www.elsevier.com/locate/ibmb
Characterization of subclones of the epithelial cell line from
Chironomus tentans resistant to the insecticide RH 5992, a
non-steroidal moulting hormone agonist
Marco Grebe, Peter Rauch, Margarethe Spindler-Barth
*Lehrstuhl fu¨r Entwicklungs- und Molekularbiologie der Tiere, Heinrich-Heine-Universita¨t, D-40225 Du¨sseldorf, Germany
Received 24 May 1999; received in revised form 2 February 2000; accepted 15 February 2000
Abstract
Selection of hormone resistant subclones in the continuous presence of the insecticide and ecdysteroid mimick RH 5992 (tefubenozide) resulted preferentially in clones with defects in ecdysteroid receptor function. RH 5992 is already degraded to polar products in wild-type cells; no increase in metabolism of tefubenozide is observed in resistant clones. According to Western blots, ecdysteroid receptor (EcR) and its heterodimerization partner ultraspiracle (USP) are present in all resistant clones. The concen-trations are comparable to wild-type cells, but in three clones the extent of phosphorylation of USP is diminished. With regard to hormone binding several types of hormone resistance are distinguished: (1) The same two high-affinity hormone recognition sites are present as in wild-type cells (KD1=0.31±0.28 nM, KD2=6.5±2.4 nM) but the number of binding sites is reduced. (2) The binding
site with the lower affinity (KD2) is missing. (3) The binding site with the higher affinity (KD1) is missing. (4) No specific binding
is observed. Ponasterone A binding can be rescued by addition of EcR but not by USP. (5) Ligand specificity is altered. RH 5992 can not compete [3H]-ponasterone A as efficient as in wild-type cells.2000 Elsevier Science Ltd. All rights reserved.
Keywords: Ecdysteroid; Receptor; Tefubenozide
1. Introduction
Resistant cell lines with defects in the hormonal sig-naling pathway are suited tools to study the molecular mechanism of hormone action (Feyereisen, 1998). In addition, interference with the insect’s endocrine system is considered as target for the development of new insec-ticides with presumably low vertebrate toxicity. This approach was first used with juvenile hormone analogues and since a few years also with moulting hormone (ecdysteroid) mimicks, especially diacylhydrazines. Since these compounds are used as pesticides (for review see Dhadialla et al., 1998) resistant cell lines may also help to anticipate the mechanisms for resistance against these compounds, which may arise under field conditions after prolonged treatment. Meanwhile, loss of sensitivity
* Corresponding author. Tel.: +41-211-811-2236; fax: + 41-211-811-5113.
E-mail address: [email protected] (M.
Spin-dler-Barth).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 3 2 - 1
to RH 5992 is already reported in a field population of
Cydia pomonella (Sauphanor and Bouvier, 1995).
In previous reports we have demonstrated that enhanced metabolism of 20-OH-ecdysone is associated with hormone resistance in clones of the epithelial cell line from Chironomus tentans, selected under the con-tinuous presence of 20-OH-ecdysone (Spindler-Barth and Spindler, 1998; Kayser et al., 1997). In this paper we show that selection in the presence of RH 5992 results mainly in clones with target site resistance; that means defects in the hormone receptor itself. The func-tional ecdysteroid receptor (Yao et al., 1993) is a hetero-dimer of ecdysteroid receptor (EcR) and ultraspiracle (USP). Therefore we characterized these two transcrip-tion factors in resistant sublones.
2. Experimental procedures
2.1. Cell culture and selection of resistant subclones
medium (Wyss, 1982) at 25°C and propagated by dis-sociation of the multicellular vesicles by pipetting and dilution with fresh medium 1:10 every 10–14 days. According to the method described for Drosophila Kc
-cells (Stevens and O’Connor, 1982) growth inhibition was used to select resistant subclones starting from 0.1 nM RH 5992 up to a final concentration of 0.1 µM. Gradually increasing concentrations of the hormone mimick were added over a period of about two years. After this time individual multicellular spheroids, which originate from single cells by cell division were picked under microscopical control, propagated in microtiter plates and recloned (Spindler-Barth and Spindler, 1998).
2.2. Enzyme assay
Acetylcholinesterase activity was performed as described (Spindler-Barth et al., 1988) using a fluori-metric assay (Parvari et al., 1983).
2.3. Metabolism of RH 5992
Two milliliters cell culture (7–10 days after dilution) were harvested by centrifugation (20 s, 10,000 g, 4°C). The pellets were washed once with PBS (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4,
pH=6.7) and resuspended in 1 ml fresh culture medium. After addition of [14C]-RH 5992 (spec. activity 329
KBq/mmol, kindly provided by Dr Carlson and Dr Dhadialla from Rohm and Haas Company, Spring House, PA, USA) in a final concentration of 2.5µM the cells were incubated for 24 h at 25°C. According to a modified procedure of Sundaram et al. (1998) 9 ml cold methanol containing 1% acetic acid was added and stored overnight at 220°C. After short treatment with ultrasonic power the samples were centrifuged (10 min, 10,000 g, 4°C). The supernatant was evaporated to 1 ml, filtered through a 0.22 µm filter, evaporated to dryness (Speed-Vac concentrator, Bachofer, Reutlingen, Germany) and finally dissolved in 1 ml methanol. An aliquot was taken for determination of radioactivity with a 1600 TR Packard liquid scintillation counter (Packard Canberra, Frankfurt, Germany). About 15,000 c.p.m. were separated on a reversed phase column (Radial-Pak, type 8NVC186, Waters, Milford, MA, USA) using a Waters 600TM HPLC (Waters, Eschborn, Germany)
coupled to a detector (Radiomatic 500 TR, Canberra Packard, Frankfurt, Germany). The separation was per-formed with an acetonitrile: water gradient (each with 0.1% acetic acid) and a flow rate of 1 ml/min.
2.4. Hormone binding studies
Cells were harvested by low speed centrifugation and washed once with PBS. The pellet was resuspended in buffer (20 mM HEPES, 0.4 M NaCl, 1 mM EDTA, 1
mM 2-mercaptoethanol, 20% glycerol, pH=7.9, sup-plemented with aprotinin, leupeptin, pepstatin; final con-centration 1 µg/ml each) and disrupted thoroughly with an all-glass homogenizer and centrifuged (1 h, 4°C, 100,000 g). Since NaCl impedes hormone binding, supernatants were desalted immediately with a Sephadex G-25 column (Pharmacia, Uppsala, Sweden) using the same buffer supplemented only with 20 mM NaCl.
Ligand binding was determined with [3
H]-ponasterone A (specific activity 7.9 Tbq/mmol; a kind gift of Prof. H. Kayser, Novartis, Switzerland) using a filter assay as already described in detail (Turberg and Spindler, 1992) with slight modifications (Rauch et al., 1998). KD-values
and Bmaxwere calculated according to Scatchard (1949).
Non-specific binding was determined by addition of 0.1 mM 20-OH-ecdysone or according to Chamness and McGuire (1975) and subtracted for calculation of spe-cific binding. For competition experiments receptor preparations were incubated with 2 to 5 nM [3
H]-ponas-terone A for 1 h at room temperature without or with unlabelled hormone or agonist in various concentrations.
2.5. Expression of nuclear receptors in E. coli
DNA fragments encoding EcR were cloned into the
BamH1 site of E. coli expression plasmid pGEX-KT
(Hakes and Dixon, 1992) and those for USP were cloned into the Hind III site of pGEX-KG (Guan and Dixon, 1991) as already described (Elke et al., 1997). Overnight cultures of E. coli strain BL21 (DE3) (Studier et al., 1990) transformed with the respective expression plas-mid were diluted 1:50 with fresh medium and grown at 37°C to an OD600=0,6. Expression was induced by the addition of IPTG (final concentration=0.2 mM) and the culture was incubated further for 3 h at 28°C. Bacteria were collected by centrifugation, washed once with PBS (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.5 mM
KH2PO4, pH=7.3) and suspended in buffer (20 mM
HEPES/NaOH, pH=7.9, 20 mM NaCl, 20% glycerol, 1 mM EDTA, 1 mM 2-mercaptoethanol, 1 µg/ml of each aprotinin, leupeptin and pepstatin). Cells were disrupted at 0°C by sonification (Branson Sonifier B-12, Branson, Danbury, CT, USA) using a microtip for 3×3 s with 90 W. Homogenates were centrifuged (30 min, 10,000 g, 4°C) and the supernatants used for rescue experiments. 280 ng EcR or 770 ng USP, quantified by silver stained gels, were added to 350µl cell extract from the resistant clone rh-r.3
2.6. Electrophoresis and Western blots
for 5 min (La¨mmli, 1970). 20 µg protein/lane (BCA-reagent, Pierce, Rockford, Illinois, USA) were loaded on a 1% SDS-gel (0.6×MDE gel solution, Boehringer, Ingelheim, Germany; AT Biochem., Minigel Twin, 8.6×7.2×0.1 cm, Biometra, Go¨ttingen, Germany). Gels were electroblotted on nitrocellulose membranes (BA 85, 45 µm pore size, Schleicher and Schuell, Dassel, Germany). The membranes were soaked in blocking buffer (5% milk powder, 1% fat, in PBS, 0.05% Tween 20, pH=6.8) and probed with either an affinity purified polyclonal antibody directed against the D-domain of the cEcR, designated pABcE/D (Wegmann et al., 1995) (kind gift of Prof. M. Lezzi, ETH Zu¨rich, Switzerland) or with a monoclonal antibody (AB 11) directed against the conserved 59 end of the DNA binding domain com-mon to all USPs (Khoury-Christianson et al., 1992) (kindly provided by Prof. F.C. Kafatos, EMBL, Heidel-berg, Germany) (Khyse-Andersen, 1984) as described in detail (Rauch et al., 1998). Protein bands were visualised with an ECL detection kit (Amersham-Pharmacia, Uppsala, Sweden) on X-ray films (Kodak Biomax) according to the instructions of the supplier. Quantitative evaluation of receptor concentration was done as described (Rauch et al., 1998).
3. Results
Clones resistant to RH 5992 were obtained by the same selection procedure (Stevens and O’Connor, 1982) as applied previously to select ecdysteroid resistant clones (Spindler-Barth and Spindler, 1998). A subclone was considered to be resistant, if no morphological response (Spindler-Barth et al., 1992) and no increase in acetylcholinesterase activity (Spindler-Barth and Spin-dler, 1998) was observed after treatment with 0.1 µM RH 5992 or 1µM 20-OH-ecdysone (Fig. 1). Subclasses selected after about two years in the continuous presence of tefubenozide were kept for 20 days in hormone free
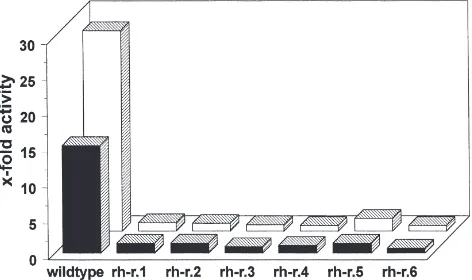
Fig. 1. Influence of 1µM 20-OH-ecdysone (black bars) and 0.1µM RH 5992 (white bars) on acetylcholinesterase activity in wild-type cells and resistant subclones of Chironomus tentans. Cells were incu-bated with hormone or agonist for seven days. (n=3, S.D.,5%).
medium to rule out transient receptor down-regulation. Once acquired, resistance was stable, since cultivation of e.g., rh-r.3 in the absence of the diacylhydrazine for about one year did not restore hormone sensitivity (data not shown).
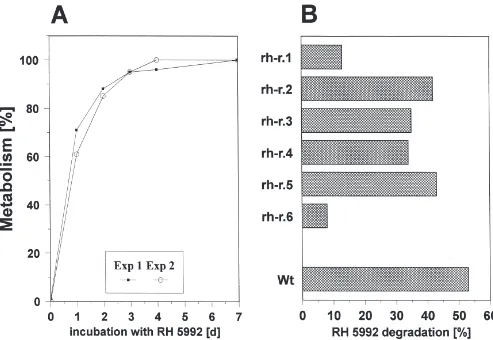
Hormone resistance is frequently accompanied by increased hormone metabolism in those clones, which were selected under the continuous presence of 20-OH-ecdysone (Kayser et al., 1997); therefore we checked metabolism of RH 5992. The hormone mimick was degraded even in wild-type cells to polar compounds (Fig. 2). Since products with the same retention time under identical separation conditions were biologically inactive (Dhadialla, personal communication) these compounds were not analysed further. The pattern of degradation products in all resistant subclones tested so far was the same as for sensitive cells (data not shown). The extent of metabolism of tefubenozide varies about five-fold between different resistant clones, but the determination of the metabolic rate in the same clone is highly reproducible (Fig. 3A, B). RH 5992 metabolism did never exceed the rate found in wild-type cells and was sometimes considerably lower (Fig. 3B).
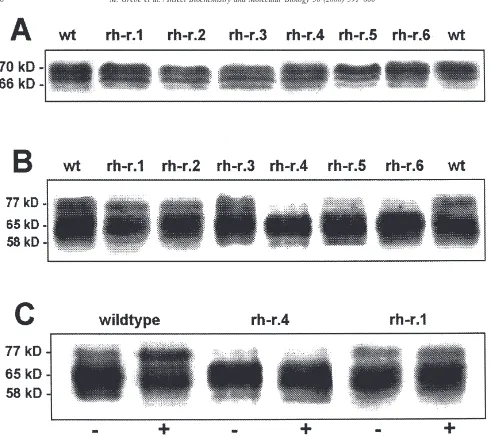
According to Western blots in all resistant clones EcR and USP were expressed in comparable concentrations as in wild-type cells (Rauch et al., 1998), but in three cases (rh-r.4, rh-r.5 and rh-r.6) the intensity of the phos-phorylated band with the lowest electrophoretic mobility was reduced in USP (Fig. 4B, Table 1). Enhanced expression of EcR and hyperphosphorylation of USP after treatment with 20-OH-ecdysone (Rauch et al., 1998), as observed in wild-type cells was not found in resistant clones (Fig. 4).
Binding of [3H]-ponasterone A was affected in
differ-ent ways. Reduced binding capacities, but normal affin-ity constants were observed (Table 1, Fig. 5). The selec-tive loss of one high-affinity binding site (either KD1 or
KD2) as well as complete loss of hormone binding were
also found. In clone rh-r.3 [3H]-ponasterone A binding
was rescued by addition of bacterially expressed EcR as shown in Fig. 6. Addition of in vitro translated EcR (data not shown) gave identical results. In contrast addition of USP has no effect on ligand binding
In one resistant clone (rh-r.2) RH 5992 competed [3H]-ponasterone A less efficiently compared to
wild-type cells. In addition to the considerably reduced num-ber of binding sites this clone had an altered ligand bind-ing specificity compared to wild-type cells. (Fig. 7, Table 2).
4. Discussion
pre-Fig. 2. Metabolism of RH 5992. Wild-type cells (7–10 days after dilution) were incubated with about 2.5 µM [14C]-RH 5992 (spec. activity=329 KBq/mmol) for 24 h at 25°C. (A) Degradation products were separated on a RP18column with acetonitrile: water gradient, flow rate 1 ml/min. Radioactivity was monitored with an in line detec-tor. (B) Control with incubation time 0 h. (C) Incubation time 24 h, retention time of non-degraded RH 5992 49.30 min, retention time of the main metabolite 5.00 min.
viously (Spindler-Barth and Spindler, 1998), monitoring of acetylcholinesterase activity is well suited to deter-mine resistance against ecdysteroids and hormone mim-icks, since hormonal stimulation of this enzyme up to about 30-fold (Spindler-Barth et al., 1988) allows the detection of very weak residual hormone responses. Monitoring of an early gene (Sundaram et al., 1998) lets expect preferentially the detection of defects in the hor-mone signalling pathway itself. Increase in
aceytcholine-sterase activity is a late hormone effect. Nevertheless, with this selection procedure using tefubenozide, clones with receptor defects were obtained. Selection in the continuous presence of 20-OH-ecdysone (Spindler-Barth and Spindler, 1998) resulted first in resistant clones with enhanced ecdysteroid metabolism (Kayser et al., 1997) and only at a later stage additional receptor defects were observed (own unpublished results). This means that the two most important mechanisms for insecticide resist-ance, metabolism and target site resistance (Mullin and Scott, 1992) against ecdysteroids and agonists arise spontaneously in the Chironomus cell line.
The ability to handle excessive concentrations of moulting hormones varies considerably among insect species and is mainly due to different efficiencies for inactivation and excretion (Blackford and Dinan, 1997a,b). Induction of inactivating enzymes after appli-cation of ecdysteroids or RH 5849 seems to be a nat-urally occurring mechanism as observed in Manduca (Williams et al., 1997). Increased susceptibility to tefub-enozide in the presence of a monooxygenase inhibitor demonstrates also the importance of metabolism (Smagghe et al., 1998). Meanwhile in the codling moth
Cydia pomonella cross-resistance with tefubenozide was
observed in a field population resistant to benzoylphe-nylureas (Sauphanor and Bouvier, 1995) which is most likely explained by an increase in inactivating enzymes involved in the degradation of both insecticides. Altered RH metabolism does not play a role in the resistant sub-clones of the Chironomus cell line, since the degradation of tefubenozide never exceeds RH 5992 metabolism in wild-type cells. This is in contrast to clones selected in the presence of 20-OH-ecdysone which show enhanced ecdysteroid metabolism but normal ligand binding.
Variation in the efficiency of metabolising enzymes doesn’t seem to be the cause for the selective action of diacylhydrazines (Smagghe and Degheele, 1993; Smagghe et al., 1998) but is caused mainly by active export of these compounds (Sundaram et al., 1998) or altered ligand binding specificity to the ecdysteroid receptor (Smagghe et al., 1996).
Fig. 3. (A) Time-course of RH 5992 metabolism in wild-type cells. Two independent experiments were shown. (B) Rate of RH 5992 metabolism in resistant subclones of the epithelial cell line from Chironomus tentans. Cells (7–10 days after dilution) were incubated with ca. 2.5µM [14 C]-RH 5992 (spec. activity=329 KBq/mmol) for 24 h at 25°C. Degradation products were separated as described in Fig. 2 and peak areas of RH 5992 and its metabolites integrated with the software Flo-One 2.0 (Packard, Frankfurt, Germany). Metabolic rates were compared with wild-type cells (Wt). Protein content of all samples was about the same (0.84±0.06 mg/ml). n=3 for wt and rh-r.6 S.D.,34%
function may be hardly compatible with life. In contrast to knock out mutations of RXR in mice (Kastner et al., 1995) which revealed that the abundance of a second isoform prevents defects, in Drosophila even one iso-form of the ecdysteroid receptor can not replace the other (Bender et al., 1997).
Selection of stable hormone resistant subclones of the epithelial cell line in the continuous presence of tefub-enozide took a period of about two years. Problems of reduced viability occurring in animals subjected to chemical mutagenesis were avoided in the cell line. This indicates that at least target site resistance at the hormone receptor level will not readily arise spontaneously and is an additional advantage of this environmentally fri-endly and safe class of insecticides. This is confirmed by treatment of Spodoptera larvae for over 12 gener-ations with tefubenozide without loss of susceptibility for up to five generations (Smagghe et al., 1998).
For Chironomus cells cultivation in the continuous presence of hormone or agonist for about two years was necessary to obtain a number of stable hormone resistant
subclones. In these clones receptor function but not expression is changed. Hormone resistance in these clones is not caused by down-regulation of receptor pro-tein or receptor specific mRNA as described previously for Drosophila cells (Stevens and O’Connor, 1982; Koelle et al., 1991).
Addition of bacterially expressed receptor, which is still functional, clearly demonstrate that rescue of ligand binding is only possible with EcR but not with USP. This identifies EcR as cause for resistance. Not yet ident-ified factors may play an additional role. This is reported for resistent clones, which arise spontaneously in
Droso-phila cells, which can not be completely rescued by
ecdysteroid receptor in contrast to mutants, which were created by parahomologeous targeting with EcR frag-ments (Cherbas and Cherbas 1996, 1997). In this case transfection studies were used, since bacterially expressed ecdysteroid receptor from Drosophila is still not available in a functional state.
Fig. 4. Western blot of 0.4 M NaCl extracts from wild-type and resistant subclones of Chironomus tentans cell line. Detection of cEcR with antiserum pABcE/D. (A) Resistant subclones show the same phosphorylation pattern as in wild-type cells. (B) Detection of cUSP with monoclonal antibody AB11. In contrast to wild-type cells the phosphorylated band (Rauch et al., 1998) with the lowest electrophoretic mobility is diminished in rh-r.4, rh-r.5 and rh-r.6. (C) In wild-type cells incubation with 20-OH-ecdysone enhances the intensity of the phosphorylated band with the lowest electrophoetic mobility (77 kD), wheras in resistant subclones no hormone effect is observed.2=control;+=hormone treated (1µM 20-OH-ecdysone, four days). Two wild-type EcR (A) and four wild-type USP (B and C) were shown to demonstrate the reproducibility of the Western blots.
data obtained with gel mobility shift assays (Elke et al., unpublished results) let assume mutations in the DNA-binding domain (DBD) also. In Drosophila, chemically induced mutagenesis with EMS (Bender et al., 1997) produced missence mutations mainly in the LBD and DBD. These regions seem to be most susceptable for mutational variation of nuclear receptors in general, since natural occurring mutations in other steroid hor-mone receptors e.g., the androgen receptor of patients with partial or complete insensitivity syndrome revealed that these two domains are also preferentially affected (McPhaul et al., 1993; Spindler et al., 1998).
However, it must be considered that the functional
597
Grebe
et
al.
/
Insect
Biochemistry
and
Molecular
Biology
30
(2000)
591–600
Table 1
Characterization of resistant subclones from the epithelial cell line from Chironomus tentansa
Clone KD1[nM] Bmax1 KD2[nM] Bmax2 “n” for KD Complementation with GST- cEcR hyperphosphorylation* [% of cUSP hyperphosphorylation* [% of
[fmol/mg [fmol/mg and Bmax cEcR [spec. bdg. of ponA in % total receptor] total receptor]
protein] protein] of pure extract]
Wild-type 0.31±0.28 9.7±6.5 6.5±2.4 142±88 10 n.d. 55±5 (n=7) 23±4 (n=5) rh-r.1 0.4±0.2 5.3±3.7 – – 3 395 50±8 (n=7) 18±3 (n=4)
rh-r.2 0.06 1.2 3.3 15 1 358 50±5 (n=7) 17±3 (n=4)
rh-r.3 – – – – 6 see Fig. 6B 46±6 (n=6) 17±2 (n=4)
rh-r.4 0.08±0.02 1.6±0.6 3.6±2.6 9.3±5.0 3 145 60±6 (n=7) 8±2 (n=4) rh-r.5 – – 3.8±2.9 26±12 6 393 58±6 (n=7) 12±5 (n=6) rh-r.6 0.96±0.57 7.0±1.3 – – 5 736 60±12 (n=7) 9±3 (n=6)
a In all clones acetylcholinesterase is no longer induced. RH 5992 metabolism in no case exceeds tefubenozide metabolism in wild-type cells. In contrast to EcR, USP never restores ligand
Fig. 5. [3H]-ponasterone A binding of 0.4 M NaCl extracts from resistant cell clones were calculated according to Scatchard (1949). Non-specific binding was determined and subtracted according to Chamness and McGuire (1975). (A) The same two high-affinity hormone recognition sites (KD1=0.1 nM, KD2=7.1 nM) are present as in wild-type cells (Rauch et al., 1998). The number of binding sites is reduced. (B) Only the binding site with the higher affinity (KD1=0.1 nM) is observed. (C) Only the binding site with the lower affinity (KD2=2.5 nM) is present. (D) No specific binding is detectable.
EcR/USP in Manduca (Song and Gilbert, 1998) is diminished.
From vertebrate receptors it is known that interactions of nuclear receptor domains either intramolecularly or intermolecularly can modify receptor function (Scheller et al., 1998). In contrast to Drosophila, where only one USP isoform has been found, in some insects two iso-forms are expressed (Kapitskaya et al., 1996; Song and Gilbert, 1998) and this seems also be the case in
Chiron-omus (Rauch et al., 1998; Voegtli et al., 1999). Although
both isoforms confer hormone binding to EcR it is not known, whether these isoforms are associated with dif-ferent functions and whether they can replace each other in vivo. The fact that both isoforms (Song and Gilbert, 1998) or specific mRNAs (Jindra et al., 1996) are expressed differently during development in Manduca indicates, that there may be also functional differences. Rescue experiments with either in vitro translated
(data not shown) or bacterially expressed nuclear recep-tors (Fig. 6) identified defects in EcR as cause for resist-ance and demonstrates that EcR can be expressed in E.
coli in a functional state. In order to correlate the
observed functional defects with changes in the receptor structure we are now engaged in sequencing the ecdys-teroid receptor and ultraspiracle from resistant subclones.
Acknowledgements
The work was supported by the “Deutsche Forschung-sgemeinschaft” (Teilprojekt A5, SFB 351). The skilful technical assistance of Mrs K. Himmelberg is gratefully acknowledged. [14C]-RH 5992 and the corresponding
non labelled compound was a kind gift from Dr Carlson (Rohm and Haas Company, Spring House, Pennsylvania, USA). [3
Fig. 6. Reconstitution of specific [3H]-ponasterone A binding (KD=2.5 nM) calculated according to Scatchard (1949). (e) Incubation of 0.4 M NaCl extract from the resistant subclone rh-r.3 with bac-terially expressed GST-cEcR. (D) Same incubation conditions as before with the exception, that GST-cEcR was replaced by GST-cUSP.
Fig. 7. Altered specificy of hormone binding in the resistant subclone rh-r.2 (s) in comparison to the wild-type of Chironomus tentans (D).
0.4 M NaCl extracts were incubated with 2–5 nM [3H]-ponasterone A and increasing amounts of non-labelled RH 5992 for 1 h at room temperature (Means±S.D., wild-type, n=3; rh-r.2, n=8).
Table 2
Change in specificity of hormone binding in resistant clone rh-r.2 in comparison to wild-type cells
Compound KD-values [µM] KD-values [µM] KDrh-r.2/KD wild-type rh-r.2 wild-type
Muristerone A 0.003 0.08±0.02 27
20-OH- 0.4±0.2 19±12 48
ecdysone
RH 5992 0.02±0.01 193±269 9,700
Kayser, Novartis, Basel, Switzerland). Antiserum pABcE/D, pGBX-kG-ctEcR and pGBX-kG-ctMSP were kind gifts from Prof M. Lezzi (ETH Zu¨rich, Switzerland) and antibody AB11 was obtained from Prof F.C. Kafatos (EMBL, Heidelberg, Germany).
References
Bender, M., Imam, F.B., Talbot, W.S., Ganetzky, B., Hogness, D.S., 1997. Drosophila ecdysone receptor mutations reveal functional differences among receptor isoforms. Cell 91, 777–788.
Blackford, M., Dinan, L., 1997a. The effects of ingested ecdysteroid agonists (20-hydroxyecdysone, RH5849 and RH5992) and an ecdy-steroid antagonist (cucurbitcacin B) on the larval development of two polyphagous lepidopterans (Acherontia atrops and Lacanobia
oleracea). Entomol. Exp. Appl. 83, 263–276.
Blackford, M., Dinan, L., 1997b. The effects of ingested 20-hydrox-yecdysone on the larvae of Aglais uritcae, Inachis io, Cynthia
car-dui (Lepidoptera: Nymphalidae) and Tyria jacobaeae (Lepidoptera:
Arctiidae). J. Insect Physiol. 43, 315–327.
Cherbas, P., Cherbas, L., 1996. Molecular aspects of ecdysteroid hor-mone action. In: Gilbert, L.I., Tata, J.R., Atkinson, B.G. (Eds.), Metamorphosis; Postembryonic Reprogramming of Gene Expression in Amphibian and Insect Cells. Academic Press, San Diego, pp. 175–221.
Cherbas, L., Cherbas, P., 1997. “Parahomologeous” gene targeting in
Drosophila cells: an efficient, homology-dependent pathway of
illegitimate recombination near a target site. Genetics 145, 349– 358.
Chamness, G.C., McGuire, W.L., 1975. Scatchard plots: common errors in correction and interpretation. Steroids 26, 538–542. Chrousos, G.P., Loriaux, D.L., Lipsett, M.B., 1986. Mechanisms and
clinical aspects of steroid hormone resistance. In: Adv. exp. Med. Biol., 196. Plenum Press, New York.
Dhadialla, T.S., Carlson, G.R., Le, D.P., 1998. New insecticides with ecdysteroidal and juvenile hormone activity. A. Rev. Entomol. 43, 545–569.
Elke, C., Vo¨gtli, M., Rauch, P., Spindler-Barth, M., Lezzi, M., 1997. Expression of EcR and USP in Escherichia coli: purification and functional studies. Arch. Insect. Biochem. Physiol. 35, 59–69. Elke, C., Rauch, P., Spindler-Barth, M., Spindler, K.-D., 1999.
DNA-binding properties of the ecdysteroid receptor-complex (EcR/USP) of the epithelial cell line from Chironomus tentans. Arch. Insect Biochem. Physiol. 41, 124–133.
Feyereisen, R., 1998. Juvenile hormone resistance! No PASaran! Proc. Natl. Acad. Sci. USA 95, 2725–2726.
Guan, K.L., Dixon, J.E., 1991. Eukaryotic proteins expressed in
Escherichia coli: an improved thrombin cleavage and purification
procedure of fusion proteins with gluatathione S-transferase. Ana-lyt. Biochem. 192, 262–267.
Hakes, D.J., Dixon, E., 1992. New vectors for high level expression of recombinant proteins in bacteria. Analyt. Biochem. 202, 293–298. Jindra, M., Malone, F., Hiruma, K., Riddiford, L.M., 1996. Develop-mental profiles and ecdysteroid regulation of the mRNAs for two ecdysone receptor isoforms in the epidermis and wings of the tob-acco hornworm Manduca sexta. Dev. Biol. 180, 258–272. Kapitskaya, M., Wang, S., Cress, D.E., Dhadialla, T.S., Raikhel, A.S.,
1996. The moscito ultraspiracle homologue, a partner of ecdys-teroid heterodimer: cloning and characterization of isoforms expressed during vitellogenesis. Molec. Cell. Endocrinol. 121, 119–132.
Kayser, H., Winkler, T., Spindler-Barth, M., 1997. 26-Hydroxylation of ecdysteroids is catalyzed by Cytochrom P450. Relation to ecdys-teroid resistance in an insect cell line. Eur. J. Biochem. 248, 707–716.
Khoury-Christianson, A.M., King, D.L., Hatzivassiliou, E., Casas, J.E., Hallenbeck, P.L., Nikodem, V.M., Mitsialis, S.A., Kafatos, F.C., 1992. DNA binding and heterdimerization of the Drosophila tran-scription factor chorion factor 1/ultraspiracle. Proc. Natl. Acad. Sci. USA 89, 11503–11507.
Khyse-Andersen, J., 1984. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J. Biochem. Biophys. Meth. 10, 203–209.
Koelle, M.R., Talbot, W.S., Segraves, W.A., Bender, M.T., Cherbas, P., Hogness, D.S., 1991. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor super-family. Cell 67, 59–77.
La¨mmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 27, 680–685. McPhaul, M.J., Marcelli, M., Zoppi, S., Griffin, J.E., Wilson, J.D.,
1993. Genetic basis of endocrine disease. 4. The spectrum of mutations in the androgen receptor gene that causes androgen resistance. J. Clin. Endocrinol. Metabol. 76, 17–23.
Mullin, C.A., Scott, J.G. (Eds), 1992. Molecular mechanisms of insec-ticide resistance. Diversity among insects. ACS Symposium Series, pp. 1–13. American Chemical Society, Washington.
Parvari, R., Pecht, I., Soreq, H., 1983. A microfluorimetric assay for cholinesterases, suitable for multiple kinetic determinations of pico-moles of released thiocholine. Analyt. Biochem. 133, 450–456. Rauch, P., Grebe, M., Elke, C., Spindler, K.-D., Spindler-Barth, M.,
1998. Ecdyssteroid receptor and ultraspiracle from Chironomus
tentans (Insecta) are phosphoproteins and are regulated differently
by molting hormone. Insect Biochem. Molec. Biol. 28, 265–275. Sauphanor, B., Bouvier, J.C., 1995. Cross-resistance between
ben-zoylureas and benzoylhydrazines in the codling moth Cydia
pomonella. Pestic. Sci. 45, 369–375.
Scatchard, G., 1949. The attractions of proteins for small molecules and ions. Ann. N.Y. Acad. Sci. 51, 660–672.
Scheller, A., Hughes, E., Golden, K.L., Robins, D.M., 1998. Multiple receptor domains interact to permit, or restrict, androgen-specific gene activation. J. Biol. Chem. 273, 24216–24222.
Smagghe, G., Degheele, D., 1993. Metabolism, pharmacokinetics and toxicity of the first nonsteroidal ecdysteroidal agonist RH 5849 to
Spodoptera exempta (Walker), Spodoptera exigus (Hu¨bner), and Leptinotarsa decemlineata (Say). Pestic. Biochem. Physiol. 46,
149–160.
Smagghe, G., Dhadialla, T.S., Derycke, S., Tirry, L., Degheele, D., 1998. Action of ecdysteroid agonist tebufenozide in susceptble and artificially selected beet armyworm. Pestic. Sci. 54, 27–34. Smagghe, G., Eelen, H., Verschelde, E., Richter, K., Degheele, D.,
1996. Differential effects of nonsteroidal ecdysteroid agonists in Coleoptera and Lepidoptera: analysis of evagination and receptor binding in imaginal discs. Insect Biochem. Molec. Biol. 26, 687– 695.
Song, Q., Gilbert, L.I., 1998. Alterations in the ultraspircacle (USP) content and phosphorylation state accompany feedback regulation of ecdysone synthesis in the insect prothoracic gland. Insect Biochem. Molec. Biol. 28, 849–860.
Spindler, K.-D., Schweikert, H.U., Weidemann, W.W., 1998. The human androgen receptor: mutations and diseases. Current Trends in Steroid Hormone Research 1, 83–91.
Spindler-Barth, M., Junger, E., Spindler, K.D., 1992. Vesicle formation and ecdysteroid induced cellular differentiation in the epithelial cell line from Chironomus tentans. Tissue and Cell 24, 919–934. Spindler-Barth, M., Spindler, K.-D., 1998. Ecdysteroid resistant
sub-clones of the epithelial cell line from Chironomus tentans (Insecta, Diptera). I. Selection and characterization of resistant clones. In Vitro Cell Dev. Biol. Animal 36, 116–122.
Spindler-Barth, M., Schmidt, H., Drews, U., Spindler, K.D., 1988. Increase in the activity of acetylcholinesterase by 20-OH-ecdysone in a Chironomus tentans cell line. Roux’s Arch. Dev. Biol. 197, 366–369.
Stevens, B., O’Connor, J.D., 1982. The acquisition of resistance to ecdysteroids in cultured Drosophila cells. Dev. Biol. 94, 176–182. Studier, F.W., Rosenberg, A.H., Dunn, J.I., Dubendorff, J.W., 1990. Use of T7 polymerase to direct expression of cloned genes. Meth. Enzymol. 185, 60–89.
Sundaram, M., Palli, S.R., Krell, P.J., Sohi, S.S., Dhadialla, T.S., Retnakaran, A., 1998. Basis for selective action of a synthetic molt-ing hormone agonist RH-5992 on lepidopteran insects. Insect Biochem. Molec. Biol. 28, 693–704.
Turberg, A., Spindler, K.-D., 1992. Properties of nuclear and cytosolic ecdysteroid receptors from an epithelial cell line from Chironomus
tentans. J. Insect Physiol. 38, 81–91.
Voegtli, M., Elke, C., Imhof, M.O., Lezzi, M., 1998. High level trans-activation by the ecdysone receptor complex at the core recognition motif. Nucl. Acids Res. 26, 2407–2414.
Voegtli, M., Imhof, M.O., Brown, N.E., Rauch, P., Spindler-Barth, M., Lezzi, M., Henrich, V.C., 1999. Functional characterization of two
Ultraspiracle forms (CtUSP-1 and CtUSP-2) from Chironomus tentans. Insect Biochem. Molec. Biol. 29, 931–942.
Wegmann, I., Quack, S., Spindler, K.-D., Dorsch-Ha¨ßler, K., Vo¨gtli, M., Lezzi, M., 1995. Immunological studies on the developmental and chromosomal distribution of ecdysteroid receptor protein in
Chironomus tentans. Arch. Insect Biochem. Physiol. 30, 95–114.
Williams, D.R., Chen, J.-H., Fisher, M.J., Rees, H.H., 1997. Induction of enzymes involved in molting hormone (ecdysteroid) inactivation by ecdysteroids and an agonist, 1,2-dibenzoyl-1-tert-butylhydrazine (RH 5849). J. Biol. Chem. 272, 8427–8432.
Wilson, T.G., Fabian, J., 1986. A Drosophila melanogaster mutant resistant to a chemical analog of juvenile hormone. Dev. Biol. 118, 190–201.
Wyss, C., 1982. Chironomus tentans epithelial cell lines sensitive to ecdysteroids, juvenile hormones, insulin and heat shock. Exp. Cell. Res. 139, 309–319.
![Fig. 2.Metabolism of RH 5992. Wild-type cells (7–10 days afterdilution) were incubated with about 2.5 µM [14C]-RH 5992 (spec.activity=329 KBq/mmol) for 24 h at 25°C](https://thumb-ap.123doks.com/thumbv2/123dok/3120830.1379304/4.598.48.288.68.522/fig-metabolism-wild-cells-afterdilution-incubated-spec-activity.webp)

![Fig. 5.[3H]-ponasterone A binding of 0.4 M NaCl extracts from resistant cell clones were calculated according to Scatchard (1949)](https://thumb-ap.123doks.com/thumbv2/123dok/3120830.1379304/8.598.47.554.69.449/ponasterone-binding-extracts-resistant-clones-calculated-according-scatchard.webp)
![Fig.6.Reconstitutionofspecific[3H]-ponasteroneAbinding(KD=2.5 nM) calculated according to Scatchard (1949)](https://thumb-ap.123doks.com/thumbv2/123dok/3120830.1379304/9.598.47.285.657.734/fig-reconstitutionofspecic-h-ponasteroneabinding-kd-calculated-according-scatchard.webp)