Lysko, W.J. Price, G. Feuerstein, K.A. Al- trophysiology and neuroprotection studies, Neuropharmacology 35
Baracanji, C.D. Benham, D.C. Harrison, M.H. Harries, S.J. Bailey, (1996) 1681–1688.
A.J. Hunter, SB 201823-A antagonizes calcium currents in central [21] K.S. Mathews, L. Ziabari, P.C. Street, E. Hisgrove, C.C. Toner, D.P. neurons and reduces the effects of focal ischemia in rats and mice, McLaughlin, J.A. Stamford, Semiquantitative assessment of is-Stroke 26 (1995) 1683–1690. chaemic damage in brain slices by Tetrazolium Red, Br. Neurosci. [5] C.P. Bath, L.N. Farrell, J. Gilmore, M.A. Ward, C.A. Hicks, M.J. Assoc. Abs. 15 (1999) 122.
O’Neill, D. Bleakman, The effects of ifenprodil and eliprodil on [22] J. McCulloch, Excitatory amino acid antagonists and their potential 21
voltage-dependent Ca channels and in gerbil global cerebral for the treatment of ischaemic brain damage in man, Br. J. Clin. ischaemia, Eur. J. Pharmacol. 299 (1996) 103–112.
Pharmac. 34 (1992) 106–114. [6] L. Belayev, O.F. Alonso, R. Busto, W. Zhao, M.D. Ginsberg, Middle
[23] E. Morikawa, M.D. Ginsberg, W.D. Dietrich, R.C. Duncan, R. Busto, cerebral artery occlusion in the rat by intraluminal suture.
Neuro-Postischemic (S )-emopamil therapy ameliorates focal ischemic brain logical and pathological evaluation of an improved model, Stroke 27
injury in rats, Stroke 22 (1991) 355–360. (1996) 1616–1622.
[24] H. Nakayama, M.D. Ginsberg, W.D. Dietrich, (S )-Emopamil, a novel [7] C.D. Benham, T.H. Brown, D.G. Cooper, M.L. Evans, M.H. Harries,
calcium channel blocker and serotonin S2 antagonist, markedly H.J. Herdon, J.E. Meakin, K.L. Murkitt, S.R. Patel, J.C. Roberts,
reduces infarct size following middle cerebral artery occlusion in the A.L. Rothaul, S.J. Smith, N. Wood, A.J. Hunter, SB 201823-A, a
21 rat, Neurology 38 (1988) 1667–1673.
neuronal Ca antagonist is neuroprotective in two models of
[25] D.W. Nicholson, N.A. Thornberry, Caspases: killer proteases, Trends cerebral ischaemia, Neuropharmacology 32 (1993) 1249–1257.
Biochem. Sci. 22 (1997) 299–306. [8] A. Bond, N. Ragumoorthy, J.A. Monn, C.A. Hicks, M.A. Ward, D.
[26] M.J. O’Neill, C.P. Bath, C.P. Dell, C.A. Hicks, J. Gilmore, S.J. Lodge, M.J. O’Neill, LY379268, a potent and selective Group II
21 1
Ambler, M.A. Ward, D. Bleakman, Effects of Ca and Na channel metabotropic glutamate receptor agonist, is neuroprotective in gerbil
inhibitors in vitro and in global cerebral ischaemia in vivo, Eur. J. global cerebral ischaemia, Neurosci. Lett. 273 (1999) 191–194.
Pharmacol. 332 (1997) 121–131. [9] A.M. Buchan, S.Z. Gertler, H. Li, D. Xue, Z.G. Huang, K.E.
21 [27] M.J. O’Neill, C. Hicks, M. Ward, J.A. Panetta, Neuroprotective Chaundy, K. Barnes, H.J. Lesiuk, A selective N-type Ca -channel
effects of the antioxidant LY231617 and NO synthase inhibitors in blocker prevents CA1 injury 24 h following severe forebrain
global cerebral ischaemia, Brain Res. 760 (1997) 170–178. ischemia and reduces infarction following focal ischemia, J. Cereb.
[28] M.J. O’Neill, A. Bond, P.L. Ornstein, M.A. Ward, C.A. Hicks, K. Blood Flow Metab. 14 (1994) 903–910.
Hoo, D. Bleakman, D. Lodge, Decahydroisoquinolines: novel com-[10] D. Choi, Excitotoxic cell death, J. Neurobiology 23 (1992) 1261–
petitive AMPA / kainate antagonists with neuroprotective effects in 1276.
global cerebral ischaemia, Neuropharmacology 37 (1998) 1211– [11] R. Gill, A. C Foster, G.N. Woodruff, Systemic administration of
1222. MK-801 protects against ischemia-induced hippocampal
neurode-[29] M.J. O’Neill, L. Bogaert, C.A. Hicks, A. Bond, M.A. Ward, G. generation in the gerbil, J. Neurosci. 7 (1987) 3343–3349.
Ebinger, P.L. Ornstein, Y. Michotte, D. Lodge, LY377770, a novel [12] R. Gill, The pharmacology ofa
-amino-3-hydroxy-5-methyl-4-isox-iGlu5 kainate antagonist with neuroprotective properties in global azole propionate (AMPA) / kainate antagonists and their role in
and focal cerebral ischaemia, Neuropharmacology 39 (2000) 1575– cerebral ischaemia, Cerebrovasc. Brain Metab. Rev. 6 (1994) 225–
1588. 256.
[30] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, [13] M.Y-T. Globus, R. Busto, W.D. Dietrich, E. Martinez, I. Valdes,
Academic Press, San Diego, CA, 1986. M.D. Effect of ischemia on in vivo release of striatal dopamine,
[31] M.A. Perez-Pinzon, M.A. Yenari, G.H. Sun, D.M. Kunis, G.K. glutamate and gamma-aminobutyric acid studied by intracerebral
Steinberg, SNX-111, a novel presynaptic N-type calcium channel microdialysis, J. Neurochem. 51 (1988) 1455–1464.
antagonist, is neuroprotective against focal cerebral ischemia in [14] S.M. Goldin, K. Subbarao, R. Sharma, A.G. Knapp, J.B. Fischer, D.
rabbits, J. Neurol. Sci. 153 (1997) 25–31. Daly, G.J. Durant, N.L. Reddy, L.-Y. Lu, S. Magar, M.E. Perlman, J.
[32] A.K. Pringle, C.D. Benham, L. Sim, J. Kennedy, F. Iannotti, L.E. Chen, S.h. Graham, W.F. Holt, D. Berlove, L.D. Margolin,
Neuro-1 21
Sundstrom, Selective N-type calcium channel antagonist omega protective use-dependent blockers of Na and Ca channels
conotoxin MVIIA is neuroprotective against hypoxia neurodegene-controlling presynaptic release of glutamate, Ann. NY Acad. Sci.
ration in organotypic hippocampal-slice cultures, Stroke 27 (1996) 765 (1995) 210–228.
2124–2130. [15] S. Gomi, J.H. Greenburg, S. Croul, M. Revich, Failure of
[33] A. Rami, J. Krieglstein, Neuronal protective effects of calcium levemopamil to improve histological outcome following tempory
channel antagonists in cerebral ischaemia, Life Sci. 55 (1994) occlusion of the middle cerebral artery in cats, J. Neur. Sci. 130
2105–2113. (1995) 128–133.
[34] J. Sharkey, S.P. Butcher, J.S. Kelly, Endothelin-1 induced middle [16] C.A. Hicks, M.A. Ward, N. Ragumoorthy, S.J. Ambler, C.P. Dell, D.
cerebral artery occlusion: pathological consequences and neuro-Dobson, M.J. O’Neill, Evaluation of glycine site antagonists of the
protective effects of MK-801, J. Autonomic System 49 (1994) NMDA receptor in global cerebral ischaemia, Brain Res. 819 (1999)
S177–S185. 65–74.
[17] E.M. Johnson, L.J.S. Greenlund, P.T. Akins, C.Y. Hsu, Neuronal [35] J. Sharkey, I.M. Ritchie, P.A.T. Kelly, Perivascular microapplication apoptosis: Current understanding of molecular mechanisms and of endothelin-1: A new model of focal cerebral ischaemia in the rat, potential role in ischemic brain injury, J. Neurotrauma 12 (1995) J. Cereb. Blood Flow Metab. 13 (1993) 865–871.
´ 843–852. [36] M.J. Sheardown, E.O. Nielson, A.J. Hansen, P. Jacobsen, T. Honore, [18] B. Lin, W.D. Dietrich, R. Busto, M.D. Ginsberg, (S )-Emopamil 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline: a
neuro-protects against global brain injury in rats, Stroke 21 (1990) 1734– protectant for cerebral ischemia, Science 247 (1990) 571–574.
Jakobsen, The neuroprotective effect of NNC 09-0026, a new on calcium channel diversity, 1988–1994, Trends Neurosci. 18 calcium channel blocker in global ischemia, J. Cereb. Blood Flow (1995) 52–54.
Metab. 13 (1993) S662. [48] K. Valentino, R. Newcomb, T. Gadbois, T. Singh, S. Bowersox, S. [38] M.J. Sheardown, P.D. Suzdak, L. Nordholm, AMPA, but not Bitner, A. Justice, D. Yamashiro, B.B. Hoffman, R. Ciaranello, G. NMDA, receptor antagonism is neuroprotective in gerbil global Miljanich, J. Ramachandran, A selective N-type calcium channel ischaemia, Eur. J. Pharmacol. 236 (1993) 347–353. antagonist protects against neuronal loss after global cerebral
¨
[39] B.K. Siesjo, Pathophysiology and treatment of focal cerebral ischemia, Proc. Natl. Acad. Sci. USA 90 (1993) 7897–7897. ischemia, Part I: Pathophysiology, J. Neurosurg. 77 (1992) 337– [49] T. Varming, P. Christophersen, A. Moller, A. Axelsson, E.O.
354. Nielson, Synthesis and biological activity of the neuronal calcium
¨
[40] B.K. Siesjo, Pathophysiology and treatment of focal cerebral blocker 2-amino-1-(2,5-dimethoxyphenyl)-5-trifluoromethyl benz-ischemia, Part II: Mechanisms and drug treatment, J. Neurosurg. 77 imidazole (NS-649), Biorg. Med. Chem. Lett. 6 (1996) 245–248. (1992) 1247–1252. [50] P. Villa, S.H. Kaufmann, W.C. Earnshaw, Caspases and caspase [41] D.L. Small, R. Monette, A.M. Buchan, P. Morley, Identification of inhibitors, Trends Biochem. Sci. 22 (1997) 388–393.
calcium channels involved in neuronal injury in rat hippocampal
[51] J.C. Watkins, H.J. Olverman, Agonists and antagonists for excitatory slices subjected to oxygen and glucose deprivation, Brain Res. 753
amino acid receptors, Trends Neurosci. 10 (1987) 265–272. (1997) 209–218.
[52] N.I. Wood, F.C. Barone, C.D. Benham, T.H. Brown, C.A. Campbell, ¨
[42] M.L. Smith, B.K. Siesjo, Postischemic treatment with the
omega-D.G. Cooper, M.L. Evans, G.Z. Feuerstein, T.C. Hamilton, M.H. conopeptide SNX-111 protects the rat brain against ischemic
Harroes, P.D. King, J.E. Meakin, K.L. Murkitt, S.R. Patel, W.J. damage, Pharmacology of Cerebral Ischemia 1992, Krieglstein, J.
Price, J.C. Roberts, A.L. Rothaul, N.A. Samson, S.J. Smith, A.J. (Ed.) pp. 161–166, 1992.
Hunter, The effects of SB 206284A, a novel neuronal calcium [43] S. Takizawa, K. Matsushima, H. Fujita, K. Nanri, S. Ogawa, Y.
channel antagonist, in models of cerebral ischemia, J. Cereb. Blood Shinohara, A selective N-type calcium channel antagonist reduces
Flow Metab. 17 (1997) 421–429. extracellular glutamate release and infarct volume in focal cerebral
[53] M.A. Yenari, J.T. Palmer, G.H. Sun, A. de Crespigny, M.E. ischemia, J. Cereb. Blood Flow. Metab. 15 (1995) 611–618.
Moseley, G.K. Steinberg, Time-course and treatment response with [44] K. Thomas, Y. Chen, G. McPhie, C. Bath, M. Kemp, B. Small, M.
SNX-11, an N-type channel blocker, in a rodent model of focal Turvey, J. Gilmore, E. Sher, Electrophysiological characterization of
cerebral ischema using diffusion-weighted MRI, Brain Res. 739 NCC1048, a novel calcium channel blocker, Br. Neurosci. Abstracts
(1996) 36–45. 53.02 (1999) p127.
[54] E. Zea Longa, P.R. Weinstein, S. Carlson, R. Cummins, Reversible [45] C.C. Toner, J.A. Stamford, Real time measurement of dopamine
middle cerebral artery occlusion without craniectomy in rats, Stroke release in an in vitro model of neostriatal ischaemia, J. Neurosci.
20 (1989) 84–91. Methods 67 (1996) 133–140.
¨
![Fig. 1. Illustrates the structure of (N-Butyl-[5,5-bis-(4-fluorophenyl)tetra-hydrofuran-2-yl]methylamine hydrochloride, LY393615).](https://thumb-ap.123doks.com/thumbv2/123dok/3137074.1382292/4.612.78.257.598.702/illustrates-structure-butyl-uorophenyl-tetra-hydrofuran-methylamine-hydrochloride.webp)